Abstract
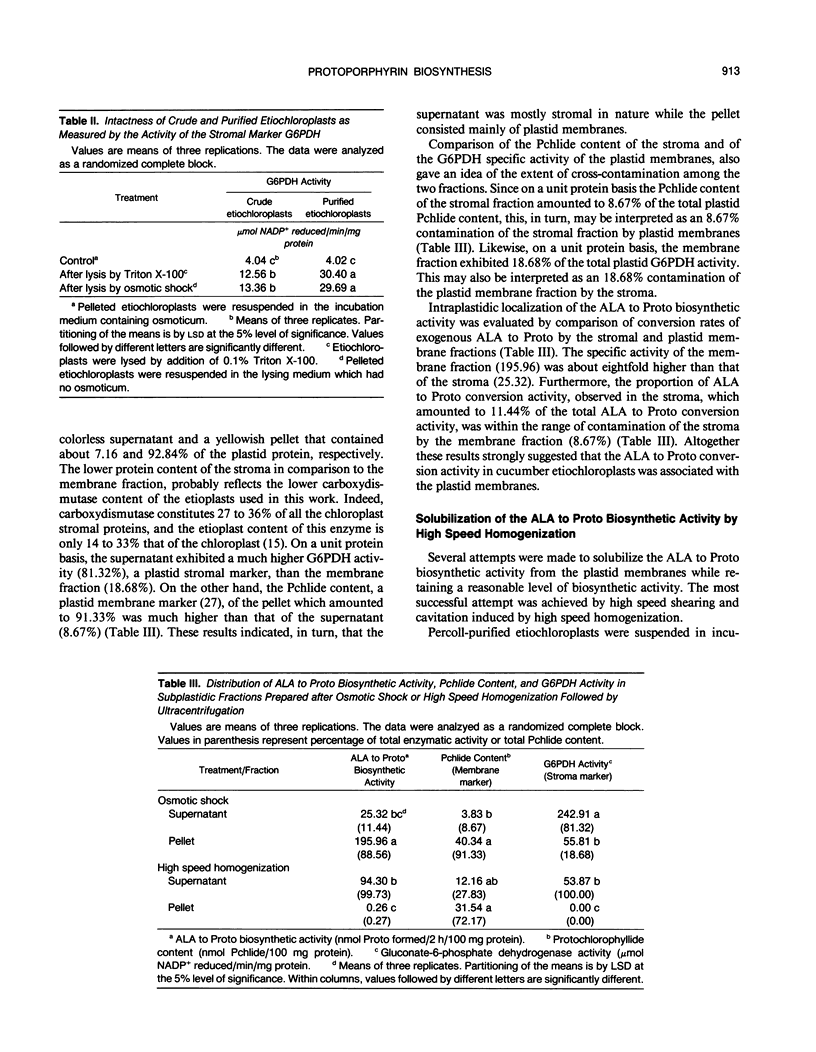
The intraplastidic localization of the enzymes that catalyze the conversion of δ-aminolevulinic acid (ALA) to protoporphyrin IX (Proto) is a controversial issue. While some researchers assign a stromal location for these enzymes, others favor a membranebound one. Etiochloroplasts were isolated from etiolated cucumber cotyledons (Cucumis sativus, L.) by differential centrifugation and were purified further by Percoll density gradient centrifugation. Purified plastids were highly intact, and contamination by other subcellular organelles was reduced five- to ninefold in comparison to crude plastid preparations. Most of the ALA to Proto conversion activity was found in the plastids. On a unit protein basis, the ALA to Proto conversion activity of isolated mitochondria was about 2% that of the purified plastids, and could be accounted for by contamination of the mitochondrial preparation by plastids. Lysis of the purified plastids by osmotic shock followed by high speed centrifugation, yielded two subplastidic fractions: a soluble clear stromal fraction and a pelleted yellowish one. The stromal fraction contained about 11% of the plastidic ALA to Proto conversion activity while the membrane fraction contained the remaining 89%. The stromal ALA to Proto conversion activity was in the range of stroma contamination by subplastidic membrane material. Complete solubilization of the ALA to Proto activity was achieved by high speed shearing and cavitation, in the absence of detergents. Solubilization of the ALA to Proto conversion activity was accompanied by release of about 30% of the membrane-bound protochlorophyllide. It is proposed that the enzymes that convert ALA to Proto are loosely associated with the plastid membranes and may be solubilized without the use of detergents. It is not clear at this stage whether the enzymes are associated with the outer or inner plastid membranes and whether they form a multienzyme complex or not.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman A., Gardeström P., Ericson I. Method to Obtain a Chlorophyll-free Preparation of Intact Mitochondria from Spinach Leaves. Plant Physiol. 1980 Sep;66(3):442–445. doi: 10.1104/pp.66.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovarnick J. G., Chang S. W., Schiff J. A., Schwartzbach S. D. Events surrounding the early development of Euglena chloroplasts: experiments with streptomycin in non-dividing cells. J Gen Microbiol. 1974 Jul;83(0):51–62. doi: 10.1099/00221287-83-1-51. [DOI] [PubMed] [Google Scholar]
- CARELL E. F., KAHN J. S. SYNTHESIS OF PORPHYRINS BY ISOLATED CHLOROPLASTS OF EUGLENA. Arch Biochem Biophys. 1964 Oct;108:1–6. doi: 10.1016/0003-9861(64)90347-9. [DOI] [PubMed] [Google Scholar]
- Castelfranco P. A., Thayer S. S., Wilkinson J. Q., Bonner B. A. Labeling of porphobilinogen deaminase by radioactive 5-aminolevulinic acid in isolated developing pea chloroplasts. Arch Biochem Biophys. 1988 Oct;266(1):219–226. doi: 10.1016/0003-9861(88)90253-6. [DOI] [PubMed] [Google Scholar]
- Daniell H., Rebeiz C. A. Chloroplast culture. IX. Chlorophyll(ide) a biosynthesis in vitro at rates higher than in vivo. Biochem Biophys Res Commun. 1982 May 31;106(2):466–470. doi: 10.1016/0006-291x(82)91133-0. [DOI] [PubMed] [Google Scholar]
- Daniell H., Rebeiz C. A. Chloroplast culture. VIII. A new effect of kinetin in enhancing the synthesis and accumulation of protochlorophyllide in vitro. Biochem Biophys Res Commun. 1982 Jan 29;104(2):837–843. doi: 10.1016/0006-291x(82)90713-6. [DOI] [PubMed] [Google Scholar]
- Davies D. D., Davies S. Purification and properties of L(+)-lactate dehydrogenase from potato tubers. Biochem J. 1972 Oct;129(4):831–839. doi: 10.1042/bj1290831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fufsler T. P., Castelfranco P. A., Wong Y. S. Formation of Mg-Containing Chlorophyll Precursors from Protoporphyrin IX, delta-Aminolevulinic Acid, and Glutamate in Isolated, Photosynthetically Competent, Developing Chloroplasts. Plant Physiol. 1984 Apr;74(4):928–933. doi: 10.1104/pp.74.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. I., Castelfranco P. A., Rebeiz C. A. Effect of the hypocotyl hook on greening in etiolated cucumber cotyledons. Plant Physiol. 1970 Nov;46(5):705–707. doi: 10.1104/pp.46.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Microbody enzymes and carboxylases in sequential extracts from c(4) and c(3) leaves. Plant Physiol. 1972 Aug;50(2):242–248. doi: 10.1104/pp.50.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J. M., Jacobs N. J. Protoporphyrinogen oxidation, an enzymatic step in heme and chlorophyll synthesis: partial characterization of the reaction in plant organelles and comparison with mammalian and bacterial systems. Arch Biochem Biophys. 1984 Feb 15;229(1):312–319. doi: 10.1016/0003-9861(84)90157-7. [DOI] [PubMed] [Google Scholar]
- Journet E. P., Douce R. Enzymic capacities of purified cauliflower bud plastids for lipid synthesis and carbohydrate metabolism. Plant Physiol. 1985 Oct;79(2):458–467. doi: 10.1104/pp.79.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo A. D., Chereskin B. M., Castelfranco P. A., Franceschi V. R., Wezelman B. E. ATP requirement for mg chelatase in developing chloroplasts. Plant Physiol. 1980 May;65(5):956–960. doi: 10.1104/pp.65.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Castelfranco P. A. Chlorophyll biosynthesis in a cell-free system from higher plants. Plant Physiol. 1971 Jan;47(1):33–37. doi: 10.1104/pp.47.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Mattheis J. R., Smith B. B., Rebeiz C. C., Dayton D. F. Chloroplast biogenesis. Biosynthesis and accumulation of protochlorophyll by isolated etioplasts and developing chloroplasts. Arch Biochem Biophys. 1975 Dec;171(2):549–567. doi: 10.1016/0003-9861(75)90065-x. [DOI] [PubMed] [Google Scholar]
- Rebeiz C. A., Mattheis J. R., Smith B. B., Rebeiz C., Dayton D. F. Chloroplast biogenesis. Biosynthesis and accumulation of Mg-protoprophyrin IX monoester and longer wavelength metalloporphyrins by greening cotyledons. Arch Biochem Biophys. 1975 Feb;166(2):446–465. doi: 10.1016/0003-9861(75)90408-7. [DOI] [PubMed] [Google Scholar]
- Simcox P. D., Reid E. E., Canvin D. T., Dennis D. T. Enzymes of the Glycolytic and Pentose Phosphate Pathways in Proplastids from the Developing Endosperm of Ricinus communis L. Plant Physiol. 1977 Jun;59(6):1128–1132. doi: 10.1104/pp.59.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. G. Subcellular localization of two porphyrin-synthesis enzymes in Pisum sativum (pea) and Arum (cuckoo-pint) species. Biochem J. 1988 Jan 15;249(2):423–428. doi: 10.1042/bj2490423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. B., Rebeiz C. A. Chloroplast Biogenesis: XXIV. Intrachloroplastic Localization of the Biosynthesis and Accumulation of Protoporphyrin IX, Magnesium-Protoporphyrin Monoester, and Longer Wavelength Metalloporphyrins during Greening. Plant Physiol. 1979 Feb;63(2):227–231. doi: 10.1104/pp.63.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Srere P. A. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Tripathy B. C., Rebeiz C. A. Chloroplast biogenesis. Demonstration of the monovinyl and divinyl monocarboxylic routes of chlorophyll biosynthesis in higher plants. J Biol Chem. 1986 Oct 15;261(29):13556–13564. [PubMed] [Google Scholar]


