Abstract
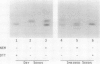
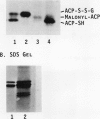
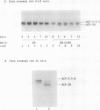
Acyl carrier protein (ACP) contains an essential sulfhydryl group in its phosphopantetheine prosthetic group. We have investigated the state of this sulfhydryl in developing and mature spinach seed (Spinacia oleracea). Seed extracts were separated on sodium dodecyl sulfate or native polyacrylamide gels, blotted to nitrocellulose, and probed with antibodies raised against spinach ACP-I. In extracts of mature seeds prepared with reducing agents, ACP-II migrated as a single major band, whereas extracts prepared without reducing agents gave two major bands. The additional band was identified as a conjugate of ACP-II to glutathione (ACP-S-S-G) on the basis of its sensitivity to reducing agents and its comigration with standards in both native and sodium dodecyl sulfate gel electrophoresis. In developing spinach seeds ACP-II exists primarily in its free sulfhydryl form or as acyl derivatives, with essentially no ACP-S-S-G present. During later stages of seed development, as seed water content declines, ACP-S-S-G accumulates to approximately 50% of the total ACP. Seed imbibition results in a rapid decline in ACP-S-S-G levels. The ACP-S-S-G:ACP-SH ratio of seeds during storage was found to be a function of seed water content and this could be manipulated by controlling the relative humidity under which the seeds were stored. We speculate that conjugation of ACP to glutathione protects the ACP from sulfhydryl oxidative damage in dry seeds.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fahey R. C., Brody S., Mikolajczyk S. D. Changes in the glutathione thiol-disulfide status of Neurospora crassa conidia during germination and aging. J Bacteriol. 1975 Jan;121(1):144–151. doi: 10.1128/jb.121.1.144-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey R. C., Mikolajczyk S. D., Brody S. Correlation of enzymatic activity and thermal resistance with hydration state in ungerminated Neurospora conidia. J Bacteriol. 1978 Sep;135(3):868–875. doi: 10.1128/jb.135.3.868-875.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Acetoacetyl-acyl carrier protein synthase, a potential regulator of fatty acid biosynthesis in bacteria. J Biol Chem. 1987 Jun 5;262(16):7927–7931. [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Altered molecular form of acyl carrier protein associated with beta-ketoacyl-acyl carrier protein synthase II (fabF) mutants. J Bacteriol. 1987 Apr;169(4):1469–1473. doi: 10.1128/jb.169.4.1469-1473.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Ohlrogge J. B., Prestegard J. H. Motional effects on NMR structural data. Comparison of spinach and Escherichia coli acyl carrier proteins. Biochem Pharmacol. 1990 Jul 1;40(1):7–13. doi: 10.1016/0006-2952(90)90171-g. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas P. L., Brody S. Neurospora crassa mitochondria contain two forms of a 4'-phosphopantetheine-modified protein. J Biol Chem. 1986 Apr 15;261(11):4785–4788. [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuo T. M. Plants have isoforms for acyl carrier protein that are expressed differently in different tissues. J Biol Chem. 1985 Jul 5;260(13):8032–8037. [PubMed] [Google Scholar]
- Post-Beittenmiller D., Jaworski J. G., Ohlrogge J. B. In vivo pools of free and acylated acyl carrier proteins in spinach. Evidence for sites of regulation of fatty acid biosynthesis. J Biol Chem. 1991 Jan 25;266(3):1858–1865. [PubMed] [Google Scholar]
- Setlow B., Setlow P. Levels of acetyl coenzyme A, reduced and oxidized coenzyme A, and coenzyme A in disulfide linkage to protein in dormant and germinated spores and growing and sporulating cells of Bacillus megaterium. J Bacteriol. 1977 Nov;132(2):444–452. doi: 10.1128/jb.132.2.444-452.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Levels of oxidized and reduced pyridine nucleotides in dormant spores and during growth, sporulation, and spore germination of Bacillus megaterium. J Bacteriol. 1977 Feb;129(2):857–865. doi: 10.1128/jb.129.2.857-865.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skujins J. J., McLaren A. D. Enzyme reaction rates at limited water activities. Science. 1967 Dec 22;158(3808):1569–1570. doi: 10.1126/science.158.3808.1569. [DOI] [PubMed] [Google Scholar]
- Wetlaufer D. B., Saxena V. P., Ahmed A. K., Schaffer S. W., Pick P. W., Oh K. J., Peterson J. D. Protein thiol-disulfide interchange and interfacing with biological systems. Adv Exp Med Biol. 1977;86A:43–50. doi: 10.1007/978-1-4684-3282-4_3. [DOI] [PubMed] [Google Scholar]