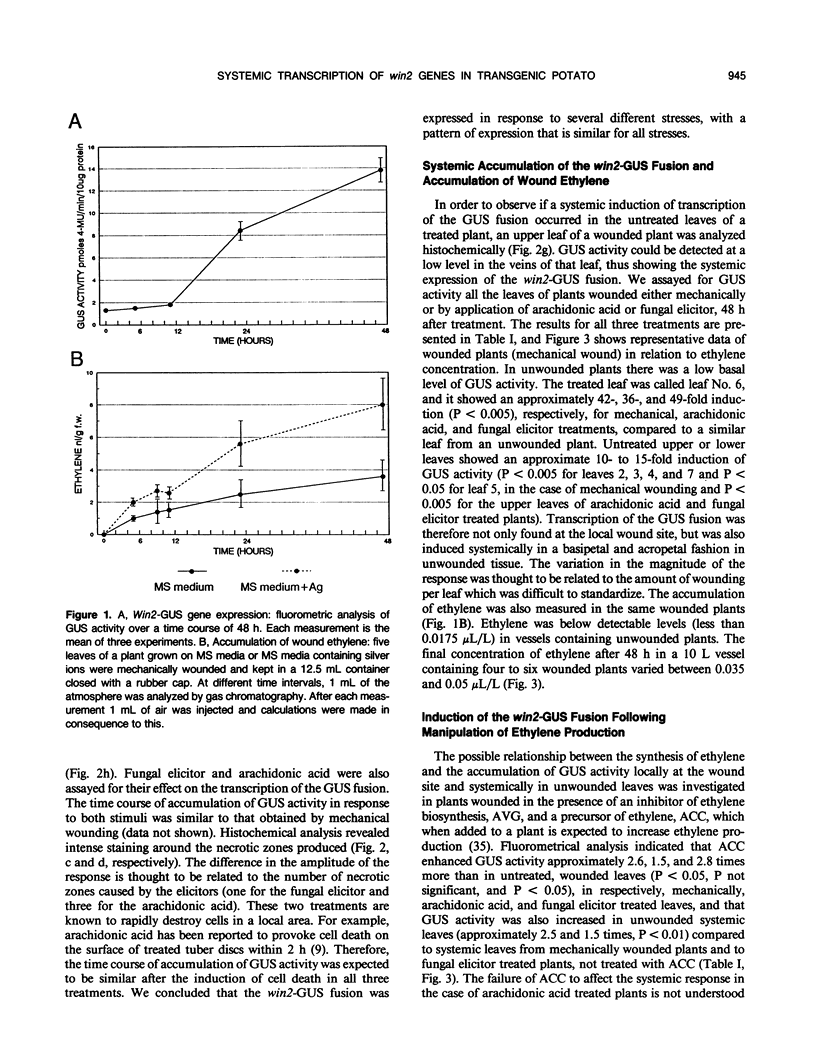
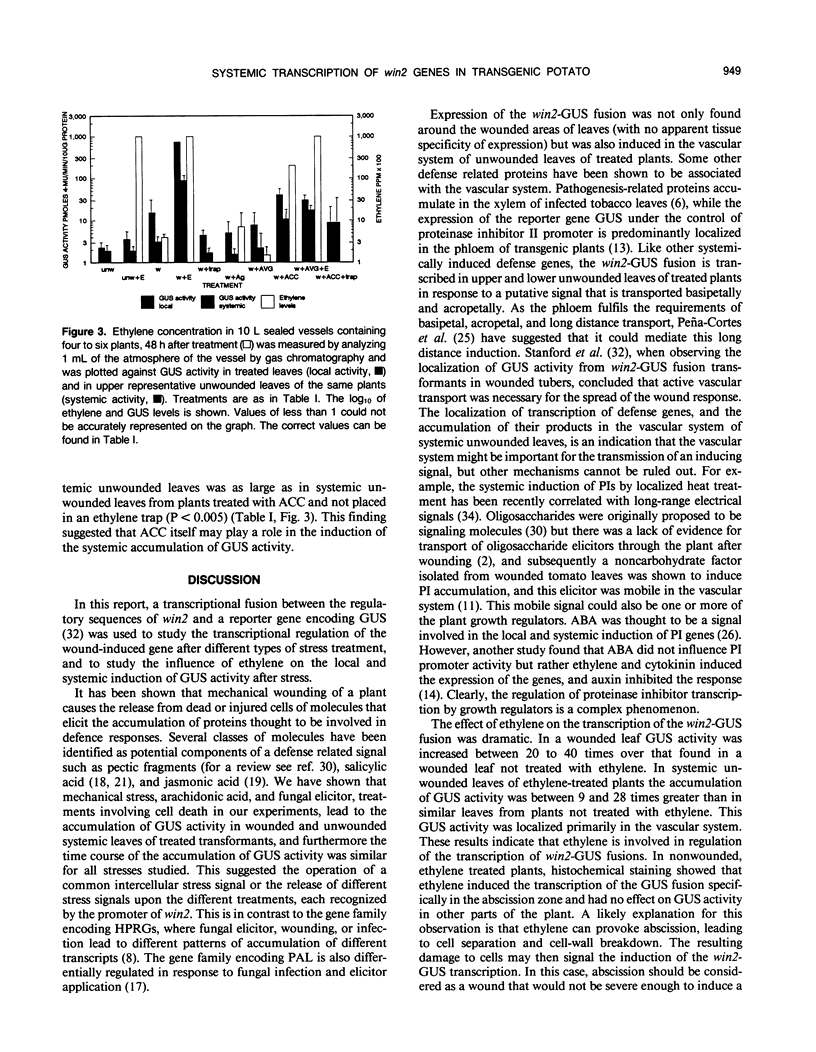
Abstract
The transcriptional regulation of a win2-β-glucuronidase gene fusion in transgenic potato (Solanum tuberosum) plants by wounding and ethylene has been analyzed. In common with other genes that are expressed in response to mechanical or chemical stress, win2 is transcribed at the site of injury and also in distant undamaged parts of the wounded plant. Similar kinetics of induction and patterns of transcription were observed in response to mechanical, wounding, elicitor, or arachidonic acid application. Experiments involving the use of chemicals that inhibited ethylene action, and those that increased ethylene production, showed that local induction of win2 transcription did not have an absolute requirement for ethylene, but ethylene was necessary for high levels of expression. In contrast, systemic expression of win2 required both a putative wound signal and ethylene. Ethylene alone had no direct effect on win2 gene expression in the absence of wounding.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Desai N. N., Neuberger A., Creeth J. M. Properties of potato lectin and the nature of its glycoprotein linkages. Biochem J. 1978 Jun 1;171(3):665–674. doi: 10.1042/bj1710665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. N., Ryder T. B., Wingate V. P., Bailey J. A., Lamb C. J. Differential accumulation of plant defense gene transcripts in a compatible and an incompatible plant-pathogen interaction. Mol Cell Biol. 1986 May;6(5):1615–1623. doi: 10.1128/mcb.6.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. Effect of silver ion, carbon dioxide, and oxygen on ethylene action and metabolism. Plant Physiol. 1979 Jan;63(1):169–173. doi: 10.1104/pp.63.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford K. J., Yang S. F. Xylem Transport of 1-Aminocyclopropane-1-carboxylic Acid, an Ethylene Precursor, in Waterlogged Tomato Plants. Plant Physiol. 1980 Feb;65(2):322–326. doi: 10.1104/pp.65.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J. P., Dixon D. C., Nikolau B. J., Voelkerding K. V., Klessig D. F. Synthesis and localization of pathogenesis-related proteins in tobacco. Mol Cell Biol. 1987 Apr;7(4):1580–1583. doi: 10.1128/mcb.7.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalutz E. Ethylene-induced Phenylalanine Ammonia-Lyase Activity in Carrot Roots. Plant Physiol. 1973 Jun;51(6):1033–1036. doi: 10.1104/pp.51.6.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin D. R., Sauer N., Lamb C. J. Differential regulation of a hydroxyproline-rich glycoprotein gene family in wounded and infected plants. Mol Cell Biol. 1987 Dec;7(12):4337–4344. doi: 10.1128/mcb.7.12.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker J. R., Davis R. W. Plant defense genes are regulated by ethylene. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5202–5206. doi: 10.1073/pnas.84.15.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Pearce G., Ryan C. A. In vitro phosphorylation of plant plasma membrane proteins in response to the proteinase inhibitor inducing factor. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1539–1542. doi: 10.1073/pnas.86.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil M., Sánchez-Serrano J. J., Willmitzer L. Both wound-inducible and tuber-specific expression are mediated by the promoter of a single member of the potato proteinase inhibitor II gene family. EMBO J. 1989 May;8(5):1323–1330. doi: 10.1002/j.1460-2075.1989.tb03512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan A., Thornburg R. W. Auxin Levels Regulate the Expression of a Wound-Inducible Proteinase Inhibitor II-Chloramphenicol Acetyl Transferase Gene Fusion in Vitro and in Vivo. Plant Physiol. 1989 Sep;91(1):73–78. doi: 10.1104/pp.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach J. E., Cantrell M. A., Sequeira L. Hydroxyproline-rich bacterial agglutinin from potato : extraction, purification, and characterization. Plant Physiol. 1982 Nov;70(5):1353–1358. doi: 10.1104/pp.70.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. W., Dron M., Cramer C. L., Dixon R. A., Lamb C. J. Differential regulation of phenylalanine ammonia-lyase genes during plant development and by environmental cues. J Biol Chem. 1989 Aug 25;264(24):14486–14492. [PubMed] [Google Scholar]
- Malamy J., Carr J. P., Klessig D. F., Raskin I. Salicylic Acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990 Nov 16;250(4983):1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Mason H. S., Mullet J. E. Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell. 1990 Jun;2(6):569–579. doi: 10.1105/tpc.2.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F., Hadwiger L. A., Boller T. Ethylene: Symptom, Not Signal for the Induction of Chitinase and beta-1,3-Glucanase in Pea Pods by Pathogens and Elicitors. Plant Physiol. 1984 Nov;76(3):607–611. doi: 10.1104/pp.76.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J. P., Signer H., Ryals J., Ward E., Wyss-Benz M., Gaudin J., Raschdorf K., Schmid E., Blum W., Inverardi B. Increase in salicylic Acid at the onset of systemic acquired resistance in cucumber. Science. 1990 Nov 16;250(4983):1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- Paradies I., Konze J. R., Elstner E. F. Ethylene: indicator but not inducer of phytoalexin synthesis in soybean. Plant Physiol. 1980 Dec;66(6):1106–1109. doi: 10.1104/pp.66.6.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. J., Bradshaw H. D., Jr, Gordon M. P. Systemic accumulation of specific mRNAs in response to wounding in poplar trees. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7895–7899. doi: 10.1073/pnas.86.20.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pēna-Cortés H., Sánchez-Serrano J. J., Mertens R., Willmitzer L., Prat S. Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9851–9855. doi: 10.1073/pnas.86.24.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. Oligosaccharide signalling in plants. Annu Rev Cell Biol. 1987;3:295–317. doi: 10.1146/annurev.cb.03.110187.001455. [DOI] [PubMed] [Google Scholar]
- Stanford A. C., Northcote D. H., Bevan M. W. Spatial and temporal patterns of transcription of a wound-induced gene in potato. EMBO J. 1990 Mar;9(3):593–603. doi: 10.1002/j.1460-2075.1990.tb08151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford A., Bevan M., Northcote D. Differential expression within a family of novel wound-induced genes in potato. Mol Gen Genet. 1989 Jan;215(2):200–208. doi: 10.1007/BF00339718. [DOI] [PubMed] [Google Scholar]



