Abstract
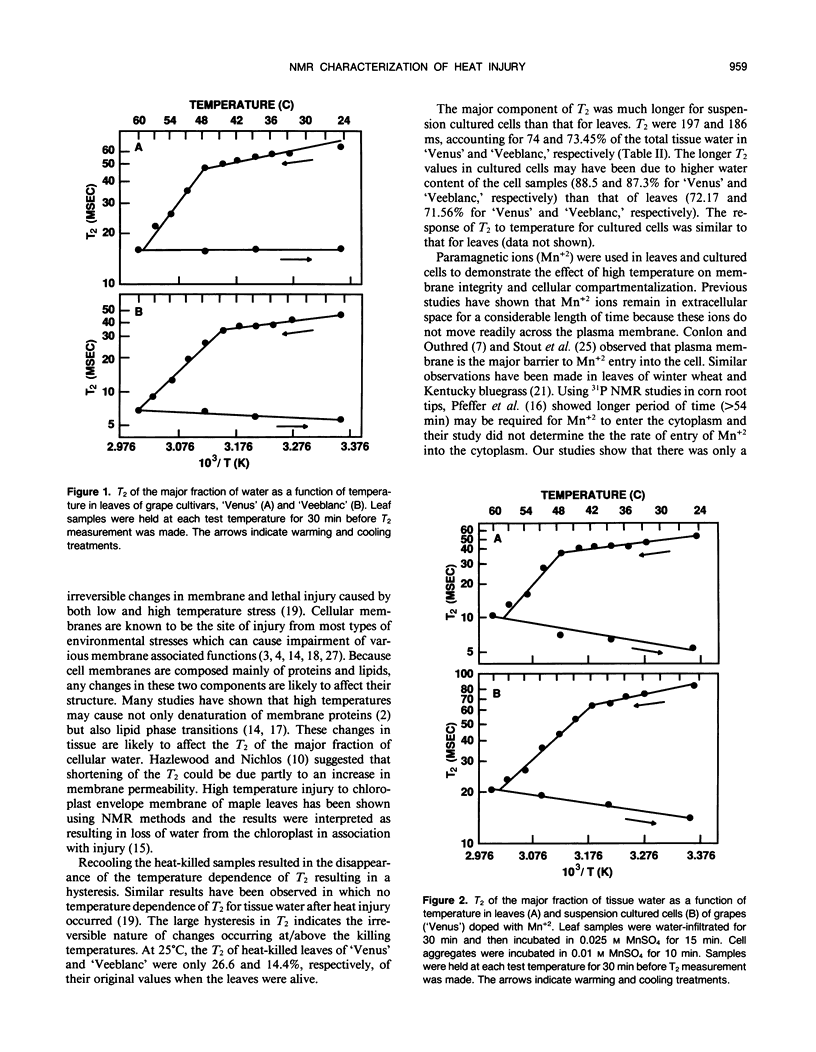
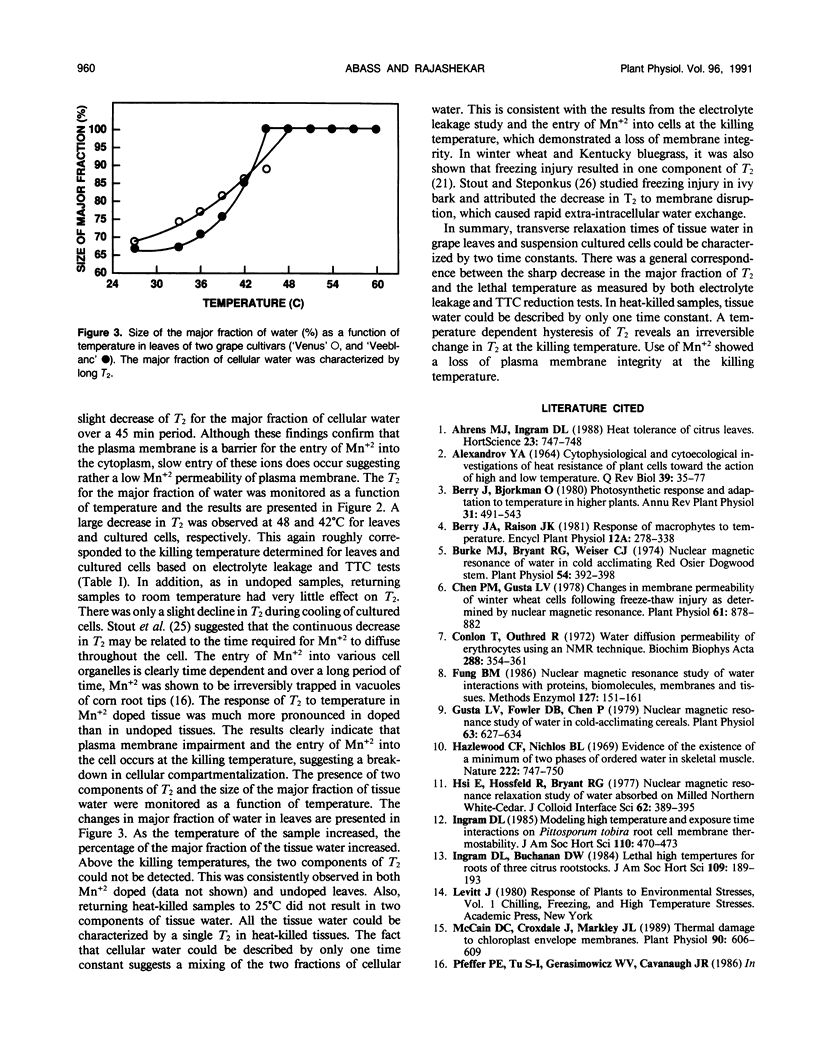
Transverse relaxation times (T2) of tissue water (1H) in leaves and suspension cultured cells of grape hybrids (Vitis spp. cv `Venus' and `Veeblanc') were measured by nuclear magnetic resonance at various temperatures. The tissue water was characterized by two T2 time constants. A sharp decrease in T2 for the major fraction of tissue water was observed in association with heat injury, as measured by electrolyte leakage and triphenyltetrazolium chloride reduction in both leaves and suspension cultured cells. The changes in T2 as a result of heat injury were irreversible, as indicated by a temperature dependent hysteresis of T2. Studies using a paramagnetic probe (Mn+2) indicated that the plasma membrane was irreversibly damaged at the killing temperature, resulting in a loss of cell compartmentalization. Tissue water in heat-killed samples was characterized by only a single T2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke M. J. Nuclear magnetic resonance of water in cold acclimating red osier dogwood stem. Plant Physiol. 1974 Sep;54(3):392–398. doi: 10.1104/pp.54.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. M., Gusta L. V. Changes in Membrane Permeability of Winter Wheat Cells following Freeze-Thaw Injury as Determined by Nuclear Magnetic Resonance. Plant Physiol. 1978 Jun;61(6):878–882. doi: 10.1104/pp.61.6.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon T., Outhred R. Water diffusion permeability of erythrocytes using an NMR technique. Biochim Biophys Acta. 1972 Nov 2;288(2):354–361. doi: 10.1016/0005-2736(72)90256-8. [DOI] [PubMed] [Google Scholar]
- Fung B. M. Nuclear magnetic resonance study of water interactions with proteins, biomolecules, membranes, and tissues. Methods Enzymol. 1986;127:151–161. doi: 10.1016/0076-6879(86)27013-5. [DOI] [PubMed] [Google Scholar]
- Gusta L. V., Fowler D. B., Chen P. A Nuclear Magnetic Resonance Study of Water in Cold-acclimating Cereals. Plant Physiol. 1979 Apr;63(4):627–634. doi: 10.1104/pp.63.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlewood C. F., Nichols B. L., Chamberlain N. F. Evidence for the existence of a minimum of two phases of ordered water in skeletal muscle. Nature. 1969 May 24;222(5195):747–750. doi: 10.1038/222747a0. [DOI] [PubMed] [Google Scholar]
- McCain D. C., Croxdale J., Markley J. L. Thermal damage to chloroplast envelope membranes. Plant Physiol. 1989 Jun;90(2):606–609. doi: 10.1104/pp.90.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. S., Berry J. A. Membrane phospholipid phase separations in plants adapted to or acclimated to different thermal regimes. Plant Physiol. 1980 Aug;66(2):238–241. doi: 10.1104/pp.66.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout D. G., Steponkus P. L. Nuclear magnetic resonance relaxation times and plasmalemma water exchange in ivy bark. Plant Physiol. 1978 Oct;62(4):636–641. doi: 10.1104/pp.62.4.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thebud R., Santarius K. A. Effects of high-temperature stress on various biomembranes of leaf cells in situ and in vitro. Plant Physiol. 1982 Jul;70(1):200–205. doi: 10.1104/pp.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]


