Abstract
Neospora caninum is a coccidial protozoan parasite that appears morphologically indistinguishable from Toxoplasma gondii and that infects a large range of mammals. Both inbred and outbred strains of mice exhibit a high degree of resistance to infection with N. caninum. Three inbred strains of mice (A/J, BALB/c, and C57BL/6) that were infected intraperitoneally with N. caninum were protected against a lethal challenge from T. gondii. Vaccine-induced protection was Neospora dose dependent. A rise in the CD8+ T-cell population in mice that had been vaccinated with N. caninum and challenged with T. gondii was observed. Adoptive transfer of CD8+ T-cell splenocytes from N. caninum-infected mice was protective against challenge with Toxoplasma. The CD8+ T cells from Neospora-infected mice proliferate to both Neospora and Toxoplasma antigens in vitro and secrete substantial quantities of gamma interferon when pulsed with the parasite antigen. These observations demonstrate that N. caninum protects against lethal T. gondii infection by the induction of CD8+ T cells that are immunoreactive to both parasites.
Neospora caninum is a recently described parasite belonging to the Apicomplexa that has been reported to infect a wide range of mammals, although human infection has not yet been described. Infection with this parasite causes a variety of clinical disorders including severe transplacentally acquired neurologic disease in dogs (2, 20). Neospora infection has been observed in other domestic animals (6) and in livestock (1, 5), where it is considered to be an important pathogen in causing abortion. In mice, N. caninum is able to induce clinical infection, producing such diseases as primary pneumonia, myositis, encephalitis, radiculoneuritis, and pancreatitis (18).
Although N. caninum and Toxoplasma gondii are morphologically similar, they are genetically distinct (19) and probably divergent (8). There is considerable antigenic distinction between these two parasite species. In particular, Neospora lacks both major Toxoplasma surface proteins, SAG1 (p30) and SAG2 (p22) (4). While the host immune response to Toxoplasma has been well studied, only recently have the mechanisms of immunity to Neospora been analyzed. In vitro observations suggest that gamma interferon (IFN-γ) may be involved in host immunity (11). Recently we have reported that mice are highly resistant to infection with large numbers (106 or more) of N. caninum tachyzoites. T cells from Neospora-infected mice proliferate in response to Toxoplasma antigen. Both interleukin 12 (IL-12) and IFN-γ appear to be important modulators of immunity to this parasite since inhibition of either of these cytokines significantly increases mouse susceptibility to infection (17).
A variety of approaches have been attempted to develop an effective vaccine against Toxoplasma. Earlier studies utilizing vaccines made from killed (e.g., by heat or formalin) organisms (12) have been unsuccessful in producing effective host immunity. Those studies have shown that partial protection was obtainable but that complete protection against challenge with even an avirulent parasite was not possible. Alternative approaches using either attenuated parasites (13) or the temperature-sensitive mutant (ts-4) (21) have produced resistance against virulent parasite challenge (9).
Immunity to T. gondii is dependent upon the early production of IFN-γ and CD8+ T cells. CD8+ T cells are considered to be major effector cells responsible for protection against T. gondii (reviewed in reference 13). Isolated CD8+ T cells exhibit cytotoxic activity in vitro against parasite-infected macrophages (9). CD8+ T cells obtained from ts-4-immunized mice are cytotoxic for the T. gondii-infected P815 mastocytoma cell line (23). Depletion of the CD8+ population but not the CD4+ population reduces the cytotoxic T-lymphocyte activity. CD8+ T cells secrete IFN-γ when stimulated with the parasite antigen. Both of these antimicrobial functions may be essential since neutralization of either IFN-γ or CD8+ T cells reverses protection. Mice vaccinated with the ts-4 mutant strain of T. gondii develop a strong protective response against subsequent challenge with a virulent RH strain. The immunity stimulated by this mutant can be adoptively transferred with CD8+ T cells. We have described an antigen-specific, cytotoxic T-cell response against T. gondii. In vivo, CD8+ T cells from mice immunized with SAG1 (p30), the major surface antigen of T. gondii, confer 100% protection against lethal parasite challenge. SAG1-specific CD8+ T-cell clones protect against T. gondii infection (15).
In this paper, we demonstrate that vaccination of mice with intact N. caninum protects mice against a lethal challenge from T. gondii. This protection is mediated by antigen-cross-reactive CD8+ T cells obtained from the spleens of N. caninum-vaccinated mice.
MATERIALS AND METHODS
Mice and parasites.
Five- to six-week-old female A/J, C57BL/6, and BALB/c mice were used for these studies (Jackson Laboratory, Bar Harbor, Maine). All studies were carried out with A/J mice except as otherwise noted in the text or legends. The NC-1 strain of N. caninum (kindly provided by David Lindsey, Auburn University) was used for vaccination. All challenge experiments were carried out 1 month postvaccination unless otherwise noted in the text or legends. Challenge experiments were performed with either the moderately virulent type II (10) PLK strain (clonally derived from Me49) or the more-virulent type I (10) RH strain of T. gondii. Both parasites were maintained by regular serial passage in human foreskin fibroblasts.
Phenotypic analysis.
Splenocytes were analyzed for cell phenotype by flow cytometric analysis using an direct immunofluorescence assay. The homogenized splenocytes (106/ml) were incubated with 1 μg of fluorescein isothiocyanate (FITC)-labeled anti-CD4+, anti-CD8+, anti-γδ (Pharmingen, San Diego, Calif.), or anti-NK cell (antiasailo GM1) rabbit polyclonal antibody. For the rabbit antibody, indirect labelling was done with FITC-conjugated anti-rabbit immunoglobulin G (IgG) as the second reagent (Sigma Chemical Co., St. Louis, Mo.). After incubation on ice for 45 min, the cells were washed in cold 3% phosphate-buffered saline–bovine serum albumin, fixed with 2% paraformaldehyde, and analyzed by fluorescence-activated cell sorter (FACS) scan.
Lymphoproliferation assay.
Mice were infected with 106 tachyzoites of N. caninum, and 14 days later their splenocytes were isolated. Purified CD8+ T cells were obtained from this population with magnetic beads (see below). The purified CD8+ T cells were cultured in 96-well plates at the concentration of 105 cells per well in a 200-μl volume. The cells were stimulated with 5 μg of concanavalin A (Con A; Sigma Chemical Co.) or 15 μg of parasite lysate per ml. Parasite lysate was prepared by sonication of 108 Percoll gradient-purified parasites. The sonicate was centrifuged at 2,500 × g, and the supernatant was measured for protein content and used at a concentration of 15 μg/ml. Feeder cells were prepared by using irradiated (3,000 rads) splenocytes from syngenic mice at a concentration of 5 × 105 irradiated feeder cells per well. After 72 h the cells were pulse labeled with tritiated thymidine (0.5 μCi/well; Amersham, Arlington Heights, Ill.) for 8 h. The cells were harvested on a glass filter by an automated multiple-cell harvester, and the incorporation of radioactive thymidine was determined by liquid scintillation.
Purification of CD8+ T cells and adoptive transfer.
Mice were vaccinated with 106 N. caninum tachyzoites. One month after vaccination, the CD8+ T cells from the infected and naive mice were isolated. Splenocytes were homogenized, and contaminating erythrocytes were lysed in buffer (Sigma Chemical Co.). For isolation of the CD8+ T-cell subset, a magnetic bead cell sorter column was used (Miltenyl Biotech, Auburn, Calif.). The cells were incubated with anti-CD8+ monoclonal antibody according to the manufacturer’s instructions. Both CD8+-enriched and -depleted fractions were collected. The CD8+ population was >95% pure as determined by FACS. Next, 107 cells from the CD8+-enriched and -depleted fractions were used to adoptively vaccinate naive mice via tail vein inoculation. Twenty four hours later the vaccinated mice were challenged with 104 PLK strain tachyzoites. The mice were observed daily for morbidity or mortality until the experiment was terminated at day 21 postchallenge.
Cytokine analysis.
For cytokine mRNA expression, splenocytes were obtained from mice at 4-day intervals postvaccination with N. caninum (from days 1 to 21) and postinfection with T. gondii (from day 1 until death). For vaccination studies, mice were first vaccinated with 106 N. caninum tachyzoites and 7 days later were challenged with a 100% lethal dose of T. gondii PLK (5 × 104 tachyzoites) via intraperitoneal inoculation. Beginning at day 1 postchallenge and continuing every fourth day through day 21 when the experiment was terminated, the splenocytes were collected and cytokine mRNA levels were determined as previously described (14, 17). Briefly, total RNA was extracted with TRIzol (Bethesda Research Laboratories) and reverse transcription was performed with random hexamer primers (Promega, Madison, Wis.). The determination of cytokine production was made by quantitative PCR. Aliquots of cDNA were assayed for IFN-γ and IL-10 by examining the competitor-to-wild-type band intensity ratio following amplification of each primer set. Separation of the PCR products was done by electrophoresis on a 3% agarose gel. The splenocytes from uninfected mice were used to establish a baseline value of 1.0 against which the levels of mRNA for the cytokines in the test mice were quantitated. The data are presented as a graph rather than as individual PCR gels for clarity. The assay for the protein concentration of IFN-γ was performed with a commercially prepared kit (Endogen, Cambridge, Mass.) in accordance with the manufacturer’s instructions.
Western blot analysis.
Western blot analysis was performed with either Neospora or Toxoplasma lysate antigen (50 μg of protein/lane). The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose paper. For T-cell Western blotting, the nitrocellulose paper was cut into 1-cm-wide strips and placed into a 96-well plate (in triplicate). The wells were treated with 1% azide–saline for 1 h followed by extensive rinsing in saline. Purified CD8+ T cells from N. caninum-vaccinated mice (105) were added to each well in the presence of irradiated feeder cells (5 × 105) obtained from the spleen cells of a naive host. After incubation for 5 days the wells were harvested and DNA synthesis was measured as described above.
For antibody Western blotting, the nitrocellulose strips containing the transferred proteins were incubated with anti-Neospora polyclonal antibody. This antibody was raised in rabbits by immunization with the equivalent of 5 × 107 purified N. caninum parasites/dose (17). Rabbits were immunized twice per week over a 3-week period. The first immunization was done in the presence of Freund’s complete adjuvant. The third and sixth immunizations were done with Freund’s incomplete adjuvant, and the remainder of the immunizations were done with parasite antigen alone. Rabbits were bled 2 weeks after the final immunization, and their sera were pooled. The IgG fraction was purified from the sera by protein A affinity, and the protein concentration was standardized with a Bio-Rad system. Alternatively, sera were obtained from N. caninum-infected mice and used for Western blotting.
RESULTS
N. caninum protects against acute infection with T. gondii.
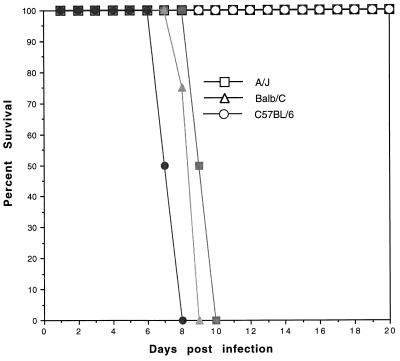
Three inbred strain of mice (C57BL/6, A/J, and BALB/c) were infected via intraperitoneal inoculation with N. caninum at 106 tachyzoites/mouse. One month later the mice were challenged with tachyzoites of the PLK strain of T. gondii. None of the N. caninum-vaccinated mice demonstrated clinical evidence of infection (ruffled fur, weight loss, huddling). As shown in Fig. 1, all of the vaccinated mice survived the infection, in contrast to nonvaccinated control mice, which died between days 8 and 10 after Toxoplasma challenge. There was no significant difference in time to death among the three inbred strains inoculated with a lethal challenge dose of Toxoplasma. Protection persists for at least 3 months postchallenge, at which point the study was terminated.
FIG. 1.
Survival of mice vaccinated with N. caninum against lethal T. gondii challenge. Inbred A/J, BALB/c, and C57BL/6 mice were vaccinated via the intraperitoneal (i.p.) route with 106 tachyzoites of N. caninum. After Neospora vaccination the mice were challenged i.p. with a 90% lethal dose of T. gondii PLK tachyzoites (open symbols). Control mice were not vaccinated but were infected with T. gondii (solid symbols). The mice were observed daily for morbidity and mortality. Results are representative of two experiments (n = 12 mice/group).
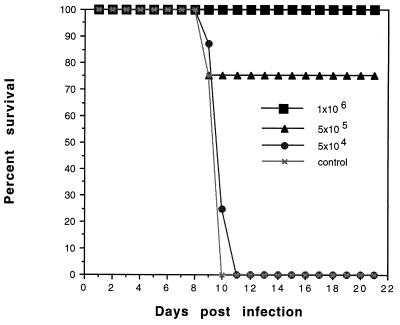
A time course study was performed to evaluate when after immunization the mice developed resistance to challenge with T. gondii. For this study, mice were vaccinated with N. caninum and challenged at 7, 14, and 30 days postvaccination with a lethal dose of T. gondii. All mice were completely protected against challenge with Toxoplasma (data not shown) beginning at day 7 postvaccination. A dose-dependent study was done to determine the number of Neospora tachyzoites required to protect against acute challenge with Toxoplasma. Mice were vaccinated with increasing numbers of viable Neospora tachyzoites. Protection against challenge with Toxoplasma was dependent upon the number of Neospora tachyzoites in the vaccination (Fig. 2). Mice vaccinated with 106 or more Neospora tachyzoites were 100% protected, whereas mice receiving fewer parasites were less well protected. Mice vaccinated with 5 × 104 Neospora tachyzoites were as susceptible as the nonvaccinated control group.
FIG. 2.
Protection by N. caninum vaccination is dose dependent. A/J mice were vaccinated with 106, 5 × 105, or 5 × 104 tachyzoites of N. caninum. Postvaccination the mice were challenged with 2.5 × 104 PLK strain tachyzoites of T. gondii via intraperitoneal inoculation. Control mice were sham vaccinated with saline. Mice were examined daily for evidence of clinical infection, and mortality was used as the end point. Results are representative of two experiments (n = 12 mice/group).
To determine if protection was dependent upon the strain and number of Toxoplasma tachyzoites used in the challenge, mice were first vaccinated with the protective dose of Neospora (106 tachyzoites). Postvaccination, the mice were challenged with either the PLK or RH strain of T. gondii. When the PLK strain was used, mice became increasingly susceptible to Toxoplasma infection as the challenge dose increased. Complete protection was observed when the challenge inoculum of PLK strain parasites was at or below 105. A similar experiment using the highly virulent RH strain as the challenge was performed. In that experiment >75% survival was observed when the challenge dose was 5 × 102 tachyzoites of T. gondii RH. Above 2 × 103 RH strain parasites, the mice were as susceptible as nonvaccinated controls (data not shown).
CD8+ T cells from Neospora-infected mice are protective.
A phenotypic analysis was carried out to evaluate the shift in immune cell subsets following parasite exposure. Mice were vaccinated with N. caninum (106 tachyzoites), followed by challenge with T. gondii PLK (104 tachyzoites). Seven days following challenge (Table 1), increased expression of the CD8+ and NK cell populations was observed in all test conditions, most notably with both vaccination and challenge. Also noted was the dramatic rise in the γδ T-cell population following exposure to either T. gondii alone or to both parasites, but not to N. caninum alone. There was a significant decrease in the CD4+ T-cell population in response to parasite infection.
TABLE 1.
Phenotypic analysis of splenocytes postvaccination and postchallengea
| Treatment | Absolute no. of cells of indicated cell type (107) ± SD
|
|||
|---|---|---|---|---|
| CD4 | CD8 | γδ | NK | |
| Saline | 1.5 ± 0.04 | 0.85 ± 0.03 | 0.16 ± 0.02 | 0.17 ± 0.04 |
| Neospora | 1.62 ± 0.08 | 1.49 ± 0.09 | 0.15 ± 0.04 | 0.65 ± 0.05 |
| Toxoplasma | 0.92 ± 0.03 | 1.12 ± 0.17 | 1.32 ± 0.04 | 0.75 ± 0.14 |
| Neospora + Toxoplasma | 1.64 ± 0.05 | 2.0 ± 0.33 | 1.25 ± 0.06 | 0.96 ± 0.11 |
Mice were infected with 106 N. caninum tachyzoites and challenged with 104 PLK strain tachyzoites of T. gondii. Phenotypic expression of CD4, CD8, γδ, and NK cells was determined either at the time of challenge (14 days postinfection with Neospora) or 7 days after Toxoplasma challenge. n = 4 mice/group.
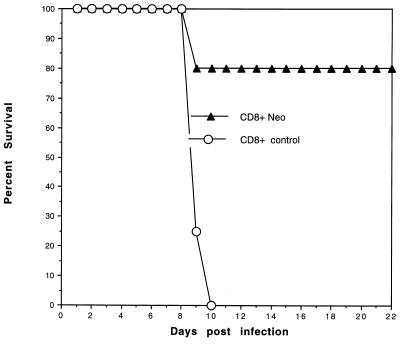
An adoptive-transfer experiment was performed to determine whether the splenocytes from vaccinated mice were able to immunize naive mice against Toxoplasma challenge. For this study (Fig. 3), mice were first vaccinated with Neospora (106 tachyzoites). One month later the isolated T cells (CD8+ and CD8−) were adoptively transferred by tail vein injection into naive mice and the mice were challenged with T. gondii PLK tachyzoites (104). Mice receiving either CD8+ T cells or whole splenocytes from Neospora-vaccinated mice were protected against Toxoplasma challenge (P < 0.001), whereas none of the controls, including CD8+ T cells from nonvaccinated mice and the residual CD8− splenocyte population from the vaccinated mice, were protective.
FIG. 3.
CD8+ T cells mediate protective immunity. A/J mice were vaccinated with 106 tachyzoites of N. caninum. Postvaccination, their splenocytes were isolated and separated with magnetic beads into a purified CD8+ population. Approximately 107 splenocytes were transferred directly into naive recipient mice via tail vein inoculation. Control mice received an equivalent number of spleen cells from saline-treated mice. Twenty-four hours after transfer the mice were challenged with 104 PLK strain tachyzoites. Results are representative of two experiments (n = 12 mice/group).
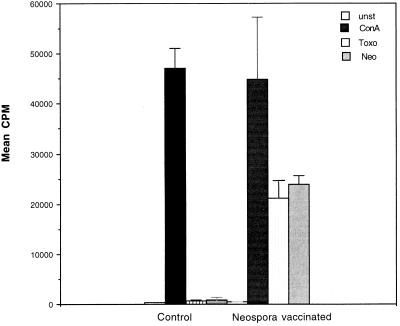
A lymphocyte DNA synthesis assay was performed to determine the antigen specificity of the CD8+ T-cell response. The CD8+ T cells from vaccinated mice were cultured in the presence of either a mitogen (Con A) or parasite antigen. As shown in Fig. 4, CD8+ T cells from Neospora-vaccinated mice were able to proliferate in vitro in response to both Neospora and Toxoplasma antigen preparations. There was no significant difference in the abilities of the lysates to induce parasite antigen-specific proliferation.
FIG. 4.
Lymphoproliferation of CD8+ T cells. Splenocytes from Neospora-vaccinated mice were isolated postvaccination. Spleen cells were pooled, and CD8+ T cells were isolated by magnetic bead separation (>95% purity as determined by FACS). The cells were cultured in 96-well plates (105 cells/well) and stimulated with mitogen or antigen plus irradiated feeder cells. At 72 (mitogen) or 96 h (antigen) postincubation DNA synthesis was measured by thymidine incorporation. unst, unstimulated; ConA, stimulated with Con A; Toxo, stimulated with Toxoplasma antigen; Neo, stimulated with Neospora antigen.
The parasite antigen specificity of the CD8+ T cells was further supported by an adoptive-transfer study. For this study, CD8+ T cells were isolated from Neospora-vaccinated mice 1 month postimmunization and cultured in the presence of the Toxoplasma antigen. Five days postculture, the CD8+ T cells were recovered (>98% purity as determined by FACS) and adoptively transferred via tail vein injection into naive mice. The adoptively immunized mice were challenged with T. gondii (Fig. 5). The CD8+ T cells from the Neospora-vaccinated mice were protective against Toxoplasma challenge, whereas naive CD8+ T cells stimulated with antigen were nonprotective.
FIG. 5.
Adoptive transfer of Toxoplasma antigen-responsive CD8+ T cells. CD8+ T cells were isolated by magnetic bead separation from the splenocytes of Neospora-vaccinated mice (Neo). The purified CD8+ T cells were 95% pure as determined by FACS and were cultured in vitro in the presence of the Toxoplasma antigen. Five days postculture, the proliferating CD8+ T cells were recovered (>98% purity as determined by FACS) and adoptively transferred (107 cells) via tail vein injection into naive mice. Mice were challenged with 104 PLK strain tachyzoites.
N. caninum modifies host cytokine response to T. gondii.
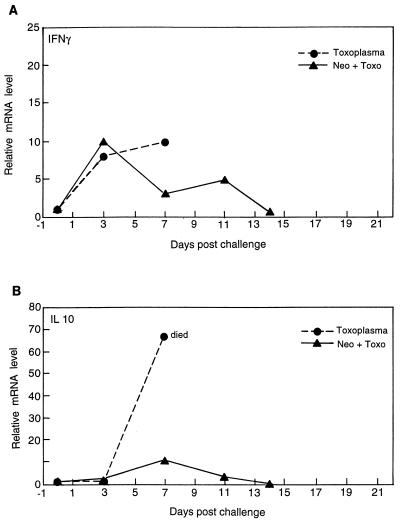
A quantitative PCR was performed to determine the level of cytokine mRNA expression in mice following infection with either Neospora, Toxoplasma, or both. Splenocytes from infected mice were collected at different time intervals postvaccination or postchallenge, and mRNA expression was measured and IFN-γ and IL-10 levels were determined. The level of mRNA for IFN-γ increased in mice vaccinated with Neospora and infected with Toxoplasma (Fig. 6A). The increased level of expression was equal to that observed for infection with Toxoplasma alone. In spite of the increased expression of mRNA, all nonvaccinated mice died by day 7 postinfection. A progressive decline in mRNA for IFN-γ after day 14 postchallenge was observed for the vaccinated and challenged group.
FIG. 6.
Cytokine mRNA expression in N. caninum-infected and T. gondii-challenged mice. Mice were infected with N. caninum, followed by challenge with T. gondii as described in Materials and Methods. The splenocytes were harvested at various time points starting at day 3 postchallenge. The spleen cells from three mice were pooled for each time point, and mRNA expression for IFN-γ (A) and IL-10 (B) was assayed by reverse transcription-PCR. The differences in the transcriptional levels for all the genes are expressed relative to those for genes of mice treated with saline (defined as 1) as previously described (16, 17). The cDNA concentration examined at each time point was standardized to the hypoxanthine phosphoribosyltransferase mRNA levels (data not shown).
A dramatic difference in the expression of mRNA for IL-10 was observed following vaccination (Fig. 6B). Mice infected with Toxoplasma alone expressed almost 70 times the background levels of mRNA for IL-10 shortly before their deaths by day 7 postinfection. In contrast, vaccinated mice demonstrated only a moderate rise in the expression of mRNA for IL-10 following Toxoplasma challenge. IL-10 expression levels returned to baseline by day 14 postchallenge.
To determine the specificity of the cytokine response, the level of IFN-γ produced by the CD8+ T cells in response to the parasite antigen was determined. For this study, the splenocytes from mice were isolated in a CD8+ T-cell population postvaccination. The CD8+ T cells were cultured in vitro in the presence of irradiated antigen-presenting cells and parasite antigen. The level of cytokine protein in the supernatant was measured. As shown in Table 2, CD8+ T cells from Neospora-infected mice produced significant quantities of IFN-γ in response to both the Toxoplasma and Neospora antigens, although there was a significantly greater quantity of IFN-γ produced in response to Neospora antigen.
TABLE 2.
IFN-γ production by CD8+ T cells from N. caninum-infected micea
| Treatment | Amt of IFN-γb (pg/ml) produced in:
|
|
|---|---|---|
| Naive mice | N. caninum-infected mice | |
| Unstimulated | N.D.c | 5.85 ± 1.4 |
| NLA | N.D. | 1,998 ± 210 |
| TLA | 6.7 ± 2.1 | 1,166 ± 43 |
Mice were infected with 106 tachyzoites of N. caninum. Purified CD8+ T cells were stimulated with 15 μg of either Neospora lysate (NLA) or Toxoplasma lysate (TLA) per ml, and the supernatant was assayed for IFN-γ by enzyme-linked immunosorbent assay.
Values are means ± SD.
N.D., not detected.
Identification of cross-reactive antigen epitopes.
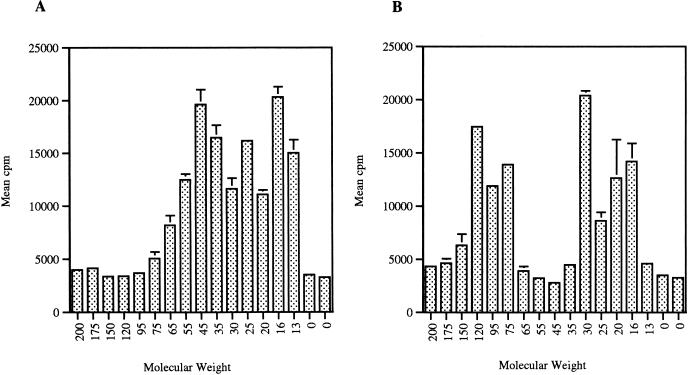
The observations above indicated that Neospora vaccination was able to protect against challenge with Toxoplasma. A T-cell Western blot analysis was performed to determine if there were T-cell antigens that were cross-reactive between the two parasite strains. Neospora and Toxoplasma lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose paper. CD8+ T cells that had been isolated from Neospora-vaccinated mice were incubated with the various parasite antigens. These CD8+ T cells proliferate in response to both Toxoplasma (Fig. 7A) and Neospora (Fig. 7B) lysates. There are Neospora antigen-reactive T cells in both the 30- and 18-kDa regions on the Western blot. Of note is the highly reactive epitope to the Neospora antigen at approximately 30 kDa, although there is no clearly defined band on the antibody-reactive blot, as shown in Fig. 8.
FIG. 7.
T-cell Western analysis of splenocytes from Neospora-vaccinated mice. Toxoplasma (A) and Neospora (B) lysates were separated by Western blotting as described in Materials and Methods. Splenocytes were isolated from Neospora-vaccinated mice, and the CD8+ population (95% pure) was incubated in the presence of the parasite antigen plus irradiated feeder cells. Lymphoproliferation in response to the parasite extract was determined by measuring thymidine incorporation. Molecular weights are in thousands. Error bars indicate SD.
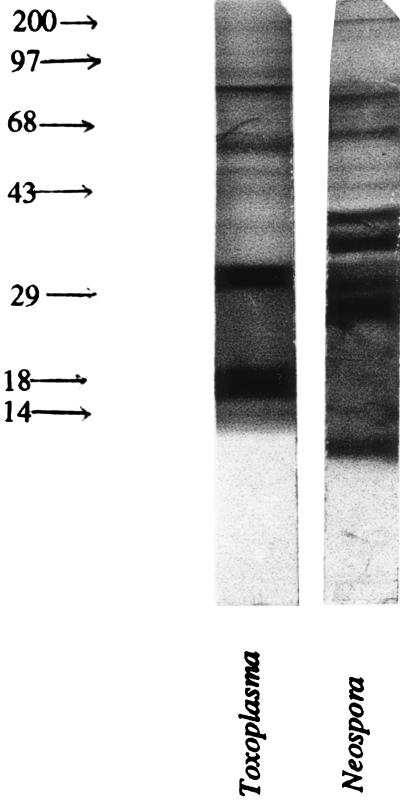
FIG. 8.
Western blot analysis of parasite antigens reactive with the anti-Neospora antibody. Toxoplasma and Neospora lysates were separated by Western blotting. The transferred proteins were reacted with a pooled sample of rabbit anti-Neospora IgG. Reactivity was determined with peroxidase-labeled goat anti-rabbit IgG. The control lanes that included human foreskin fibroblasts and peroxidase-labeled anti-rabbit IgG alone were nonreactive (data not shown).
The antigen lanes were reacted with anti-Neospora polyclonal IgG that had been isolated from the serum fraction of immunized rabbits. As shown in Fig. 8, there were multiple antigenic bands in each of the parasite preparations that were of both similar and divergent molecular weights. The most striking observation is the reactivity of the anti-Neospora antibody with the Toxoplasma lysate, in particular the reactivity with two bands in that lane of approximately 30 and 18 kDa. Neither of these bands appears as strongly reactive in the Neospora antigen lane. Similar observations were made when the sera from Neospora-infected mice were reacted with either the Toxoplasma or Neospora antigens.
DISCUSSION
In this report we demonstrate that N. caninum can mediate CD8+ T-cell immunity against acute T. gondii infection in an experimental murine model. N. caninum was first isolated from dogs (20) and infects a wide range of mammals. The development of infection in mice appears to be genetically restricted in that some mouse strains are more susceptible to infection of the central nervous system (18). Recent studies at our laboratory demonstrate that A/J mice are highly resistant to infection with this parasite (17). In the A/J mouse, Neospora infection was limited to a few scattered inflammatory foci of zoites in the brain following acute infection. Because of the strong innate resistance to Neospora infection in mice and the morphological similarity of N. caninum to T. gondii, we evaluated whether this parasite could protect against acute Toxoplasma infection.
Our studies demonstrate that vaccination with Neospora prevents death following a lethal challenge with Toxoplasma. This observation differs from those in studies by Lindsay and coworkers, who reported that N. caninum was not protective against Toxoplasma challenge (17a). In that study, the virulence of the challenge strain and the number of parasites used for challenge differed from those of ours. The RH strain (type 1) (10) of T. gondii used by Lindsay and coworkers is lethal with as few as 10 parasites. In our study, vaccinated mice were completely protected against challenge with the less-virulent PLK strain (type II) even when 1,000 times more parasites were used in the challenge. These mice were resistant to Toxoplasma challenge provided the vaccinating dose of N. caninum did not fall below 5 × 105 organisms. It appears that protection is dependent on the strain of Toxoplasma used for challenge.
The protective response elicited by Neospora is mediated by CD8+ T cells. Both Neospora and Toxoplasma can stimulate the expansion of the CD8+ T-cell subset, whereas the CD4+ T-cell subset does not expand. The CD8+ T cells proliferate in vitro in response to either parasite antigen. Adoptive transfer of CD8+ T cells but not CD8− T cells into a naive host protects against Toxoplasma challenge. Although cells from other immune compartments, most notably NK cells, are involved in innate resistance, the isolation of antigen-reactive and protective CD8+ T cells would suggest that this immunity is an antigen-specific condition.
Although Toxoplasma and Neospora may contain antigenically distinct T-cell epitopes (4), we have previously observed antigen cross-reactivity between the two parasites (17). The Western blot analysis performed with both Toxoplasma and Neospora antigens that were reacted with the IgG fractions of anti-Neospora sera demonstrates a number of shared bands, principally between 43 and 97 kDa. Of particular note are the bands in the Toxoplasma antigen lane that are not in the Neospora antigen lane and that react with the Neospora antibody. These bands are most notable at 30 and 18 kDa. Cross-reactive epitopes with similar sequence homology between Neospora and Toxoplasma may exist. Although earlier studies have suggested that these parasites belong to different genera, it is possible that there are shared immunoreactive epitopes. Recent unpublished observations have suggested that the major surface antigen of Toxoplasma (SAG1) has substantial sequence homology with a number of additional surface antigens of this parasite (3). Although the genes for these SAG1 homologs are probably distinct, it is possible that some of the expressed amino acid sequences represent T-cell epitopes common to the two organisms.
Splenocytes from Neospora-infected mice show significant proliferation in response to a Toxoplasma lysate. Of particular significance is the expansion of the CD8+ T-cell subset from Neospora-vaccinated mice in response to the presence of the Toxoplasma antigen. The T-cell Western blot assay demonstrates that there are a number of parasite antigens of various molecular masses (15 to 50 kDa) that are reactive to the CD8+ T cells. There is reactivity in the regions of 30 and 18kDa with both parasite preparations. Analyses of the relationship between different viruses and viral proteins have shown that cross-reactivity between heterologous T-cell epitopes does exist (22). Prior immunity to one virus could modulate future primary responses to another virus. A similar mechanism may be responsible for the immunity in our system, whereby an increase in the CD8+ T-cell activity directed at Neospora may provide for enhanced protection against Toxoplasma. This may account for the high level of activity in the same approximate molecular mass range as determined by T-cell Western blot analysis. These Neospora-primed CD8+ T cells may be recognizing homologs and reactive T-cell epitopes of Toxoplasma, in particular the SAG1-related sequences observed by Boothroyd (3). The importance of CD8+ T cells in host immunity to Neospora is consistent with the observations for Toxoplasma (9, 15, 23). Isolation and characterization of these cross-reactive antigens may provide a novel strategy for immunization against infection with Toxoplasma.
The importance of the cytokine response during dual infection with these two parasites must also be considered. A rise in IL-10 production at day 7 after Toxoplasma challenge has been reported and is probably involved in Toxoplasma-induced immunosuppression of the host (16). It has been further proposed that the production of IL-10 is required to prevent immune hyperactivity and that the high levels of IL-10 are a response to pathogenic levels of IFN-γ (7). A severalfold increase in the expression of IL-10 does occur at day 10 after Neospora vaccination (17). In this report, we demonstrate that during dual infection a marked reversal in the production of Toxoplasma-mediated IL-10 expression occurs. The significance of this reduced expression of IL-10 is uncertain, although it may be related to altering Toxoplasma-mediated immunosuppression (16). In contrast, vaccination with Neospora stimulates an exuberant IL-12 response (17). When Neospora-vaccinated mice are depleted of IL-12 with antibody, the level of mRNA for IL-10 rises 100-fold. It is possible that the exuberant IL-12 response observed following Neospora vaccination regulates the expression of IL-10 after Toxoplasma challenge.
It is well established that IFN-γ is essential for survival against infection with Toxoplasma. Similarly, host protection against Neospora also appears to be dependent on IFN-γ (17). During murine T. gondii infection, CD8+ T cells are an important source of IFN-γ. These studies show that following stimulation with parasite antigen, the proliferating CD8+ T cells obtained from Neospora-vaccinated mice produce a significant quantity of IFN-γ. These IFN-γ-producing CD8+ T cells are probably involved in the establishment of long-term immunity to this parasite since they are apparent for at least 1 month postvaccination.
ACKNOWLEDGMENTS
We thank Joseph Schwartzman, Sujeewa Fonseka, and Tadashi Matsuura for their assistance during this study. We appreciate the assistance of Chaitali Dutta in this study.
This work was supported in part by NIH grants AI19613, AI30000, and AI33325.
REFERENCES
- 1.Anderson M L, Blanchard P C, Barr B C, Dubey J P, Hoffman R L, Conrad P A. Neospora-like protozoan infection as a major cause of abortion in California dairy cattle. J Am Vet Med Assoc. 1991;198:241–244. [PubMed] [Google Scholar]
- 2.Bjorkman C, Lunden A, Holmdahl J, Barber J, Trees A J, Uggla A. Neospora caninum in dogs: detection of antibodies by ELISA using an iscom antigen. Parasite Immunol. 1994;16:643–648. doi: 10.1111/j.1365-3024.1994.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 3.Boothroyd J C. Molecular genetic analysis of development in Toxoplasma gondii. Park City, Utah: Keystone Symposia; 1997. [Google Scholar]
- 4.Brindley P J, Gazzinelli R T, Denkers E Y, Davis S W, Dubey J P, Belfort R, Jr, Martins M C, Silveira C, Jamra L, Waters A P, et al. Differentiation of Toxoplasma gondii from closely related coccidia by riboprint analysis and a surface antigen gene polymerase chain reaction. Am J Trop Med Hyg. 1993;48:447–456. doi: 10.4269/ajtmh.1993.48.447. [DOI] [PubMed] [Google Scholar]
- 5.Dubey J P, Lindsay D S, Anderson M L, Davis S W, Shen S K. Induced transplacental transmission of Neospora caninum in cattle. J Am Vet Med Assoc. 1992;201:709–713. [PubMed] [Google Scholar]
- 6.Dubey J P, Lindsay D S, Lipscomb T P. Neosporosis in cats. Vet Pathol. 1990;27:335–339. doi: 10.1177/030098589002700505. [DOI] [PubMed] [Google Scholar]
- 7.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 8.Guo Z G, Johnson A M. Genetic comparison of Neospora caninum with Toxoplasma and Sarcocystis by random amplified polymorphic DNA-polymerase chain reaction. Parasitol Res. 1995;81:365–370. doi: 10.1007/BF00931495. [DOI] [PubMed] [Google Scholar]
- 9.Hakim F, Gazzinelli R, Denkers E, Hieny S, Shearer G, Sher A. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J Immunol. 1991;147:2310–2316. [PubMed] [Google Scholar]
- 10.Howe D, Sibley L. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 11.Innes E A, Panton W R, Marks J, Trees A J, Holmdahl J, Buxton D. Interferon gamma inhibits the intracellular multiplication of Neospora caninum, as shown by incorporation of 3H uracil. J Comp Pathol. 1995;113:95–100. doi: 10.1016/s0021-9975(05)80075-1. [DOI] [PubMed] [Google Scholar]
- 12.Johnson A M. Toxoplasma vaccines. In: Wright I, editor. Veterinary protozoan and hemoparasite vaccines. Boca Raton, Fla: CRC Press; 1989. [Google Scholar]
- 13.Kasper L H, Boothroyd J C. Toxoplasma gondii and toxoplasmosis. In: Warren K, editor. Immunology and molecular biology of parasitic infections. Boston, Mass: Blackwell Scientific Publications; 1993. pp. 269–301. [Google Scholar]
- 14.Kasper L H, Matsuura T, Khan I A. IL-7 stimulates protective immunity in mice against the intracellular pathogen Toxoplasma gondii. J Immunol. 1995;155:4798–4804. [PubMed] [Google Scholar]
- 15.Khan I A, Ely K H, Kasper L H. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J Immunol. 1994;152:1856–1860. [PubMed] [Google Scholar]
- 16.Khan I A, Matsuura T, Kasper L H. IL-10 mediates immunosuppression following primary infection with Toxoplasma gondii in mice. Parasite Immunol. 1995;17:185–195. doi: 10.1111/j.1365-3024.1995.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 17.Khan I A, Schwartzman J D, Fonseka S, Kaspar L H. Neospora caninum: role for cytokines in host immunity. Exp Parasitol. 1997;85:24–34. doi: 10.1006/expr.1996.4110. [DOI] [PubMed] [Google Scholar]
- 17a.Lindsay D S, Blagburn B L, Dubey J P. Infection of mice with Neospora caninum does not protect against challenge with Toxoplasma gondii. Infect Immun. 1990;58:2699–2700. doi: 10.1128/iai.58.8.2699-2700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindsay D S, Lenz S D, Cole R A, Dubey J P, Blagburn B L. Mouse model for central nervous system Neospora caninum infections. J Parasitol. 1995;81:313–315. [PubMed] [Google Scholar]
- 19.Marsh A E, Barr B C, Sverlow K, Ho M, Dubey J P, Conrad P A. Sequence analysis and comparison of ribosomal DNA from bovine Neospora to similar coccidial parasites. J Parasitol. 1995;81:530–535. [PubMed] [Google Scholar]
- 20.Munday B L, Dubey J P, Mason R W. Neospora caninum infection in dogs. Aust Vet J. 1990;67:76–77. doi: 10.1111/j.1751-0813.1990.tb07706.x. [DOI] [PubMed] [Google Scholar]
- 21.Pfefferkorn E R, Pfefferkorn L C. Toxoplasma gondii: isolation and preliminary characterization of a temperature sensitive mutant. Exp Parasitol. 1976;39:365–373. doi: 10.1016/0014-4894(76)90040-0. [DOI] [PubMed] [Google Scholar]
- 22.Selin L K, Nahill S R, Welsh R M. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med. 1994;179:1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subauste C S, Koniaris A H, Remington J S. Murine CD8+ cytotoxic T lymphocytes lyse Toxoplasma gondii-infected cells. J Immunol. 1991;147:3955–3959. [PubMed] [Google Scholar]