Abstract
This perspective discussed the available evidence on the involvement of mTOR pathway in antiphospholipid syndrome (APS), from the aspects of endothelial cells, platelets, monocytes and anti-phospholipid antibodies (PLs), which may lead to future therapeutic applications of mTOR inhibition in APS.
Keywords: antiphospholipid syndrome, mTOR, autoimmune disease
Mammalian target of rapamycin (mTOR) is essential for regulating cellular metabolism and controlling cell proliferation. The mTOR enzyme has two complexes, mTOR complex (mTORC) 1 and mTORC2, with downstream target as phosphorylation of S6 ribosomal protein (S6RP) and Akt (phosphorylation at Ser473), respectively [1]. The mTOR pathway is regulated by metabolic cues, mainly glucose and amino acids, as well as by growth factors. Glucose and amino acids act through surface receptors, such as glucose transporter type 1 or type 4. Growth factor triggers PI3K-Akt axis and downstream mTOR activation (Figure 1) [1].
Figure 1:
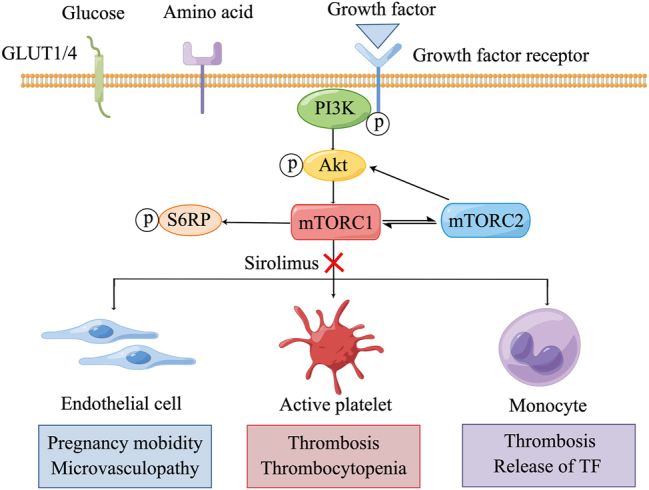
The involvement of mTOR pathway in the pathogenesis of APS. GLUT1, glucose transporter type 1; GLUT4, glucose transporter type 4; p, phosphorylation; PI3K, phosphatidylinositide 3-kinase; S6RP: ribosomal protein S6 kinase; mTORC1, mTOR complex 1; mTORC2, mTOR complex 2; TF, tissue factor. This figure was generated by Figdraw.
The antiphospholipid syndrome (APS) is a systemic autoimmune disease defined by thrombotic or obstetrical events that occur in patients with persistent antiphospholipid antibodies (aPLs) [2]. The involvement of mTOR pathway activation in APS was firstly described in 2014. However, nearly a decade has passed, the role of mTOR in pathogenic mechanisms of APS is not fully elucidated. In this perspective, we will discuss the available evidence on the involvement of mTOR pathway in APS, from the aspects of endothelial cells, platelets, monocytes and aPLs, which may lead to future therapeutic applications of mTOR inhibition in APS.
Endothelial cells
In an in vitro model, IgG isolated from APS women with both pregnancy morbidity and vascular thrombosis was observed to be able to activate the mTOR and autophagic pathways in human umbilical vein endothelial cells [3]. In addition to thrombotic and pregnancy complications, aPL-positive patients can develop microvascular manifestations, such as livedo and nephropathy. mTOR pathway was firstly reported to be involved in the development of endothelial dysfunction and proliferation in aPL-associated nephropathy [4]. Further study also found increased mTOR activity in livedoid lesions of aPL-positive patients, more significant in the lower basal layers of epidermis [5]. It seemed that both mTORC1 and mTORC2 were activated in these microvascular diseases. The favorable therapeutic effect of mTOR1 pathway inhibition by using rapamycin in APS related microangiopathy had been reported in the retrospective study and case reports [4, 6, 7].
Platelets
Platelets might play a pivotal role in both inflammation and thrombosis of APS. Thrombocytopenia is quite common in APS and is also identified as an independent risk factor for thrombotic events [8]. A vitro study had shown that inhibition of mTORC1 could prevent Fcγ-receptor IIa-mediated platelet activation by anti-β2-glycoprotein I antibody [9]. A more recent study indicated that mTORC2/Akt pathway was also involved in the activation of platelet [10]. Moreover, our pilot study had shown promising effectiveness and safety of rapamycin monotherapy in the treatment of thrombocytopenia in patients with APS [11].
Monocytes
Monocytes are the main source of circulating tissue factor, which is the main initiator of thrombosis [12]. Xia et al. had found that aPL/antigen complex could dramatically induce mTOR activation as well as expression of tissue factor and IL-8 in monocytes. The mTOR inhibitor rapamycin could attenuate the elevated expression of tissue factor and IL-8 [13].
Antiphospholipid antibodies (aPLs)
aPLs are pathogenic antibodies in APS and systemic lupus erythematosus (SLE). It has been showed that mTORC1-dependent mitochondrial dysfunction contributes to the generation of aPL in liver, and the process was blocked by rapamycin in lupus-prone mice [14]. In further human studies, diminished aPLs were also found in rapamycin treated APS or SLE patients [11, 15].
Conclusions
In conclusion, aPLs might induce a proliferative and hypercoagulable state by activation of mTOR pathway in endothelial cells, platelets, and monocytes. The limited effect of antithrombotic therapy in patients with vasculopathy and the benefit of immunosuppressive treatment for thrombocytopenia associated with APS highlight the need for comprehensive understanding of the pathogenesis of APS. The therapeutic application of mTOR inhibition is promising and needs to be explored in the future in APS. To be noted that several other signaling pathways (e.g. NFκb, p38 MAPK) besides mTOR have also been reported to be involved in the pathogenesis of APS, as well as other systemic autoimmune diseases [16], [17], [18], which deserves more attention.
Footnotes
Research ethics: Not applicable.
Informed consent: Not applicable.
Author contributions: ZLZ was responsible for topic selection, and critically revised the manuscript. AP revised the manuscript. LLJ drafted the manuscript. All the authors listed have approved the enclosed manuscript.
Competing interests: None.
Research funding: None.
Data availability: Not applicable.
Contributor Information
Zhuoli Zhang, Email: zhuoli.zhang@126.com.
Andras Perl, Email: PerlA@upstate.edu.
References
- 1.Perl A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol. 2016;12:169–82. doi: 10.1038/nrrheum.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018;378:2010–21. doi: 10.1056/nejmra1705454. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez CM, Velásquez-Berrío M, Rúa C, Viana M, Abrahams VM, Cadavid AP, et al. Antiphospholipid antibodies from women with pregnancy morbidity and vascular thrombosis induce endothelial mitochondrial dysfunction, mTOR activation, and autophagy. Front Physiol. 2021;12:706743. doi: 10.3389/fphys.2021.706743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canaud G, Bienaimé F, Tabarin F, Bataillon G, Seilhean D, Noël LH, et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med. 2014;371:303–12. doi: 10.1056/nejmoa1312890. [DOI] [PubMed] [Google Scholar]
- 5.Sevim E, Siddique S, Chalasani MLS, Chyou S, Shipman WD, O’Shea O, et al. Mammalian target of rapamycin pathway assessment in antiphospholipid antibody–positive patients with livedo. J Rheumatol. 2022;49:1026–30. doi: 10.3899/jrheum.220049. [DOI] [PubMed] [Google Scholar]
- 6.Dufour I, Venot Q, Aydin S, Demoulin N, Canaud G, Morelle J. mTORC pathway activation and effect of sirolimus on native kidney antiphospholipid syndrome nephropathy: a case report. Am J Kidney Dis. 2020;76:288–91. doi: 10.1053/j.ajkd.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 7.Sartorelli S, De Luca G, Campochiaro C, Peretto G, Sala S, Esposito A, et al. Successful use of sirolimus in a patient with cardiac microangiopathy in primary antiphospholipid syndrome. Scand J Rheumatol. 2019;48:515–6. doi: 10.1080/03009742.2019.1574022. [DOI] [PubMed] [Google Scholar]
- 8.Pardos-Gea J, Marques-Soares JR, Buján S, Ordi-Ros J, Alijotas-Reig J. Persistent thrombocytopenia predicts poor long-term survival in patients with antiphospholipid syndrome: a 38-year follow-up study. Rheumatology. 2022;61:1053–61. doi: 10.1093/rheumatology/keab475. [DOI] [PubMed] [Google Scholar]
- 9.Hollerbach A, Müller-Calleja N, Ritter S, Häuser F, Canisius A, Orning C, et al. Platelet activation by antiphospholipid antibodies depends on epitope specificity and is prevented by mTOR inhibitors. Thromb Haemostasis. 2019;119:1147–53. doi: 10.1055/s-0039-1685453. [DOI] [PubMed] [Google Scholar]
- 10.Tang Z, Shi H, Chen C, Teng J, Dai J, Ouyang X, et al. Activation of platelet mTORC2/akt pathway by anti-β2GP1 antibody promotes thrombosis in antiphospholipid syndrome. Arterioscler Thromb Vasc Biol. 2023;123:318978. doi: 10.1161/atvbaha.123.318978. [DOI] [PubMed] [Google Scholar]
- 11.Xie W, Ji L, Zhang Z. Sirolimus monotherapy for thrombocytopenia in primary antiphospholipid syndrome: a pilot study from a tertiary referral center. Front Immunol. 2022;13:857424. doi: 10.3389/fimmu.2022.857424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Åberg M, Siegbahn A. Tissue factor non-coagulant signaling – molecular mechanisms and biological consequences with a focus on cell migration and apoptosis. J Thromb Haemostasis. 2013;11:817–25. doi: 10.1111/jth.12156. [DOI] [PubMed] [Google Scholar]
- 13.Xia L, Zhou H, Wang T, Xie Y, Wang T, Wang X, et al. Activation of mTOR is involved in anti-β2GPI/β2GPI-induced expression of tissue factor and IL-8 in monocytes. Thromb Res. 2017;157:103–10. doi: 10.1016/j.thromres.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Oaks Z, Winans T, Caza T, Fernandez D, Liu Y, Landas SK, et al. Mitochondrial dysfunction in the liver and antiphospholipid antibody production precede disease onset and respond to rapamycin in lupus-prone mice. Arthritis Rheumatol. 2016;68:2728–39. doi: 10.1002/art.39791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai ZW, Kelly R, Winans T, Marchena I, Shadakshari A, Yu J, et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet. 2018;391:1186–96. doi: 10.1016/s0140-6736(18)30485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong M, Kayani T, Jones DM, Salmon JE, Whirledge S, Chamley LW, et al. Antiphospholipid antibodies increase endometrial stromal cell decidualization, senescence, and inflammation via toll-like receptor 4, reactive oxygen species, and p38 MAPK signaling. Arthritis Rheumatol. 2022;74:1001–12. doi: 10.1002/art.42068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandt KJ, Fickentscher C, Boehlen F, Kruithof EK, de Moerloose P. NF-κB is activated from endosomal compartments in antiphospholipid antibodies-treated human monocytes. J Thromb Haemostasis. 2014;12:779–91. doi: 10.1111/jth.12536. [DOI] [PubMed] [Google Scholar]
- 18.Poulton K, Rahman A, Giles I. Examining how antiphospholipid antibodies activate intracellular signaling pathways: a systematic review. Semin Arthritis Rheum. 2012;41:720–36. doi: 10.1016/j.semarthrit.2011.09.004. [DOI] [PubMed] [Google Scholar]


