Abstract
Among important candidates for babesial vaccines are apical complex proteins, including rhoptry-associated protein 1 (RAP-1) from Babesia bovis and B. bigemina, which have been shown to induce partial immunity. Four variant B. bigemina rap-1 transcripts identified in a clone of the Mexico strain have highly conserved sequence in the central region but vary in sequence at the amino and carboxy termini (NT and CT) of the predicted proteins, resulting in different combinations of NT and CT domains in the individual gene products. Cattle were immunized with native protein consisting of the RAP-α1 variant, which contains NT-1 and CT-1 domains, and T-cell responses were characterized. We previously reported the identification of two T helper (Th) cell epitopes in B. bigemina RAP-1α1 protein (I. Hötzel, W. C. Brown, T. F. McElwain, S. D. Rodriguez, and G. H. Palmer, Mol. Biochem. Parasitol. 81:89–99, 1996). One epitope mapped to the constant domain of RAP-1 (amino acids [aa] 144 to 187), and one mapped to the CT-1 variable domain (aa 386 to 480). Th1-like clones responding to these epitopes proliferated differentially to different strains of B. bigemina, raising the possibilities that the T-cell epitopes may vary antigenically and that CT-1 may be differentially expressed with respect to the other RAP-1 CT domains in the different strains. In this report, we definitively map the T-cell epitope identified in the constant domain of RAP-1 to aa 159 to 187 (FVVSLLKKNVVRDPESNDVENFASQYFYM) and show that the predicted amino acid sequence is completely conserved among seven strains. The T-cell epitope in the CT-1 domain was mapped to aa 436 to 465 (VNSEKVDADDAGNAETQQLPDAENEVRADD), which is also completely conserved among eight strains of B. bigemina. We further show that the RAP-1α1-immunized cattle were protected against homologous B. bigemina challenge, thus suggesting an association between protective immunity and the helper T-cell response against the two epitopes. The immunogenic and highly conserved nature of these T-cell epitopes and their ability to stimulate functionally relevant Th cells that express gamma interferon support their inclusion in a vaccine.
Proteins of the apical complex, which in Babesia sp. include micronemes, spherical bodies, and rhoptries, are often soluble and secreted by the parasite and are believed to play a major role in host erythrocyte invasion, nutrient acquisition, and/or egression (31, 36). Their existence as homologs in different parasite genera is suggestive of their functional relevance (29, 31, 36). Apical complex antigens have been shown to induce protection and are among the targeted vaccine antigens for malarial and babesial parasites.
Rhoptries are complex organelles and contain numerous proteins, many of which are immunogenic (31). In Babesia bigemina and B. bovis, the best-characterized rhoptry protein is the 58- to 60-kDa rhoptry-associated protein 1 (RAP-1), which is also detected on the merozoite surface in each species (24, 40). These proteins were shown to be highly immunogenic for both T and B cells and to possess epitopes conserved among strains of each species but not between species (3, 14, 23, 24, 30, 34, 37). RAP-1 is encoded by two genes in B. bovis and multiple polymorphic genes in B. bigemina. RAP-1-encoding genes have also been identified in B. canis, B. ovis, B. divergens, and B. caballi (8, 9, 36, 38). Members of this family have retained four conserved cysteine residues and considerable sequence homology. For example, B. bovis and B. bigemina RAP-1 homologs have approximately 45% amino acid sequence identity and a completely conserved 14-amino-acid (aa) sequence in the amino-terminal half of the protein (8, 39). Furthermore, several conserved oligopeptide motifs are shared by the different RAP-1 proteins and the malaria rhoptry protein, apical membrane antigen 1 (AMA-1)/pf83 (41). At or near the time of merozoite release, the 83-kDa malarial rhoptry protein is processed to a 66-kDa component which is expressed on the merozoite surface (42). However, there is no evidence for similar processing of the Babesia RAP-1 proteins, and the secretion pathways for these proteins have not been determined. Conservation of apical complex organelles and amino acid sequences in rhoptry proteins within and across genera indicate their functional significance and immunological relevance.
The ability of rhoptry-associated proteins to induce partial protective immunity against parasite challenge has been documented for several apicomplexan parasites. Protection was demonstrated with Plasmodium sp. by using rhoptry protein AMA-1, RAP-1, or RAP-2 (7; reviewed in reference 17), with B. bovis by using partially purified native RAP-1 protein or a recombinant glutathione S-transferase fusion protein consisting of a fragment of the B. bovis RAP-1 protein (45), and with affinity-purified native B. bigemina RAP-1 protein (24). It was more recently demonstrated that purified B. bigemina rhoptries conferred significant protection against B. bigemina challenge in Brazil, with the majority of antibody in immune animals directed at RAP-1 (20). However, in the studies with Babesia, the titer of specific antibody to RAP-1 did not consistently correlate with the degree of protective immunity (43), which underscores the importance of characterizing both the helper and effector cell functions of CD4+ T cells specific for RAP-1 and other babesial apical complex proteins. As helper cells, T cells that produce gamma interferon (IFN-γ) can induce isotype switching to immunoglobulin G2 (IgG2) (1, 11), the opsonizing antibody subclass in cattle (27). Through the production of this same cytokine, T cells can additionally act as effector cells to activate macrophages to produce molecules, such as reactive nitrogen intermediates, that are toxic for intraerythrocytic apicomplexan protozoa (32, 33). For these reasons, antigens that induce both opsonizing antibody and the macrophage-activating cytokine, IFN-γ, are good vaccine candidates (4).
Four different variants of the B. bigemina RAP-1 protein which have highly conserved sequence in the central region but vary in sequence at the amino and carboxy termini of the protein were found in a biological clone (JG-29) from the Mexico strain (25, 26). Two N-terminal (NT-1 and NT-2) and three C-terminal (CT-1, CT-2, and CT-3) variant domains have now been identified and shown to be present in different strains of B. bigemina (16). Transcripts of all four genes (rap-1α1, which encodes NT-1 and CT-1; rap-1β1, which encodes NT-2 and CT-1; rap-1β2, which encodes NT-2 and CT-2; and rap-1β3, which encodes NT-2 and CT-3) were identified in the Mexico JG-29 clone (16). To understand the nature of protective immunity against this complex antigen, T-cell responses were characterized in calves immunized with native RAP-1 protein. The immunogen was affinity purified by using an antibody specific for an epitope in NT-1 and thus consisted of one of the four potentially expressed gene products, RAP-1α1, which contains the NT-1 and CT-1 variant domains. RAP-1-specific immune lymph node cells and peripheral blood-derived T helper (Th) cell clones expressed predominantly type 1 cytokines, consisting of low levels of interleukin-4 (IL-4) and IL-10 and relatively high levels of IFN-γ, in response to antigenic stimulation (34, 35). RAP-1-specific Th cell clones were also shown to provide antigen-dependent help to B cells to secrete both IgG1 and IgG2 (1), which reflected the mixed IgG subclass response against RAP-1 in the immune sera of the donor cattle (34).
When lymphocytes from these RAP-1-immunized cattle were tested for recognition of different B. bigemina strains, peripheral blood mononuclear cells (PBMC) and Th cell clones proliferated differentially to Mexico, Puerto Rico, Texcoco, and St. Croix (34). Among the seven RAP-1-specific T-cell clones tested, five clones had levels of proliferation that were less than 20% of the response to the Mexico strain, whereas two clones exhibited less dramatic differences in the levels of proliferation to the different strains. These data suggested recognition of at least two distinct epitopes by the two sets of T-cell clones, which was confirmed in later studies (15). Th cell clones that responded more similarly to the different strains recognized an epitope within aa 144 to 187 in the constant region of the RAP-1 conserved among the known rap-1 gene family members of the JG-29 clone of the Mexico strain. In contrast, the five Th cell clones which exhibited differential responses to Mexico versus additional strains of B. bigemina were shown to recognize an epitope within aa 386 to 480, which comprises the CT-1 domain. CT-1-specific T cells did not cross react with CT-2. These observations raised the question of whether the reduced Th cell response to other strains of B. bigemina was due to sequence polymorphism in either T-cell epitope, which could result in reduced antigenicity for the Th cells (12). An alternative explanation for the results with CT-1-specific clones was that CT-1 was expressed at variable levels relative to CT-2 and CT-3 in the different parasite strains. The purpose of this study was to further define the T-cell epitopes in B. bigemina RAP-1 with truncated fusion proteins and synthetic peptides, to determine whether these epitopes were conserved among geographically diverse strains of the parasite, and to evaluate recognition of these epitopes by peripheral lymphocytes from immune cattle, factors critical for rational selection of vaccine epitopes.
MATERIALS AND METHODS
B. bigemina strains.
B. bigemina strains CGA and CGP from Brazil (21), strains S1A and S2P from Argentina (10), strain UYA from Uruguay, and strains from Puerto Rico, Texcoco, and Mexico (44) were used in this study. Strain CGA is derived from strain CGP. The uncloned Mexico strain of B. bigemina was cultured in vitro, using bovine erythrocytes from nonexposed cattle as described previously (2). The biological clone of the Mexico strain, JG-29, was described previously (24).
Sequencing of the constant domain of rap-1 genes from B. bigemina strains.
Genomic DNA from various B. bigemina strains was extracted from the blood of infected splenectomized calves by the standard phenol-chloroform method. The 5′ fragment from nucleotides 269 to 843, which encodes aa 29 to aa 219 of rap-1, was amplified by using primers B269F (5′-GGGTGTTATGTCAGCAGAGGTGGTT-3′) and B822R (5′-TACCGAAACCGAACAGGCGAGT-3′). The sequences were amplified for 30 cycles in a DNA thermal cycler (GeneAmp PCR System 2400; Perkin-Elmer, Norwalk, Conn.) in a 50-μl volume, using 100 ng of genomic DNA as the template. Samples without DNA were included as controls for DNA contamination. The annealing temperature used in the PCR was 55°C during 30 s. The PCR products were cloned into the pCR2.1 vector by using a TA cloning kit (Invitrogen, Carlsbad, Calif.). Clones with inserts were sequenced by using a PRISM Ready Reaction DyeDeoxy Terminator Cycle Sequencing kit and read with an ABI PRISM 373 Genetic Analyzer (Perkin-Elmer Applied Biosystems, Foster City, Calif.). Both strands from each clone were sequenced. The sequence of the cloned JG-29 Mexico strain was reported previously (26).
Native parasite antigen.
Merozoite crude membrane (CM) antigens from the Mexico and Texcoco strains of B. bigemina were prepared with a French pressure cell (SLM Instruments, Urbana, Ill.) as described previously (2, 24). Protein concentration was determined by the Bradford assay as described elsewhere (2).
RAP-1 fusion proteins.
Escherichia coli clones encoding CT-1 sequences from B. bigemina strains have been previously described (16). Expression of CT-1 from the Mexico JG-29 clone as a maltose binding protein (MBP) fusion protein was described elsewhere (15). CT-1-encoding sequences from strains Argentina (S2P), Texcoco, and Uruguay were subcloned into pMAL2c (New England Biolabs, Beverly, Mass.) for expression as MBP fusion proteins. The CT-1 sequences (aa 386 to 486) were amplified by PCR using cloned CT-1 (16) from these three strains as the template and subcloned in frame between the XbaI and HindIII sites of pMAL2c. Two overlapping CT-1 fragments from the Mexico JG-29 clone (aa 386 to 448 and 418 to 480) were also subcloned in frame into pMAL-2c as described above. Reading frame and absence of PCR alterations in the sequence were confirmed by DNA sequencing of the subclones as described above. CT-1-MBP fusion proteins were expressed and purified on amylose resin as recommended by the manufacturer (New England Biolabs) and dialyzed against phosphate-buffered saline (150 mM NaCl, 10 mM sodium phosphate [pH 7.2]). Purity and integrity of the fusion proteins were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by silver staining. MBP fusion proteins consisting of RAP-1α1, NT-1 (aa 45 to 98), or control MBP protein alone were prepared as described previously (15, 34). Protein concentration was determined by the bicinchoninic acid assay using a Micro BCA kit (Pierce, Rockford, Ill.).
Cattle used in this study.
Two cross-bred heifers that were seronegative for Babesia (2216 and 2234) were immunized with native affinity-purified RAP-1α1 protein (34). They were immunized a total of five times subcutaneously with 20 μg of antigen in RIBI adjuvant (catalog no. R-730; RIBI Immunochem Research, Inc. Hamilton, Mont.), consisting of monophosphoryl lipid A, trehalose dimycolate, and cell wall skeleton. Babesia-seronegative control calves received four immunizations of recombinant Anaplasma marginale major surface protein 5 (MSP-5) in RIBI adjuvant. After all of the in vitro assays were completed, the animals were challenged. Cattle received an intravenous inoculation of 3 ml of freshly collected, heparinized whole blood containing 1.3 × 105 blood-stage B. bigemina parasites from a splenectomized calf experimentally infected with the Mexico strain (24). Cattle were bled daily and monitored for signs of infection, including parasitemia, temperature, and packed erythrocyte cell volume. Parasitemia was monitored by calculating the number of parasites in 20 light microscope fields (over 1,000 erythrocytes) in a modified Wright’s-stained blood smear.
RAP-1-specific T-cell clones.
The CD4+ T-cell clones used in this study were derived from RAP-1α1-immunized cattle 2216 and 2234 and were described in previous publications (15, 34). Clones 2216.1H4 and 2216.2C6 recognize an epitope (aa 144 to 187) in the constant domain of RAP-1α1, whereas clones 2216.1G8, 2216.2B2, 2216.2C2, 2234.1E3, and 2234.1F3 all recognize an epitope in the CT-1 domain of RAP-1α1 (16). These clones expressed type 1 cytokine profiles, which consisted of high levels of IFN-γ and low or undetectable levels of IL-4 and IL-10 cytokine transcripts and production of IFN-γ upon antigen stimulation. For use in T-cell proliferation assays, the cryopreserved T-cell clones were thawed and cultured with irradiated (3,000 rads) autologous PBMC as a source of antigen-presenting cells (APC), 25 μg of B. bigemina CM antigen or recombinant B. bigemina RAP-1 protein per ml, and 10% bovine T-cell growth factor in complete RPMI 1640 medium in 24-well plates (Costar, Cambridge, Mass.) as described previously (2, 34).
Lymphocyte proliferation assays.
Proliferation assays were carried out in replicate wells of either round-bottom or flat-bottom-half-area 96-well plates (Costar) for 6 days when PBMC were used and for 3 days when T-cell clones were used, essentially as described previously (2, 5, 34). Briefly, 2 × 105 PBMC were cultured in triplicate wells with antigen in a total volume of 100 μl of complete RPMI 1640 medium. T-cell clones were assayed 7 days after the last stimulation with antigen and APC, and 3 × 104 T cells were cultured in duplicate wells in a total volume of 100 μl of complete medium containing antigen and 2 × 105 autologous APC. Antigens consisted of a final concentration of 0.2 to 100 μg per ml of the following: CM prepared from different geographical strains of B. bigemina or uninfected erythrocytes, recombinant B. bigemina RAP-1 fusion proteins or control MBP, and synthetic peptides that represented specific RAP-1 regions. Peptides were prepared by Gerhardt Munske, Laboratory for Biotechnology and Bioanalysis I, Washington State University, Pullman, Wash. In some experiments with T-cell clones, 1 or 2 U of recombinant human IL-2 (Boehringer Mannheim, Indianapolis, Ind.) per ml was added to all assay wells to amplify proliferation. To measure proliferation, cells were radiolabeled either for the last 4 h of culture with 0.25 μCi of [125I]iododeoxyuridine (ICN Radiochemicals, Costa Mesa, Calif.) or for the last 6 h of culture with [3H]thymidine (catalog no. NET-27; New England Nuclear, Boston, Mass.), and radiolabeled nucleic acids were harvested onto glass filters and counted in a gamma or beta counter, respectively. Results are presented as mean cpm ± range of variation around the mean of duplicate cultures, as mean ± standard deviation of triplicate cultures, or as a stimulation index. The stimulation index was calculated as mean cpm of T cells cultured with antigen/mean cpm of T cells cultured with medium alone.
Analysis of T-cell epitopes for amphipathicity.
The amino acid sequence of B. bigemina RAP-1α1 protein was analyzed for potential T-cell epitopes by using the computer program TSites (13), provided by Vidal de la Cruz, Medimmune, Inc., Gaithersburg, Md. This program predicts amphipathic regions of the sequence characteristic of structures that might form stable alpha-helical configurations, using the AMPHI algorithm with overlapping blocks of 11 aa (22).
Nucleotide sequence accession numbers.
The GenBank database accession numbers for the sequences of the B. bigemina strains from Brazil (CGA and CGP), Argentina (S1A and S2P), Puerto Rico, and Uruguay are AF014757 to AF014768.
RESULTS
RAP-1 CT-1-specific T-cell clones respond to CT-1 from four strains of B. bigemina.
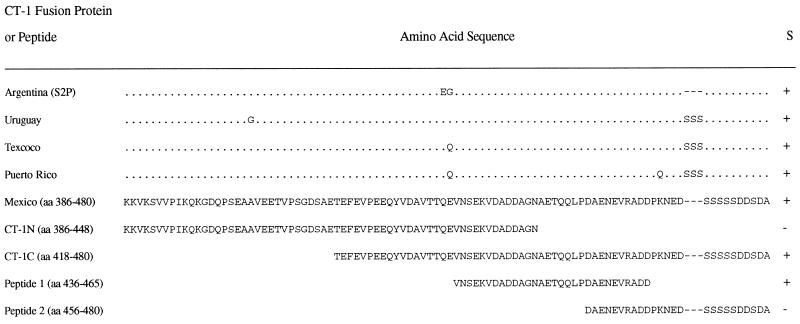
Our earlier studies indicated that the CT-1-specific Th cell clones derived from cattle immunized with RAP-1α1 (Mexico strain) responded poorly to CM antigen from other Central American strains of B. bigemina (34). One possible explanation for this result is that the CT-1 variant present in the Mexico strain is less abundantly expressed in the other strains, resulting in reduced responses to crude antigen. An alternative possibility is that the CT-1 T cell epitopes in the different strains exhibit sequence polymorphism which could result in reduced antigenicity for the Th cells. This latter possibility was addressed by the following studies. The CT-1 variant domain was sequenced in eight different strains of B. bigemina (16), and although the sequence was highly conserved, we identified several amino acid polymorphisms that could contribute to reduced T-cell responsiveness if these were residues critical for binding to either major histocompatibility complex (MHC) class II or the T-cell receptor. These included insertion of three serines in all but the Argentina S2P strain at position 471 and a glycine or glutamine substitution for glutamic acid at position 435 (reference 16 and Fig. 1). To determine if there was a variable response to CT-1 domains from different strains of B. bigemina, CT-1s (aa 386 to 480) from Argentina S2P, Uruguay, and Texcoco strains were expressed as MBP fusion proteins and compared with the CT-1 variant derived from the cloned Mexico JG-29 strain for stimulation of CT-1-specific T-cell clones. In contrast to what was observed with crude parasite antigen, the CT-1 proteins from Texcoco, Uruguay, and Argentina strains were comparable or superior to the CT-1 protein from the Mexico strain in their immunogenicity for the T-cell clones (Table 1). Thus, the differential response of these T cells to different isolates was not likely caused by antigenic variation within the T-cell epitope. However, since minor sequence polymorphism was present in CT-1 domains from the different strains, we further defined the T-cell epitope.
FIG. 1.
RAP-1 fusion proteins and peptides used in this study. CT-1 was cloned by PCR from DNA isolated from the different parasite strains indicated, and sequence analysis was performed (16). The sequences are compared with RAP-1 CT-1 (Mexico); identical amino acids are indicated by dots, while different amino acids are indicated by letters. Deletions are indicated by dashes. CT-1N and CT-1C were derived from the JG-29 cloned Mexico strain by PCR and sequenced. All CT-1 peptides were expressed in pMAL as MBP fusion proteins. Peptides 1 and 2 were synthesized. All fusion proteins and peptides were tested for stimulation (S) of CT-1-specific Th cell clones derived from cattle immunized with RAP-1α1 (containing CT-1), and the results are summarized. +, and −, positive and negative proliferative responses of CT-1-specific Th cell clones.
TABLE 1.
B. bigemina RAP-1 CT-1-specific Th cell clones respond to RAP-1 CT-1 from four strains
| Antigen (Source) | Mean cpm incorporated by RAP-1-specific Th cell clonesa
|
|||
|---|---|---|---|---|
| CT-1-specific Th clones
|
aa 144–187-specific Th clone (2216.1H4) | |||
| 2216.1G8 | 2216.2B2 | 2234.1E3 | ||
| Medium | 1,126 | 4,283 | 4,056 | 4,972 |
| Native parasite antigen | ||||
| B. bigemina (Mexico) | 6,959 (6.2) | 16,087 (3.8) | ND | 10,080 (2.0) |
| B. bigemina (Texcoco) | 3,531 (3.1) | 5,706 (1.3) | 24,455 (6.0) | 11,013 (2.2) |
| Recombinant proteins | ||||
| MBP | 1,929 (0.8) | 4,419 (1.0) | 6,633 (1.6) | 3,737 (0.8) |
| RAP-1α1 (Mexico) | 16,235 (14.4) | 29,793 (7.0) | 54,413 (13.4) | 18,068 (3.6) |
| CT-1 (Mexico) | 8,299 (7.4) | 14,094 (3.3) | ND | 3,869 (0.8) |
| CT-1 (Texcoco) | 7,569 (6.7) | 17,158 (4.0) | 42,359 (10.4) | 302 (0.1) |
| CT-1 (Uruguay) | 14,081 (12.5) | 17,366 (4.1) | 47,788 (11.8) | 4,401 (0.9) |
| CT-1 (Argentina S2P) | 11,463 (10.2) | 21,777 (5.1) | 47,221 (11.6) | 4,653 (0.9) |
T cells (3 × 104) were cultured in duplicate wells for 3 days with antigen and 105 APC. Results are presented for 5 μg of native B. bigemina CM antigen per ml and 25 μg of recombinant protein per ml. All clones except 2234.1E3 were assayed with IL-2 (2 U per ml) in the culture medium. Numbers in parentheses represent stimulation indices, calculated as mean cpm of T cells + antigen/mean cpm of T cells + medium. ND, not determined.
Mapping of Th cell epitopes with truncated fusion proteins and synthetic peptides.
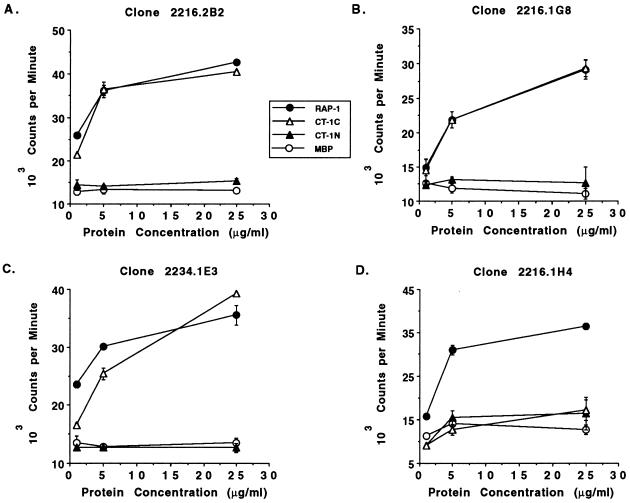
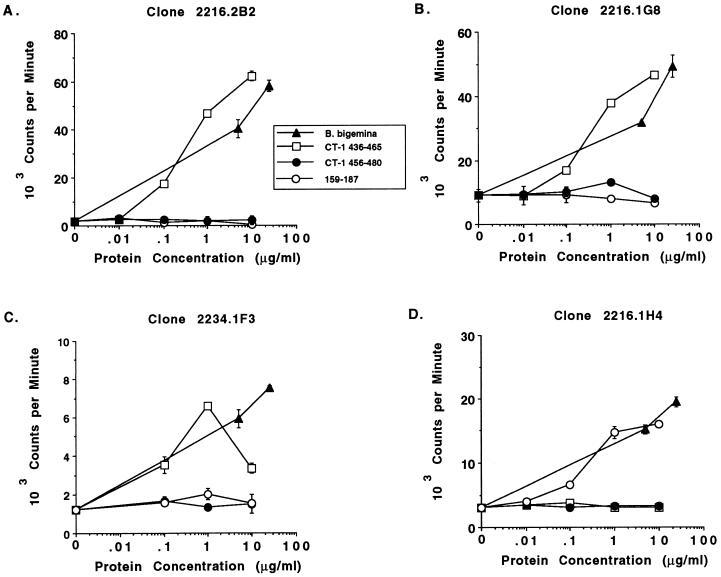
To determine whether the T-cell epitope within CT-1 was completely conserved or contained one or more polymorphic residues, truncated fusion proteins and peptides of CT-1 (Mexico) were constructed and tested for stimulation of the CT-1-specific Th cell clones. These proteins and peptides are diagramatically represented in Fig. 1. Two MBP fusion proteins consisting of aa 386 to 448 (CT-1N) and aa 418 to 480 (CT-1C) were used in T-cell proliferation assays with CT-1-specific clones 2216.1G8, 2216.2B2, and 2234.1E3, which were derived from both immunized cattle, and as a control clone 2216.1H4, which recognizes the Th cell epitope within aa 144 to 187. As shown in Fig. 2A to C, all three CT-1-specific clones responded in a dose-dependent manner to whole RAP-1 protein (which contains CT-1) and to CT-1C (aa 418 to 480) but did not respond to CT-1N (aa 386 to 448). As expected, clone 2216.1H4 proliferated against RAP-1 protein but did not respond to either CT-1 fusion protein (Fig. 2D), and none of the T-cell clones responded to control MBP protein. The responses of the clones to CT-1C were comparable to those of the whole RAP-1α1 protein. Because CT-1N and CT-1C contain 31 overlapping residues (aa 418 to 448), the T-cell epitope is not within this sequence but could contain several overlapping residues at the amino terminus of CT-1N, thus mapping the T-cell epitope to approximately aa 440 to 480. To further define the epitope, we constructed synthetic peptides consisting of aa 436 to 465 and 456 to 480 (Fig. 1). When tested for stimulation of the CT-1-specific Th cell clones (2216.2B2, 2216.1G8, 2216.2C2, and 2234.1F3), only the peptide consisting of aa 436 to 465 stimulated these Th cell clones (results for representative clones are shown in Fig. 3A to C), inducing maximal levels of proliferation with either 1 or 10 μg of protein per ml. This peptide sequence is completely conserved among the eight B. bigemina strains analyzed (reference 16 and Fig. 1), thus verifying that this Th cell epitope does not contain polymorphic residues in these strains. As anticipated, clones 2216.2C6 (data not shown) and 2216.1H4 (Fig. 3D) did not respond to either CT-1 peptide.
FIG. 2.
B. bigemina RAP-1 CT-1-specific T cells respond to aa 418 to 480. Three CT-1-specific Th cell clones (A to C) and one RAP-1-specific Th cell clone specific for an epitope within aa 144 to 187 (D) were cultured in a 3-day proliferation assay with autologous APC, 2 U of recombinant human IL-2 per ml, and 1, 5, or 25 μg of recombinant MBP fusion proteins per ml, consisting of the whole RAP-1 protein (closed circles), the N terminal fragment of CT-1 (CT-1N) consisting of aa 386 to 448 (closed triangles), the C-terminal fragment of CT-1 (CT-1C) consisting of aa 418 to 480 (open triangles), or control MBP protein (open circles). Cells were radiolabeled for 6 h with [3H]thymidine, harvested, and counted. The results are presented as the mean ± range of variation around the mean of duplicate cultures. Background proliferative responses of cells in medium plus IL-2 for the clones were as follows: 2216.2B2, 15,305 ± 639 cpm; 2216.1G8, 12,652 ± 2,376 cpm; 2234.1E3, 12,635 ± 775 cpm; and 2216.1H4, 16,353 ± 3,758 cpm.
FIG. 3.
Proliferative responses of RAP-1-specific Th cell clones to peptides within CT-1 or constant domains. Three CT-1-specific Th cell clones (A to C) and one Th cell clone specific for an epitope within aa 144 to 187 (D) were cultured in a 3-day proliferation assay with autologous APC and 0.1, 1.0, and 10.0 μg of B. bigemina RAP-1α1 peptide antigen per ml, consisting of aa 436 to 465 (open squares), aa 456 to 480 (closed circles), and aa 159 to 187 (open circles), or 5 and 25 μg of B. bigemina (Mexico) merozoite CM antigen per ml (closed triangles). Clones 2234.1F3 (C) and 2216.1H4 (D) were assayed in the presence of 1 U of recombinant human IL-2 per ml. Cells were radiolabeled for 6 h with [3H]thymidine, harvested, and counted. The results are presented as the mean ± range of variation around the mean of duplicate cultures.
Mapping of the constant-region T-cell epitope to aa 159 to 187 and conservation among strains.
We previously showed that two T-cell clones (2216.1H4 and 2216.2C6) responded to an MBP fusion protein containing a sequence from the central region (aa 144 to 187) of RAP-1α1 (15). To more precisely define this epitope, a peptide consisting of aa 159 to 187 was synthesized and assayed with the RAP-1-specific Th cell clones (Fig. 3). Whereas the RAP-1 CT-1-specific Th cell clones were not stimulated by this peptide (Fig. 3A to C), clone 2216.1H4 proliferated to this peptide vigorously and in a dose-dependent manner (Fig. 3D). Clone 2216.2C6 also responded specifically to this peptide (data not shown). The RAP-1-specific clones responded to their peptides with levels of proliferation similar to those induced by native B. bigemina (Mexico) merozoite antigen. Although it was known that aa 159 to 187 is in a region of the protein relatively conserved among the four different rap-1 gene products of the cloned JG-29 Mexico strain, the conservation of this epitope among different strains was not known. This region was therefore sequenced by PCR in genomic DNA prepared from B. bigemina strains originating from Brazil (CGA and CGP), Argentina (S1A and S2P), Mexico, Puerto Rico, and Uruguay and was found to be completely conserved among all seven uncloned and the cloned JG-29 Mexico strains (data not shown and references 25 and 26). Thus, Th cell epitopes identified in B. bigemina RAP-1α1 (Mexico), present in both constant and variable domains, are completely conserved among multiple strains of parasites from different geographical locations.
Computer-aided analysis of Th cell epitopes for amphipathicity.
Few algorithms have been tested to predict the binding of peptides to MHC class II in cattle, since little is known of bovine MHC class II molecules and few T-cell epitopes for any bovine pathogen have been identified. One algorithm broadly applicable for different mammalian species is the AMPHI algorithm, which predicts whether a peptide will comprise a T-cell site based on the alpha-helical periodicity and amphipathicity (22). Since two of three peptides used in this study contained Th cell epitopes, we subjected the RAP-1α1 protein to AMPHI analysis to determine the amphipathicity for the two peptide sequences that contained T-cell sites. Interestingly, both peptides known to contain Th cell epitopes had high amphipathic scores, whereas the peptide representing aa 456 to 480 of RAP-1 CT-1, which did not appear to stimulate T cells from these cattle, had a low amphipathic score (Table 2). Thus, this algorithm may prove useful in the prediction of additional Th cell epitopes for pathogens of cattle.
TABLE 2.
Analysis of B. bigemina RAP-1 peptide sequences for amphipathicity
| Peptide | Sequence | Amphipathic scorea | Able to stimulate T-cell clones? |
|---|---|---|---|
| RAP-1 CT-1 aa 456–480 | DAENEVRADDPKNEDSSSSSDDSDA | 5.0, 4.4 | No |
| AAA........AA............ | |||
| RAP-1 CT-1 aa 436–465 | VNSEKVDADDAGNAETQQLPDAENEVRADD | 20.0, 5.0 | Yes |
| .......AAAAAAAAAAAA.AAA....... | |||
| RAP-1 aa 159–187 | FVVSLLKKNVVRDPESNDVENFASQYFYM | 5.3, 5.2, 28.4 | Yes |
| AAA.AAAA.AAAAAAAAAAAAAAA..... |
The whole RAP-1α1 protein was analyzed with the AMPHI algorithm (13, 22) for alpha-helical periodicity and amphipathicity and assigned an amphipathic score for each set of amino acids that scored. Analysis of the peptides used in T-cell proliferation assay is presented, and the region of sequence with predicted antigenic T-cell sites is indicated below the amino acid sequence by the letter A. The oligopeptide motifs conserved in P. falciparum AMA-1 and B. bigemina RAP-1 are in boldface.
Presence of motifs broadly conserved among rhoptry proteins.
A second interesting feature of the two peptides that we have identified as immunogenic for Th cells is that each contains a short oligopeptide motif previously demonstrated by statistical analysis to be significantly conserved among rhoptry proteins, including B. bigemina RAP-1, B. bovis RAP-1, and P. falciparum AMA-1 (41). These motifs include GNAE, present in the RAP-1 CT-1 peptide consisting of aa 436 to 465, and LsKNVV, present in the peptide aa 159 to 187 conserved among rap-1 gene products (Table 2).
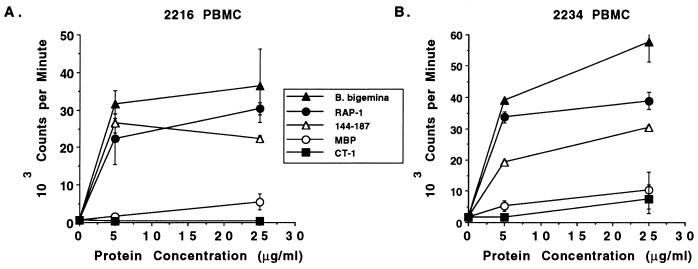
PBMC from RAP-1-immune cattle respond to the constant domain (aa 159 to 187) but not to RAP-1 CT-1 or NT-1.
The Th cell clones used in this study were derived following in vitro culture of PBMC with B. bigemina antigen for four weekly stimulations and recombinant RAP-1α1 fusion protein for an additional four stimulations (2234 clones) or by two stimulations with recombinant RAP-1α1 fusion protein (2216 clones) (34). Thus, the dominant response against either CT-1 or the constant-domain epitope (aa 159 to 187) could have arisen from in vitro selection and expansion of a minor population of memory cells. Alternatively, the responses observed by expanding antigen-specific cells ex vivo could reflect a dominant memory T-cell population in vivo. Furthermore, T-cell clones from each animal responded to CT-1, whereas the clones specific for the epitope aa 159 to 187 were from animal 2216 only, and so the ability of T lymphocytes from animal 2234 to recognize this epitope was not known. To attempt to address these questions, PBMC from both cattle immunized with RAP-1α1 were tested against MBP fusion proteins which included the whole RAP-1 sequence, the NT-1 domain, the CT-1 domain, or aa 144 to 187. PBMC from both immune cattle proliferated vigorously and in a dose-dependent manner to the fusion protein containing aa 144 to 187 and to whole RAP-1 fusion protein but surprisingly failed to respond to CT-1 fusion protein (Fig. 4). This experiment was repeated with the same results, and even protein concentrations of 50 or 100 μg/ml failed to stimulate PBMC (data not shown). In parallel experiments, this fusion protein stimulated proliferation of the Th cell clones derived from these cattle (16), ruling out a problem related to the antigenicity of the protein. RAP-1 CT-1 peptides were also tested for stimulation of 2216 and 2234 PBMC but again failed to induce significant and consistent levels of proliferation, whereas the peptide consisting of aa 159 to 187 was stimulatory (data not shown). In addition, a fusion protein containing the NT-1 domain was not stimulatory for PBMC from either RAP-1-immune animal (data not shown).
FIG. 4.
Proliferative responses of PBMC from RAP-1α1-immunized cattle to recombinant proteins containing Th cell epitopes. PBMC obtained prior to B. bigemina challenge were cultured for 6 days with 5 and 25 μg of antigen per ml consisting of B. bigemina (Mexico) merozoite CM antigen (closed triangles) or recombinant MBP fusion proteins consisting of RAP-1 protein (closed circles), aa 144 to 187 (open triangles), CT-1 (aa 386 to 480; closed squares), and MBP (open circles). Cells were radiolabeled for 4 h with [125I]iododeoxyuridine, harvested, and counted. The results are presented as the means ± 1 standard deviation of triplicate cultures.
Cattle immunized with native RAP-1 protein were protected against homologous challenge.
Native B. bigemina RAP-1 antigen was previously shown to induce partial protective immunity in cattle (24). To verify that the cattle used in this study were similarly protected and to determine the relevance of the ex vivo response to defined RAP-1 epitopes, the animals were challenged with virulent B. bigemina-infected blood which contained an estimated 1.3 × 105 parasites. Table 3 demonstrates that when compared with two control animals immunized with an irrelevant antigen (recombinant A. marginale MSP-5) administered with the same adjuvant (RIBI), the cattle immunized with native RAP-1α1 protein developed considerably lower levels of parasitemia and experienced less severe reduction in packed erythrocyte cell volumes than controls in response to homologous challenge.
TABLE 3.
Protective immunity in cattle immunized with native B. bigemina RAP-1α1 protein
| Cattlea | Parameters of infection following challengeb
|
|
|---|---|---|
| Parasitemia (day) | % reduction in PCV (day) | |
| Controls | ||
| 96B26 | 33 (6) | 56.3 (9) |
| 96B28 | 60 (6) | 39.0 (8) |
| Immunized | ||
| 2216 | 1 (6) | 12.2 (10) |
| 2234 | 2 (9) | 10.7 (9) |
Control cattle were immunized with recombinant A. marginale MSP-5 protein in RIBI adjuvant, and immunized cattle received native B. bigemina RAP-1α1 protein in RIBI adjuvant.
Cattle were challenged by intravenous inoculation of 3.0 ml of fresh bovine blood containing approximately 1.3 × 105 parasites. The maximal parasitemias (determined as number of parasites in 20 light microscope fields) and percent reduction in packed erythrocyte cell volume (PCV) are presented. Numbers in parentheses represent the day postchallenge that maximal parameters of infection were observed.
DISCUSSION
B. bigemina RAP-1 has been shown to induce partial immunity in cattle challenged with homologous parasites (reference 24 and this study) and is a candidate vaccine antigen. However, vaccine development is complicated by the presence of multiple genes encoding RAP-1 variants which contain different combinations of N-terminal and C-terminal variant domains. The biological relevance for the parasite of having multiple rap-1 gene products is not understood. Transcripts for all four variants have been detected in the cloned JG-29 Mexico strain (16, 25), but it is not known whether a single parasite simultaneously expresses more than one protein, and the relative abundance of the expressed proteins within a population has not been determined. It has been speculated that such antigenic polymorphism could favor parasite survival through alteration of B- and T-cell epitopes (15, 29). In support of this hypothesis, four B-cell epitopes were mapped to the NT-1 or CT-1 variant domains of RAP-1 which were not cross-reactive with NT-2 or CT-2 variants, and one Th cell epitope was mapped to CT-1 which did not cross-react with CT-2 (15). Interestingly, however, an additional Th cell epitope was identified in the constant region of RAP-1α1. The results presented in this paper now demonstrate complete amino acid sequence conservation of both Th cell epitopes of RAP-1α1 among multiple geographically distant strains of B. bigemina. Thus, the constant-region T-cell epitope (aa 159 to 187) conserved among RAP-1 variants and B. bigemina strains is a candidate vaccine epitope that could have widespread use. Similarly, if CT-1 is expressed by the majority of parasites within a population, this epitope could also be a useful component of a vaccine. Even though lymphocytes from each animal in this study responded to each T-cell epitope, the universality of these epitopes for other animals bearing different MHC class II haplotypes has not been evaluated and warrants investigation before consideration of inclusion of either of these epitopes in a vaccine.
The presence of additional RAP-1α1 epitopes that could stimulate T cells from these or other cattle cannot be excluded, and in fact additional sequences with high amphipathic scores predictive of T-cell sites were identified in the protein sequence (data not shown). However, all Th cell clones derived from the two immune animals in our study responded to one of the two epitopes, suggesting that they are the immunodominant RAP-1 T-cell epitopes for these animals. Although only two Th cell clones that recognize aa 159 to 187 were identified, PBMC from both cattle proliferated vigorously to fusion protein containing this sequence and to the peptide. In contrast, whereas the majority of Th cell clones from both cattle responded to CT-1, PBMC from neither animal responded by proliferation to this peptide. One interpretation of this apparent paradox is that the epitope (aa 159 to 187) in the nonpolymorphic region conserved among RAP-1 variants is more immunodominant and stimulated a larger number of memory T cells than CT-1. In a proliferation assay using 2 × 105 lymphocytes, enough memory cells specific for peptide aa 159 to 187 were present to detect proliferation, whereas perhaps there were too few memory cells specific for CT-1 to detect proliferation. However, repeated in vitro exposure to recombinant RAP-1 protein then allowed for expansion of both conserved epitope (aa 159 to 187)- and CT-1-specific memory T cells in vitro. Since T-cell clones specific for these synthetic or recombinant peptides responded comparably to the native (Mexico) parasite antigen, it is unlikely that differences in processing of native versus recombinant or synthetic peptides are occurring (18). Thus, priming with recombinant or synthetic RAP-1 should evoke vigorous anamnestic responses to the native protein.
Based on the differences in proliferation to Mexico versus Texcoco and Puerto Rico strains of B. bigemina by CT-1-specific Th cell clones (34), we expected to find variation within the Th cell epitope in these strains. However, neither of these strains exhibited amino acid polymorphism in the T-cell epitope, ruling out the possibility that an altered peptide ligand induced diminished T-cell responsiveness or T-cell anergy (12). Left unanswered is the possibility that CT-1 is expressed differentially by the different strains of B. bigemina. Current experiments are focused on identification of additional rap-1 genes and expression of their products in different parasite strains.
Of interest was the discovery that each of the stimulatory RAP-1 peptides contained a motif (GNAE or LsKNVV) that is also present in the P. falciparum AMA-1 rhoptry protein (41). The relevance of these oligopeptide motifs in the context of a Th cell epitope is not known, but it is interesting that the GNAE and KNVV motifs were present in short (17- to 18-aa) peptides of P. falciparum AMA-1 that contained T-cell epitopes recognized by lymphocytes from humans exposed to malaria (19). In addition, each of these motifs was conserved in nine strains of P. falciparum examined (28). The conservation of the motifs among rhoptry proteins of multiple strains of both P. falciparum and B. bigemina supports the contention that they are functionally important for parasite survival. A synthetic peptide including the KKNV sequence was shown by Calvo et al. (6) to possess erythrocyte binding activity.
In conclusion, we show that the dominant helper T-cell response from immunized and protected cattle was directed against two epitopes completely conserved among Central and South America strains of B. bigemina. These results demonstrated that the previously observed decreased T-cell responses to nonhomologous parasite strains is not the result of antigenic variation within these Th cell epitopes that could lead to impaired T-cell recognition. This new information, together with our previous finding that RAP-1α1-immunized cattle expressed a dominant type 1 cytokine response and RAP-1-specific IgG1 and IgG2 subclasses of antibody (34, 35), support inclusion of one or both of these epitopes in a subunit vaccine that could provide protection against a number of diverse B. bigemina strains.
ACKNOWLEDGMENTS
We thank Debby Alperin, Sue Ellen Chantler, Beverly Hunter, Kathleen Logan, and Carla Robertson for expert technical assistance.
This research was supported by U.S. Department of Agriculture-US-Israel Binational Agricultural Research and Development Fund grant US-2496-94C, National Institutes of Health grant AI30136, and USDA NRICGP grants 93-37206-9657 and 96-35204-3667.
REFERENCES
- 1.Brown W C, Estes D M, Rodriguez S D, Ruef B J, Tuo W, McElwain T F, Palmer G H. Proceedings from the Society for Tropical Veterinary Medicine, 4th Biennial Meeting, Montpellier, France. 1997. Babesia bigemina rhoptry-associated protein 1 (RAP-1) induces a type 1 cytokine response and both IgG1 and IgG2 antibody subclasses; p. 30. [Google Scholar]
- 2.Brown W C, Logan K S, Wagner G G, Tetzlaff C L. Cell-mediated immune responses to Babesia bovis merozoite antigens in cattle following infection with tick-derived or cultured parasites. Infect Immun. 1991;59:2418–2426. doi: 10.1128/iai.59.7.2418-2426.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown W C, McElwain T F, Ruef B J, Suarez C E, Shkap V, Chitko-McKown C G, Tuo W, Rice-Ficht A C, Palmer G H. Babesia bovis rhoptry-associated protein 1 is immunodominant for T helper cells of immune cattle and contains T-cell epitopes conserved among geographically distant B. bovis strains. Infect Immun. 1996;64:3341–3350. doi: 10.1128/iai.64.8.3341-3350.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown W C, Rice-Ficht A C. Use of helper T cells to identify potentially protective antigens of Babesia bovis. Parasitol Today. 1994;10:145–149. doi: 10.1016/0169-4758(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 5.Brown W C, Woods V M, Dobbelaere D A E, Logan K S. Heterogeneity in cytokine profiles of Babesia bovis-specific bovine CD4+ T cell clones activated in vitro. Infect Immun. 1993;61:3273–3281. doi: 10.1128/iai.61.8.3273-3281.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvo M, Guzman F, Perez E, Segura C H, Molano A, Patarroyo M E. Specific interactions of synthetic peptides derived from P. falciparum merozoite proteins with human red blood cells. Peptide Res. 1991;4:324–332. [PubMed] [Google Scholar]
- 7.Collins W E, Pye D, Crewther P E, Vandenberg K L, Galland G G, Sulzer A J, Kemp D J, Edwards S J, Coppel R L, Sullivan J S, Morris C L, Anders R F. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am J Trop Med Hyg. 1994;51:711–719. doi: 10.4269/ajtmh.1994.51.711. [DOI] [PubMed] [Google Scholar]
- 8.Dalrymple B P, Casu R E, Peters J M, Dimmock C M, Gale K R, Boese R, Wright I G. Characterisation of a family of multi-copy genes encoding rhoptry protein homologues in Babesia bovis, Babesia ovis and Babesia canis. Mol Biochem Parasitol. 1993;57:181–92. doi: 10.1016/0166-6851(93)90194-3. [DOI] [PubMed] [Google Scholar]
- 9.Dalrymple B P, Peters J M, Böse R, Wright I G. A polymerase chain reaction method for the identification of genes encoding members of the Bv60/p58 family of rhoptry protein homologues in the genus Babesia. Exp Parasitol. 1996;84:96–100. doi: 10.1006/expr.1996.0094. [DOI] [PubMed] [Google Scholar]
- 10.Echaide I E, de Echaide S T, Gugliemone A A. Live and soluble antigens for cattle afford protection to Babesia bigemina. Vet Parasitol. 1993;51:35–40. doi: 10.1016/0304-4017(93)90193-q. [DOI] [PubMed] [Google Scholar]
- 11.Estes D M, Closser N M, Allen G K. IFN-γ stimulates IgG2 production from bovine B cells costimulated with anti-μ and mitogen. Cell Immunol. 1994;154:287–295. doi: 10.1006/cimm.1994.1078. [DOI] [PubMed] [Google Scholar]
- 12.Evavold B D, Sloan-Lancaster J, Allen P M. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today. 1993;14:602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- 13.Feller D C, de la Cruz V F. Identifying antigenic T-cell sites. Nature. 1991;349:720–721. doi: 10.1038/349720a0. [DOI] [PubMed] [Google Scholar]
- 14.Goff W L, Davis W C, Palmer G H, McElwain T F, Johnson W C, Bailey J F, McGuire T C. Identification of Babesia bovis merozoite surface antigens by using immune bovine sera and monoclonal antibodies. Infect Immun. 1988;56:2363–2368. doi: 10.1128/iai.56.9.2363-2368.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hötzel I, Brown W C, McElwain T F, Rodriguez S D, Palmer G H. Dimorphic sequences of rap-1 genes encode B and CD4+ T helper lymphocyte epitopes in the Babesia bigemina rhoptry associated protein-1. Mol Biochem Parasitol. 1996;81:89–99. doi: 10.1016/0166-6851(96)02686-2. [DOI] [PubMed] [Google Scholar]
- 16.Hötzel I, Suarez C E, McElwain T F, Palmer G H. Genetic variation in the dimorphic regions of rap-1 genes and rap-1 loci of Babesia bigemina. Mol Biochem Parasitol. 1998;90:479–489. doi: 10.1016/s0166-6851(97)00182-5. [DOI] [PubMed] [Google Scholar]
- 17.Howard R J, Pasloske B L. Target antigens for asexual malaria vaccine development. Parasitol Today. 1993;9:369–372. doi: 10.1016/0169-4758(93)90085-t. [DOI] [PubMed] [Google Scholar]
- 18.Krzych U, Jareed T, Link H T, Loomis L D, Ballou W R. Distinct T cell specificities are induced with the authentic versus recombinant Plasmodium berghei circumsporozoite protein. J Immunol. 1992;148:2530–2538. [PubMed] [Google Scholar]
- 19.Lal A A, Hughes M A, Oliveira D A, Nelson C, Bloland P B, Oloo A J, Hawley W E, Hightower A W, Nahlen B L, Udhayakumar V. Identification of T-cell determinants in natural immune responses to the Plasmodium falciparum apical membrane antigen (AMA-1) in an adult population exposed to malaria. Infect Immun. 1996;64:1054–1059. doi: 10.1128/iai.64.3.1054-1059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machado R Z, Palmer G H, McElwain T F. Proceedings from the Society for Tropical Veterinary Medicine 4th Biennial Meeting, Montpellier, France. 1997. Vaccination of cattle with Babesia bigemina rhoptry proteins; p. 46. [Google Scholar]
- 21.Madruga C R, Suarez C E, McElwain T F, Palmer G H. Conservation of merozoite membrane and apical complex B cell epitopes among Babesia bigemina and Babesia bovis strains isolated in Brazil. Vet Parasitol. 1996;61:21–30. doi: 10.1016/0304-4017(95)00809-8. [DOI] [PubMed] [Google Scholar]
- 22.Margalit H, Spouge J L, Cornette J L, Cease K B, DeLisi C, Berzofsky J A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987;138:2213–2229. [PubMed] [Google Scholar]
- 23.McElwain T F, Perryman L E, Davis W C, McGuire T C. Antibodies define multiple proteins with epitopes exposed on the surface of live Babesia bigemina merozoites. J Immunol. 1987;138:2298–2304. [PubMed] [Google Scholar]
- 24.McElwain T F, Perryman L E, Musoke A J, McGuire T C. Molecular characterization and immunogenicity of neutralization-sensitive Babesia bigemina merozoite surface proteins. Mol Biochem Parasitol. 1991;47:213–222. doi: 10.1016/0166-6851(91)90181-5. [DOI] [PubMed] [Google Scholar]
- 25.Mishra V S, McElwain T F, Dame J B, Stephens E B. Isolation, sequence, and differential expression of the p58 gene family of Babesia bigemina. Mol Biochem Parasitol. 1992;53:149–158. doi: 10.1016/0166-6851(92)90017-e. [DOI] [PubMed] [Google Scholar]
- 26.Mishra V S, Stephens E B, Dame J B, Perryman L E, McGuire T C, McElwain T F. Immunogenicity and sequence analysis of recombinant p58: a neutralization-sensitive, antigenically conserved Babesia bigemina merozoite surface protein. Mol Biochem Parasitol. 1991;47:207–212. doi: 10.1016/0166-6851(91)90180-e. [DOI] [PubMed] [Google Scholar]
- 27.Musoke A J, Rurangirwa F R, Nantulya V M. Biological properties of bovine immunoglobulins and systemic antibody responses. In: Morrison W I, editor. The ruminant immune system in health and disease. Cambridge, England: Cambridge University Press; 1986. pp. 391–408. [Google Scholar]
- 28.Oliviera D A, Udhayakumar V, Bloland P, Shi Y P, Nahlen B L, Oloo A J, Hawley W E, Lal A A. Genetic conservation of the Plasmodium falciparum apical membrane antigen-1 (AMA-1) Mol Biochem Parasitol. 1996;76:333–336. doi: 10.1016/0166-6851(95)02548-0. [DOI] [PubMed] [Google Scholar]
- 29.Palmer G H, McElwain T F. Molecular basis for vaccine development against anaplasmosis and babesiosis. Vet Parasitol. 1995;57:233–253. doi: 10.1016/0304-4017(94)03123-e. [DOI] [PubMed] [Google Scholar]
- 30.Palmer G H, McElwain T F, Perryman L E, Davis W C, Reduker D R, Jasmer D P, Shkap V, Pipano E, Goff W L, McGuire T C. Strain variation of Babesia bovis merozoite surface-exposed epitopes. Infect Immun. 1991;59:3340–3342. doi: 10.1128/iai.59.9.3340-3342.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perkins M E. Rhoptry organelles of apicomplexan parasites. Parasitol Today. 1992;8:28–32. doi: 10.1016/0169-4758(92)90308-o. [DOI] [PubMed] [Google Scholar]
- 32.Rockett K A, Awburn M M, Aggarwal B B, Cowden W B, Clark I A. In vivo induction of nitrite and nitrate by tumor necrosis factor, lymphotoxin, and interleukin-1: possible roles in malaria. Infect Immun. 1992;60:3725–3730. doi: 10.1128/iai.60.9.3725-3730.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rockett K A, Awburn M M, Cowden W B, Clark I A. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991;59:3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez S D, Palmer G H, McElwain T F, McGuire T C, Ruef B J, Chitko-McKown C G, Brown W C. CD4+ T-helper lymphocyte responses against Babesia bigemina rhoptry-associated protein 1. Infect Immun. 1996;64:2079–2087. doi: 10.1128/iai.64.6.2079-2087.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruef B J, Rodriguez S D, Roussel A J, Palmer G H, McElwain T F, Chitko-McKown C G, Rice-Ficht A C, Brown W C. Immunization with Babesia bigemina rhoptry-associated protein 1 induces a type 1 cytokine response. J Interferon Cytokine Res. 1997;17:45–54. doi: 10.1089/jir.1997.17.45. [DOI] [PubMed] [Google Scholar]
- 36.Sam-Yellowe T Y. Rhoptry organelles of the apicomplexa: their role in host cell invasion and intracellular survival. Parasitol Today. 1996;12:308–316. doi: 10.1016/0169-4758(96)10030-2. [DOI] [PubMed] [Google Scholar]
- 37.Shompole S, McElwain T F, Jasmer D P, Hines S A, Katende J, Musoke A J, Rurangirwa F R, McGuire T C. Identification of Babesia bigemina infected erythrocyte surface antigens containing epitopes conserved among strains. Parasite Immunol. 1994;16:119–127. doi: 10.1111/j.1365-3024.1994.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 38.Skuce P J, Mallon T R, Taylor S M. Molecular cloning of a putative rhoptry associated protein homologue from Babesia divergens. Mol Biochem Parasitol. 1996;77:99–102. doi: 10.1016/0166-6851(96)02570-4. [DOI] [PubMed] [Google Scholar]
- 39.Suarez C E, McElwain T F, Stephens E B, Mishra V S, Palmer G H. Sequence conservation among merozoite apical complex proteins of Babesia bovis, Babesia bigemina and other apicomplexa. Mol Biochem Parasitol. 1991;49:329–332. doi: 10.1016/0166-6851(91)90077-j. [DOI] [PubMed] [Google Scholar]
- 40.Suarez C E, Palmer G H, Jasmer D P, Hines S A, Perryman L E, McElwain T F. Characterization of the gene encoding a 60-kilodalton Babesia bovis merozoite protein with conserved and surface exposed epitopes. Mol Biochem Parasitol. 1991;46:45–52. doi: 10.1016/0166-6851(91)90197-e. [DOI] [PubMed] [Google Scholar]
- 41.Suarez C E, Thompson S M, McElwain T F, Hines S A, Palmer G H. Conservation of oligopeptide motifs in rhoptry proteins from different genera of erythrocytic protozoa. Exp Parasitol. 1994;78:246–251. doi: 10.1006/expr.1994.1025. [DOI] [PubMed] [Google Scholar]
- 42.Thomas A W, Waters A P, Carr D. Analysis of variation in PF83, an erythrocytic merozoite vaccine candidate antigen of Plasmodium falciparum. Mol Biochem Parasitol. 1990;42:285–288. doi: 10.1016/0166-6851(90)90172-i. [DOI] [PubMed] [Google Scholar]
- 43.Ushe T C, Palmer G H, Sotomayor L, Figueroa J V, Buening G M, Perryman L E, McElwain T F. Antibody response to Babesia bigemina rhoptry-associated protein-1 surface-exposed and neutralization-sensitive epitope in immune cattle. Infect Immun. 1994;62:5698–5701. doi: 10.1128/iai.62.12.5698-5701.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vidotto O, McElwain T F, Machado R Z, Perryman L E, Suarez C E, Palmer G H. Babesia bigemina: identification of B cell epitopes associated with parasitized erythrocytes. Exp Parasitol. 1996;81:491–500. doi: 10.1006/expr.1995.1142. [DOI] [PubMed] [Google Scholar]
- 45.Wright I G, Casu R, Commins M A, Dalrymple B P, Gale K R, Goodger B V, Riddles P W, Waltisbuhl D J, Abetz I, Berrie D A, Bowles Y, Dimmock C, Hayes T, Kalnins H, Leatch G, McCrae R, Montague P E, Nisbet I T, Parrodi F, Peters J M, Scheiwe P C, Smith W, Rode-Bramanis K, White M A. The development of a recombinant Babesia vaccine. Vet Parasitol. 1992;44:3–13. doi: 10.1016/0304-4017(92)90138-y. [DOI] [PubMed] [Google Scholar]