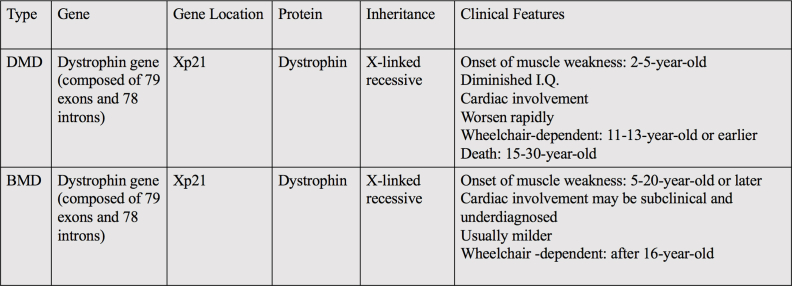
Pseudohypertrophic muscular dystrophy is a lethal X-linked recessive neuromuscular disorder caused by mutations in the dystrophin gene. This gene encodes a cytoskeletal protein that is extensively expressed in muscle cells and that enables the strength, stability, and functionality of myofibers. Muscular dystrophy can be grouped into two different categories based on the mutational rate and clinical severity, Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD). DMD is the most common and the more severe type of dystrophy. DMD patients usually become wheelchair-bound by the age of 12 years and die in their late teens to early twenties.1 In contrast, BMD is relatively less severe and the patients can potentially have a longer life expectancy.
Early diagnosis and prompt treatment of cardiac involvement in BMD patients are of great significance to delay the onset of heart failure. However, the cardiac involvement of BMD is often underdiagnosed in clinical practice due to the less prominent symptoms, thus resulting in the undertreatment of heart failure. Herein, we present a case of BMD in a 16-year-old child with early cardiac dysfunction. We used speckle tracking imaging and real-time 3D echocardiography, which is a very effective and sensitive tool, for the early detection of subclinical LV dysfunction. The early treatment of preserved reduced ejection fraction was applied to the patient; as shown in the follow-up examinations, his heart condition seemed to become stabilized.
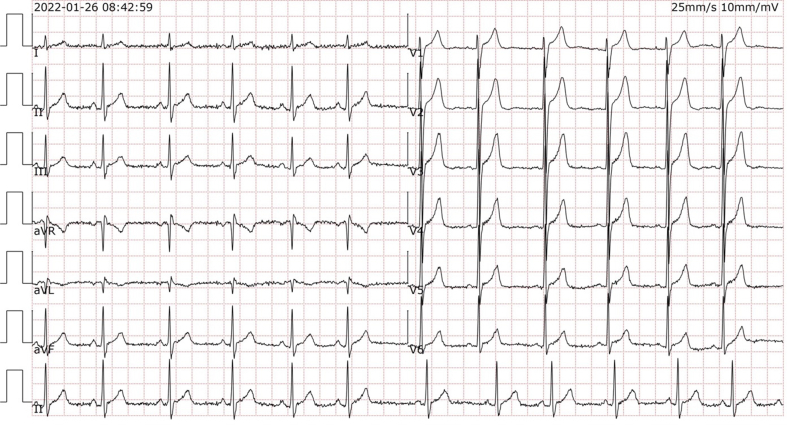
This 16-year-old boy came to the outpatient clinic for medical help with major complaints of fatigue and bilateral lower extremity weakness. Medical and family history inquiries revealed no family history of heart disease or other hereditary diseases. Laboratory tests showed creatine kinase (CK) at 5550 IU/L and CK-MB at 300 IU/L. Moreover, the electrocardiogram (ECG) showed sinus rhythm.
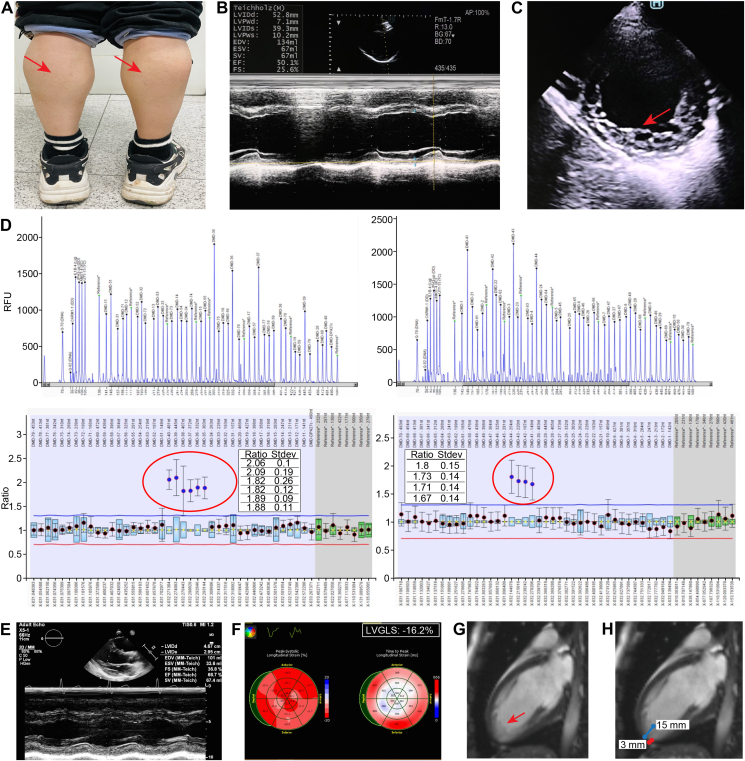
On general physical examination, the child did not present with difficulty in standing, walking, or getting up from the sitting position, but he had calf hypertrophy (Fig. 1A) and a positive Gower's sign. Cranial nerve examination was found to be normal. The patient was subjected to echocardiography, which indicated normal atrium size, mild dilated left ventricle [left ventricle end-diastolic diameter (LVEDD): 55 mm, short axis (SAX) View, 2D Mode], and reduced left ventricular ejection fraction [LVEF: 50%, parasternal long axis (PLAX) View, M-mode Teichholz method] with apical myocardial noncompaction at the inferior and lateral segments (Fig. 1B, C). There was also normal relative wall thickness without abnormal segmental wall movement. Based on the history and clinical examination, a diagnosis of some type of myopathy with early cardiac involvement was established. The child was then advised to immediately receive guideline-directed medical therapy (GDMT) for heart failure with mildly reduced ejection fraction to improve his heart structure and function.
Figure 1.
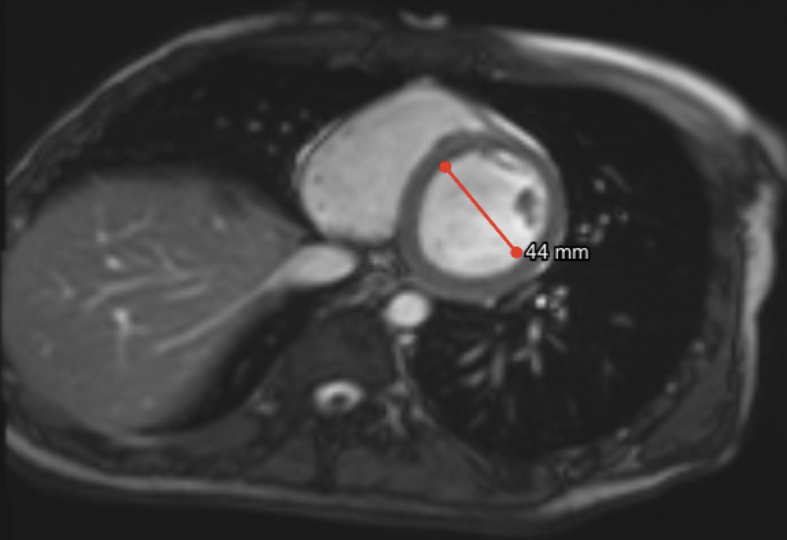
Clinical characteristics and echocardiographic and cardiovascular magnetic resonance imaging of a 16-year-old boy with BMD. (A) Calf hypertrophy (red arrows). (B) Echocardiography PLAX View: M Mode: LVEF 50%. (C) SAX View: 2D Mode: LV noncompaction (apical lateral and inferior wall). (D) The whole exome sequencing (WES) test detected duplicate variants in exons 35–44 of the DMD gene, and no other pathogenic or suspected pathogenic single-nucleotide variants (SNVs) or insertion-or-deletion (InDel) mutations were detected. Multiplex ligation-dependent probe amplification (MLPA) validated that the recipient had a duplication mutation in exons 35–44 of the DMD (NM_004006) gene (see red circle). The results of the genetic test supported the clinical diagnosis of pseudohypertrophic muscular dystrophy. (E) Echocardiography PLAX View, M-mode and 2D image analysis, 2D Mode: LVEDD 4.67 mm. M Mode: LVEF 66.7%. (improved). (F) Echocardiography (the original full image, see supplementary file), LVGLS: −16.2% (with a reduction in 2D long axis myocardial strains at inferior and lateral segments). (G, H) Contrast CMR images showing apical trabecular layer thickness (red arrow), non-compacted myocardium to compacted myocardium ratio >2.5.
After 12 weeks, the boy arrived for his second outpatient visit, with gene testing reports showing that the mutation of duplication of exons 35–44 (2 copies) in the dystrophin gene was identified (Fig. 1D). The dystrophin gene spans 2.3 Mb on chromosome X and is composed of 79 exons and 78 introns (2.1 Mb) (Homo sapiens dystrophin [DMD], transcript variant Dp427c, mRNA-Nucleotide-NCBI https://www.ncbi.nlm.nih.gov/nuccore/NM_000109.4?report=GenBank). Mutations in the dystrophin gene will lead to progressive muscle weakness and degeneration, thus causing DMD and a milder phenotype known as BMD (Table S1). In this scenario, a diagnosis of BMD was established.
During the second visit, the child was subjected to more BMD-related tests to evaluate disease progression and the effectiveness of the early treatment regarding his heart condition. The biomarkers indicating myocardial injury were much higher (CK: 3,463 IU/L, CK-MB: 55.2 IU/L, high-sensitivity troponin I: 0.3 ng/L). In addition, the volume load biomarker was normal (BNP <10 pg/mL). The ECG exhibited sinus arrhythmia and definite elevation of the J point in V1 to V4 (Fig. S1).
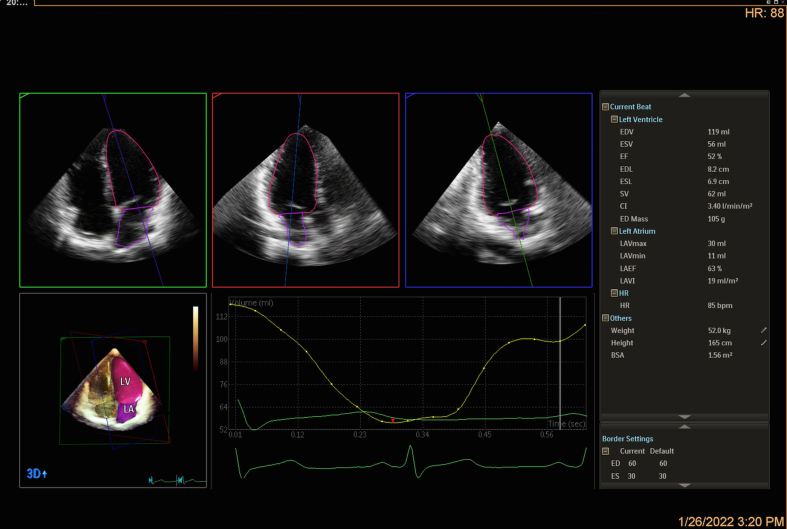
The echocardiographic images showed that cardiac systolic function improved after GDMT for heart failure (Fig. 1E; Fig. S2), whereas LV global longitudinal strain (LVGLS) was mildly decreased (−16.2%, the normal value of LVGLS is >18%) (Fig. 1F). Furthermore, biventricular diastolic function was normal (left atrial volume index: 19 mL/m2, mitral transmitral E velocity to septal e' ratio [E/e'] was 5.2, lateral E/e' was 4, and tricuspid E/e' lateral was 3.3). Apical noncompaction of the left ventricle was demonstrated via cardiac magnetic resonance (CMR) (Fig. 1G, H), and LVEF 52.5% indicated a slight reduction in systolic function (Fig. S3), consistent with the value of LVEF 52% measured by using 3D echocardiography (Fig. S2). No obvious myocardial fibrosis was detected by using late gadolinium enhancement (LGE) for the clinical assessment of disease development. This case demonstrated the effectiveness of early treatment to prevent heart failure in BMD patients, which is dependent on the early diagnosis of any heart involvement.
Based on this case, we observed early cardiac involvement in BMD. Consistent with relatively mild clinical courses and later symptom onset time, in over 70% of cases, the patients are diagnosed with asymptomatic cardiac involvement at the time when BMD is initially confirmed.2 Cardiac involvement of BMD was reported to be uncommon in patients under 16 years of age, and more than 70% of patients will not be detected with pathologic cardiac findings until the age of 40 years or more.3
Cardiac involvement in BMD patients may be underdiagnosed by using conventional echocardiographic methods, thus resulting in the undertreatment of heart failure. In clinical practice, echocardiography-based tissue Doppler or strain imaging techniques represent very effective tools for the early detection of subclinical LV dysfunction in BMD patients. In this case, the early diagnosis of cardiac involvement of BMD via this technique is of great importance, as the early management of heart failure should benefit ventricular remodeling with improvements in LV systolic function (or at least resulting in the deceleration of progressive dysfunction).4 The boy with normal LVEF exhibited mildly lower LV longitudinal strain; therefore, we suggested that he continue GDMT for heart failure. A “cocktail medication” of angiotensin receptor-neprilysin inhibitor (ARNi), beta-adrenergic receptor blockers, and aldosterone inhibitors is necessary for effective treatment.
Other techniques, such as cardiac magnetic resonance (CMR), can be an alternative powerful diagnostic tool for BMD. Compared to using conventional echocardiography, cardiac evaluation using CMR is even more sensitive in detecting subtle abnormal findings in BMD patients. Moreover, real-time 3D echocardiography provides an accurate assessment of both left and right ventricular structures and function as well. Both assessments are valuable in minimizing the underdiagnosis of cardiac involvement and in avoiding the delay of beneficial medication for potential heart failure for the boy.
Cardiac involvement is the most important cause of death in BMD patients; therefore, early detection and intervention are important. We used strain imaging and real-time 3D echocardiography (which is a very effective and sensitive tool) for the early detection of subclinical LV dysfunction, which has rarely been reported. In this case, the early diagnosis of cardiac involvement of BMD by using this technique is of great importance for the early management of heart failure. To minimize underdiagnosis and to not delay medication administration, we recommend the use of cardiomyopathy evaluation using echocardiography-based strain imaging and real-time 3D echocardiography for all BMD patients (regardless of their ages).
Ethics declaration
Our study was approved by the ethical committee of Chongqing General Hospital (No. XJS S2021-050-01). All procedures performed involving human participants were in accordance with the Health Insurance Portability and Accountability Act (HIPPA) of 1996.
Author contributions
Yuhui Luo drafted the manuscript and figures; Qinglan Shu and Yi Wang were involved in echocardiographic imaging acquisition and analyses; Min Gu and Rui Wang analyzed the cardiac magnetic resonance parameters; Siqi Hong interpreted all the lab data; Hao Zhang revised the manuscript; Lixue Yin revised the manuscript and gave the final approval of the version to be published. All authors read and approved the final manuscript.
Conflict of interests
All authors declare that they have no conflict of interests.
Funding
This case is funded by the Science Health Joint Medical Scientific Research Project of Chongqing, China (No. 2019GDRC016) and the Medical Research Foundation of Chongqing General Hospital (Chongqing, China) (No. Y2019MSXM02).
Acknowledgements
We thank the patient's family members especially Mr. Xin Gao, for his great help and support to publish this information.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2023.04.019.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Figs1.
Figs2.
Figs3.
Figs4.
References
- 1.Mercuri E., Bönnemann C.G., Muntoni F. Muscular dystrophies. Lancet. 2019;394(10213):2025–2038. doi: 10.1016/S0140-6736(19)32910-1. [DOI] [PubMed] [Google Scholar]
- 2.Finsterer J., Stöllberger C. Cardiac involvement in Becker muscular dystrophy. Can J Cardiol. 2008;24(10):786–792. doi: 10.1016/s0828-282x(08)70686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melacini P., Fanin M., Danieli G.A., et al. Myocardial involvement is very frequent among patients affected with subclinical Becker's muscular dystrophy. Circulation. 1996;94(12):3168–3175. doi: 10.1161/01.cir.94.12.3168. [DOI] [PubMed] [Google Scholar]
- 4.Kirchmann C., Kececioglu D., Korinthenberg R., Dittrich S. Echocardiographic and electrocardiographic findings of cardiomyopathy in Duchenne and Becker-Kiener muscular dystrophies. Pediatr Cardiol. 2005;26(1):66–72. doi: 10.1007/s00246-004-0689-2. [DOI] [PubMed] [Google Scholar]