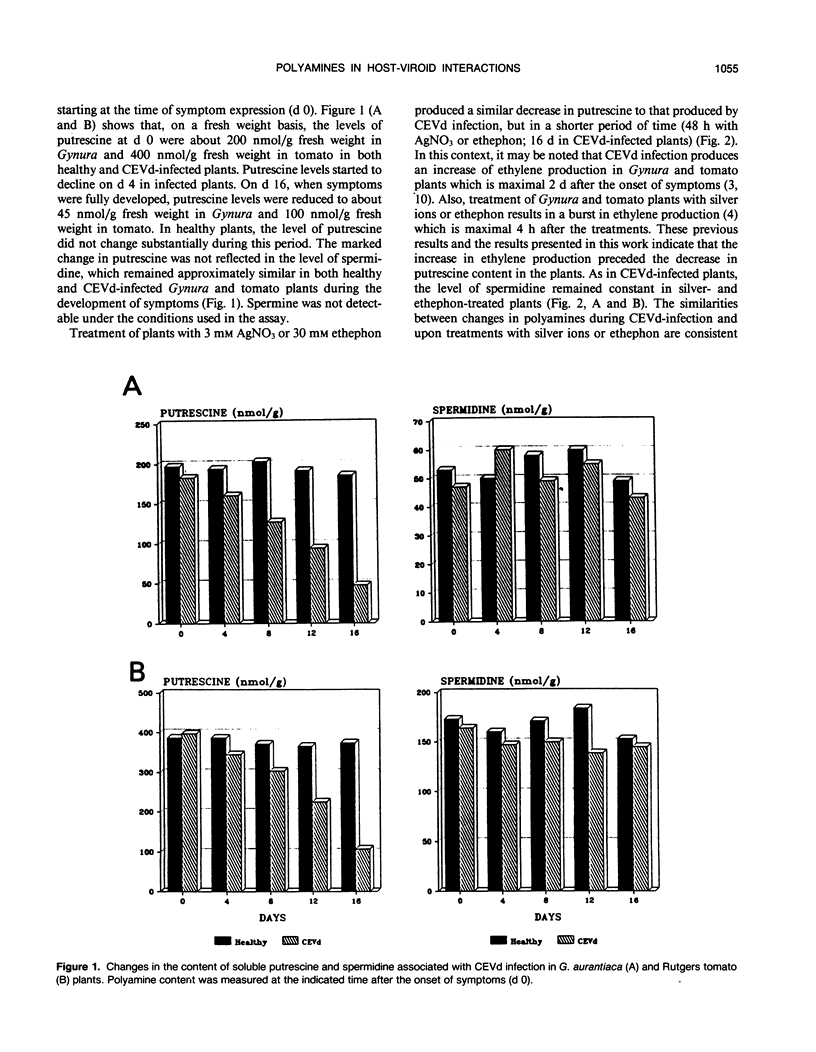
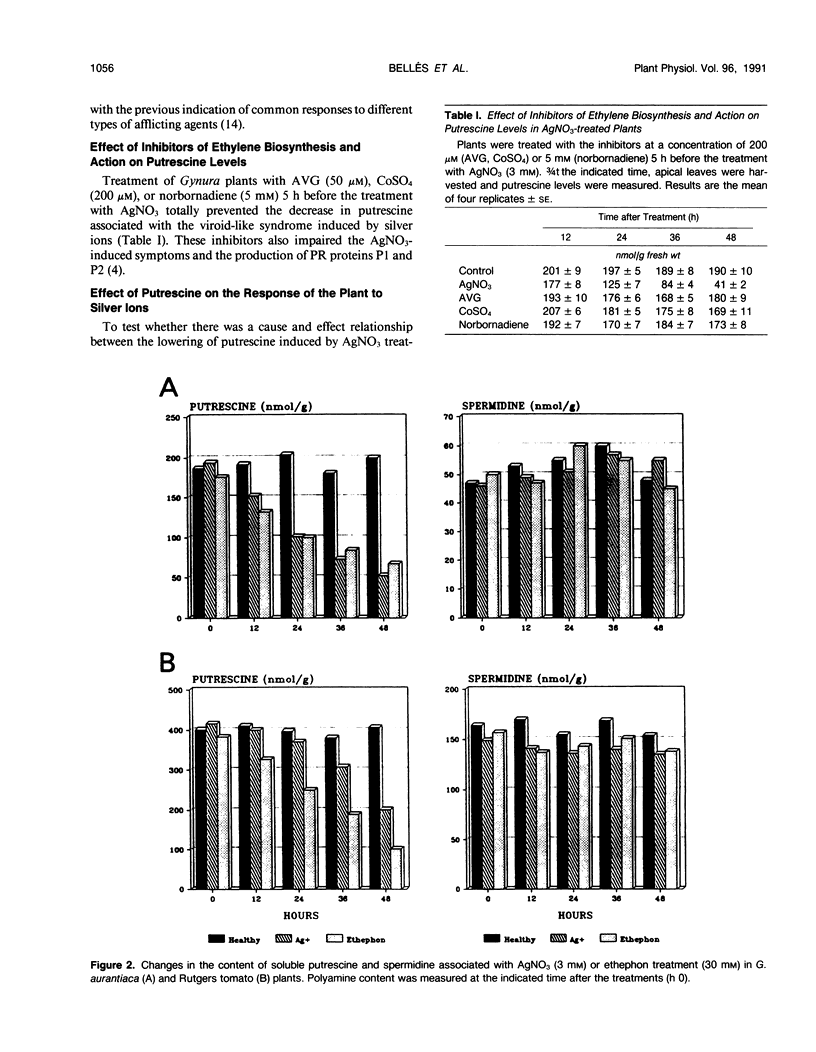
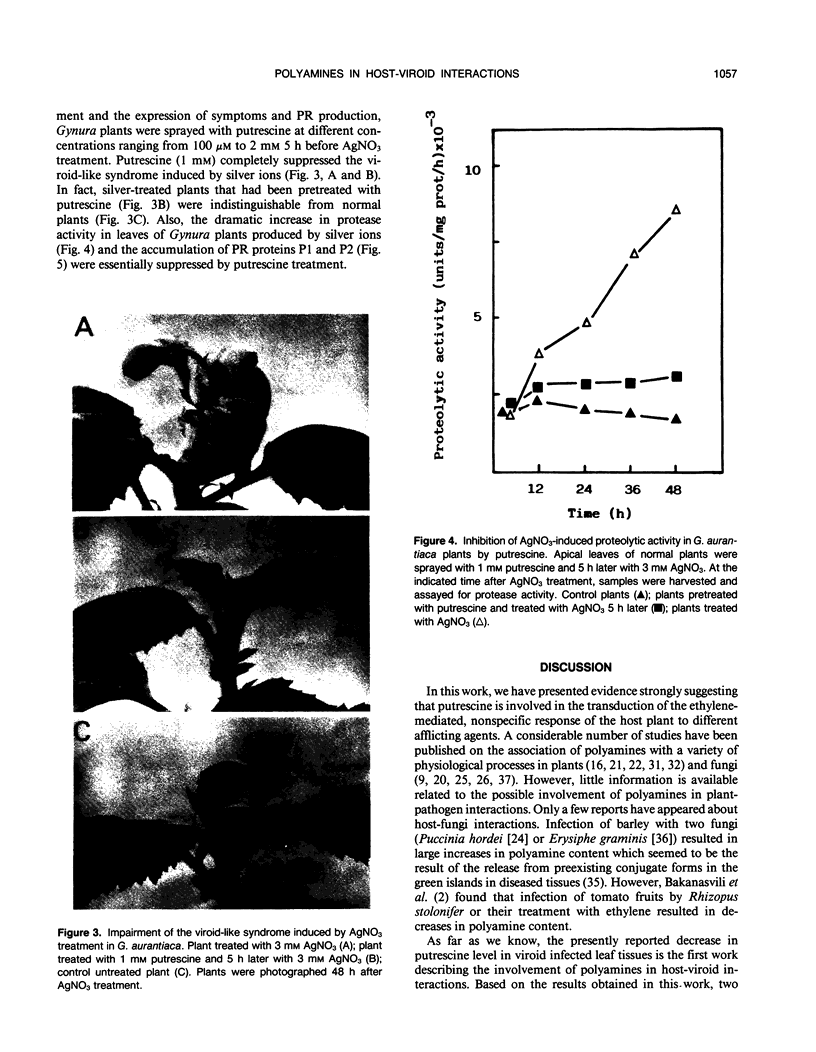
Abstract
The levels of polyamines in leaves of Gynura aurantiaca DC and tomato, Lycopersicon esculentum Mill. cv Rutgers, infected with citrus exocortis viroid (CEVd) or treated with silver nitrate or ethephon (2-chloroethylphosphonic acid) were measured by HPLC in relation to development of symptoms. Previously it had been demonstrated that treatment of G. aurantiaca plants with silver nitrate or ethephon closely mimicked the effects of viroid infection in the plants. In the studies reported here, a marked decrease in putrescine level was observed in plants infected by CEVd or treated with silver ions or ethephon. There was no significant change in either spermidine or spermine levels. Treatment of G. aurantiaca plants with specific inhibitors of ethylene biosynthesis (aminoethoxyvinylglycine, Co2+) or ethylene action (norbornadiene) prevented the decrease of putrescine associated with silver nitrate treatment and had no effect on spermidine or spermine levels. The development of viroid-like symptoms, the production of associated pathogenesis-related proteins, and the rise in protease activity induced by silver nitrate, were all suppressed by exogenous application of putrescine. The decreased level of putrescine as an ethylene-mediated step in the transduction of the viroid and silver or ethephon signaling is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apelbaum A., Goldlust A., Icekson I. Control by ethylene of arginine decarboxylase activity in pea seedlings and its implication for hormonal regulation of plant growth. Plant Physiol. 1985 Nov;79(3):635–640. doi: 10.1104/pp.79.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birecka H., Bitonti A. J., McCann P. P. Activities of arginine and ornithine decarboxylases in various plant species. Plant Physiol. 1985 Oct;79(2):515–519. doi: 10.1104/pp.79.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birecka H., Bitonti A. J., McCann P. P. Assaying ornithine and arginine decarboxylases in some plant species. Plant Physiol. 1985 Oct;79(2):509–514. doi: 10.1104/pp.79.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birecka H., Garraway M. O., Baumann R. J., McCann P. P. Inhibition of Ornithine Decarboxylase and Growth of the Fungus Helminthosporium maydis. Plant Physiol. 1986 Mar;80(3):798–800. doi: 10.1104/pp.80.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejero V., Semancik J. S. Exocortis viroid: alteration in the proteins of Gynura aurantiaca accompanying viroid infection. Virology. 1977 Mar;77(1):221–232. doi: 10.1016/0042-6822(77)90420-2. [DOI] [PubMed] [Google Scholar]
- Even-Chen Z., Mattoo A. K., Goren R. Inhibition of ethylene biosynthesis by aminoethoxyvinylglycine and by polyamines shunts label from 3,4-[C]methionine into spermidine in aged orange peel discs. Plant Physiol. 1982 Feb;69(2):385–388. doi: 10.1104/pp.69.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982 Mar;69(3):701–706. doi: 10.1104/pp.69.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajam M. V., Weinstein L. H., Galston A. W. Prevention of a plant disease by specific inhibition of fungal polyamine biosynthesis. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6874–6878. doi: 10.1073/pnas.82.20.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Morris T. J., Weathers L. G., Rodorf B. F., Kearns D. R. Physical properties of a minimal infectious RNA(viroik) associated with the exocortis disease. Virology. 1975 Jan;63(1):160–167. doi: 10.1016/0042-6822(75)90381-5. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Weathers L. G. Exocortis disease: evidence for a new species of "infectious" low molecular weight RNA in plants. Nat New Biol. 1972 Jun 21;237(77):242–244. doi: 10.1038/newbio237242a0. [DOI] [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Davies P. J. Separation and quantitation of polyamines in plant tissue by high performance liquid chromatography of their dansyl derivatives. Plant Physiol. 1985 May;78(1):89–91. doi: 10.1104/pp.78.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera P., Conejero V. Pathogenesis-related proteins of tomato : p-69 as an alkaline endoproteinase. Plant Physiol. 1988 May;87(1):58–63. doi: 10.1104/pp.87.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein L. H., Osmeloski J. F., Wettlaufer S. H., Galston A. W. Protection of wheat against leaf and stem rust and powdery mildew diseases by inhibition of polyamine metabolism. Plant Sci. 1987;51:311–316. doi: 10.1016/0168-9452(87)90208-1. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979 Nov;198(1):280–286. doi: 10.1016/0003-9861(79)90420-x. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Auxin-induced Ethylene Production and Its Inhibition by Aminoethyoxyvinylglycine and Cobalt Ion. Plant Physiol. 1979 Dec;64(6):1074–1077. doi: 10.1104/pp.64.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]