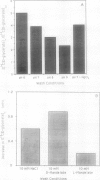
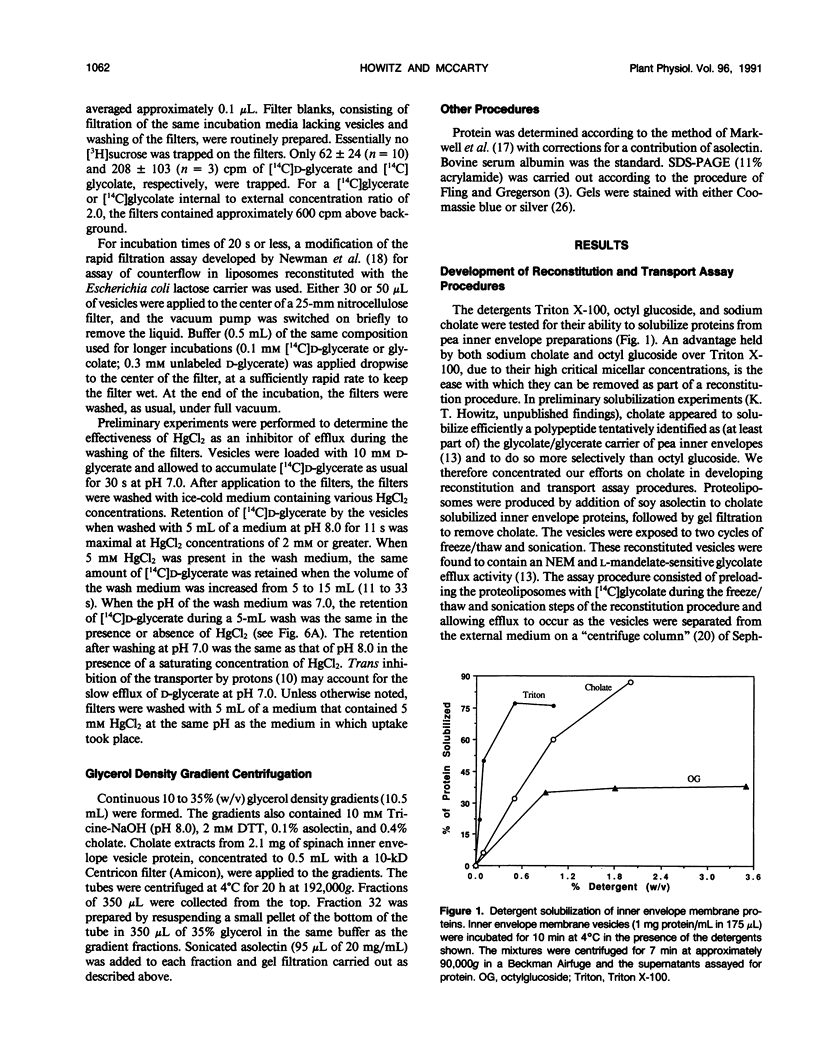
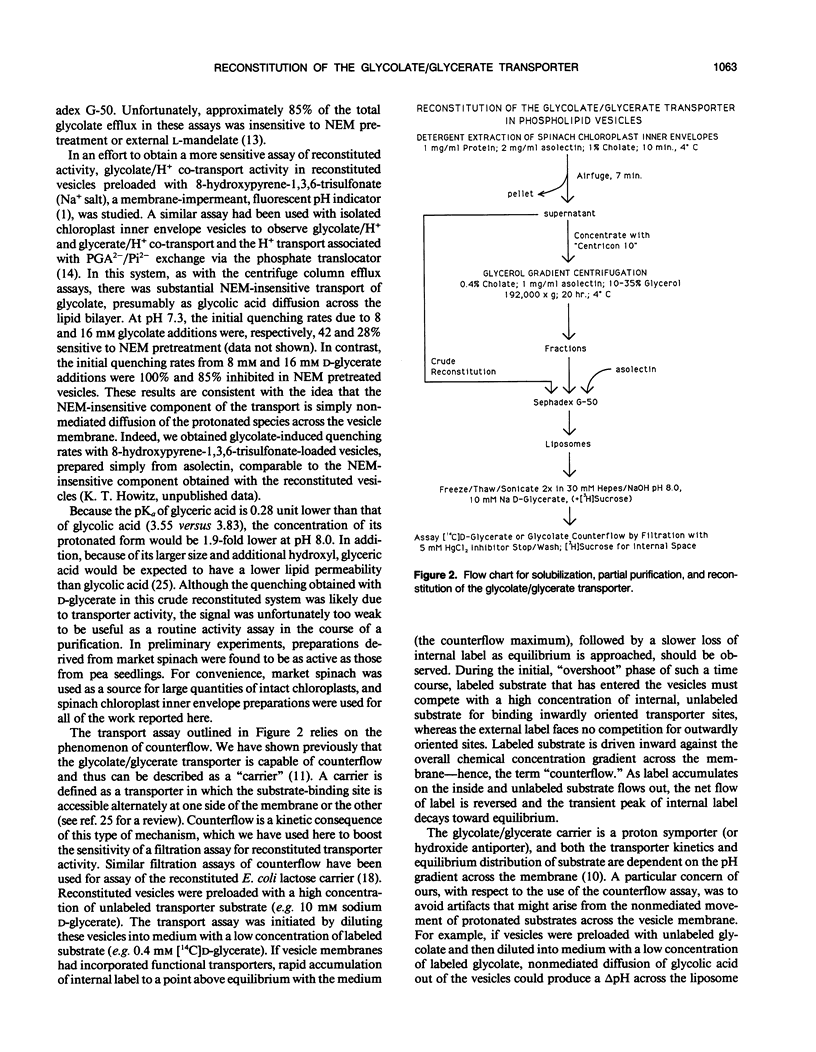
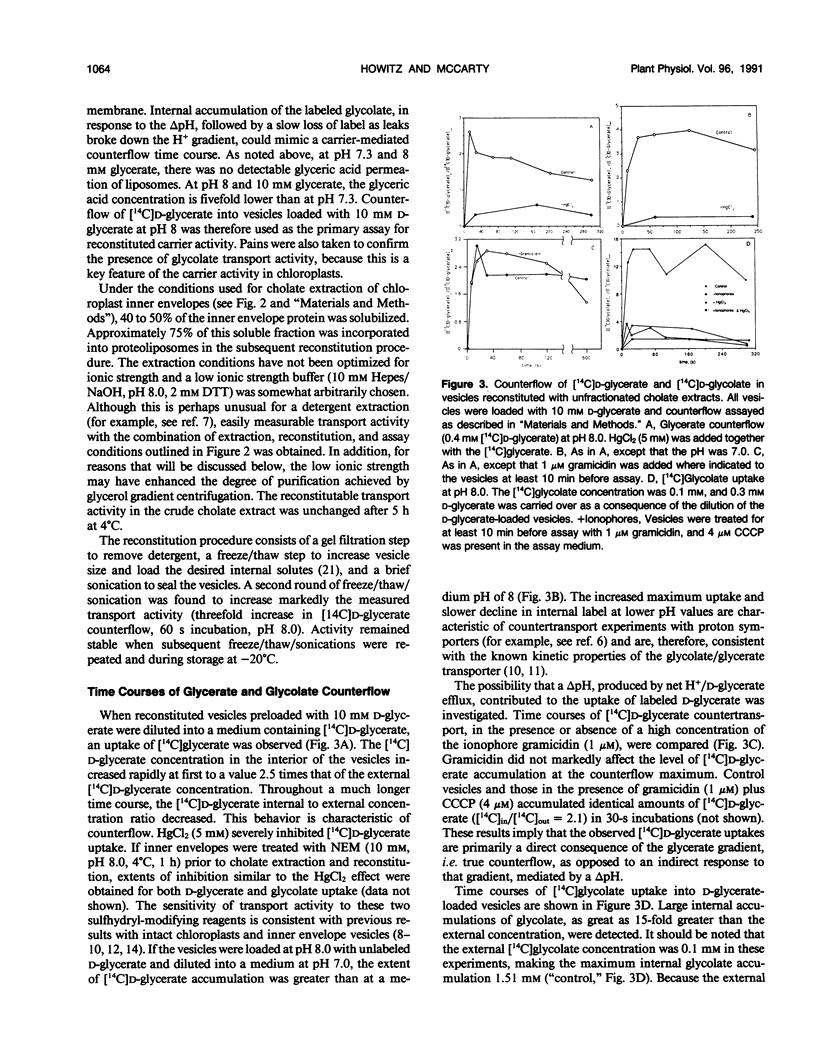
Abstract
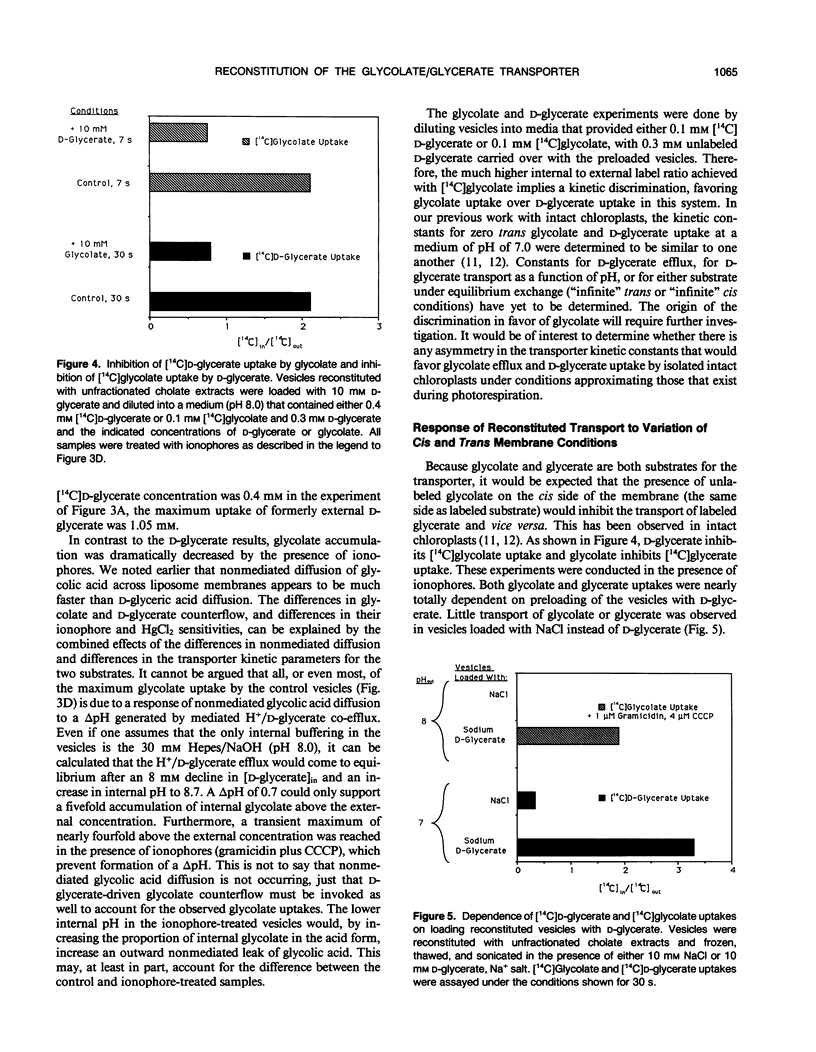
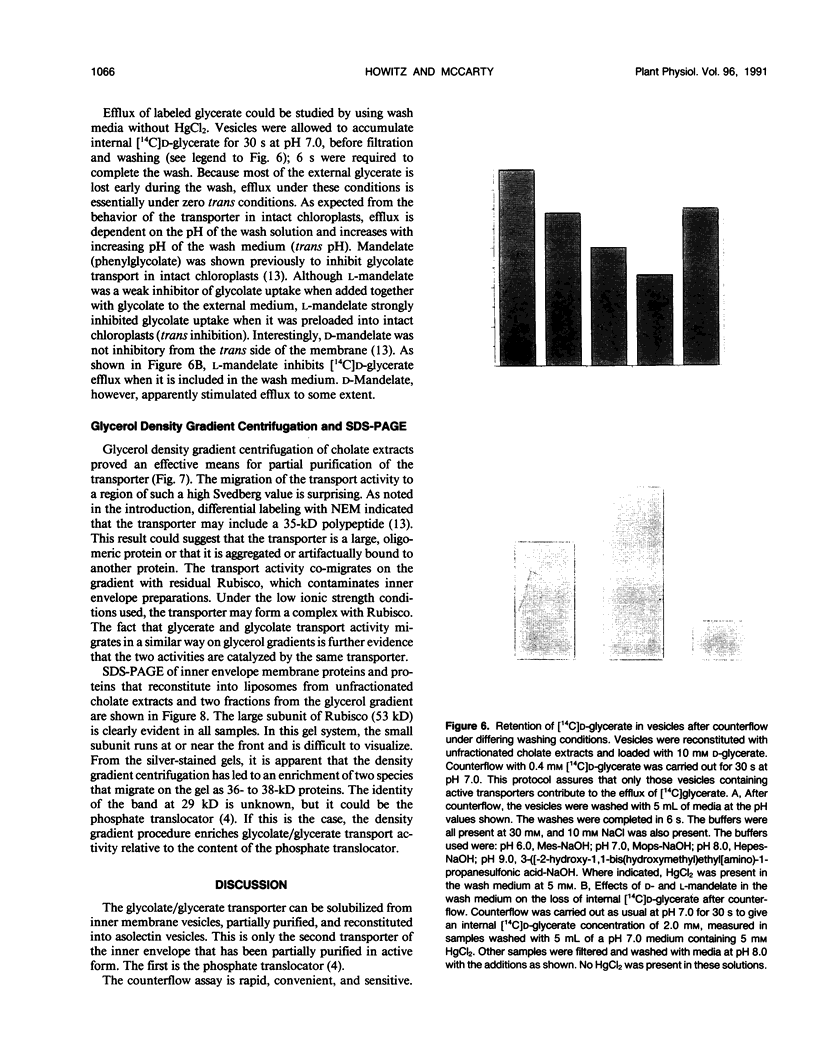
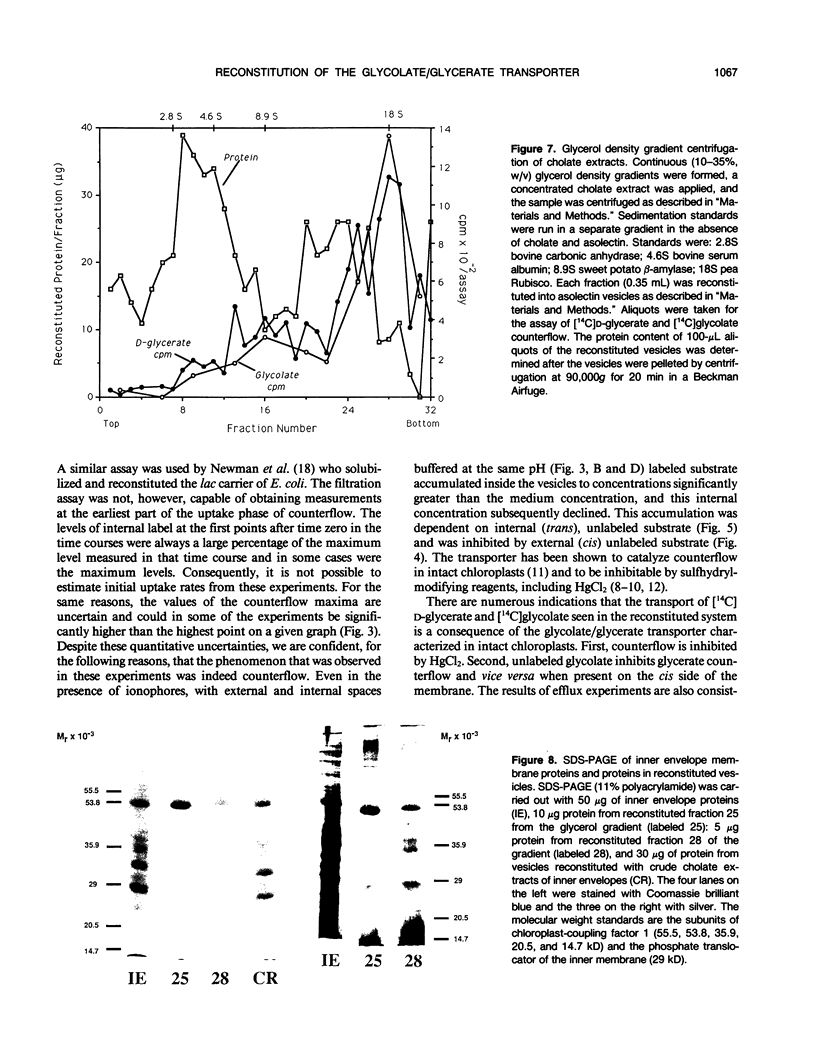
The glycolate/glycerate transporter of spinach (Spinacia oleracea L.) chloroplast inner envelope membranes was solubilized by treatment of the membranes with sodium cholate. Mixtures of the cholate extracts and soy asolectin were subjected to gel filtration to remove the detergent. The reconstituted vesicles were frozen, thawed, and sonicated in a buffer that contained 10 millimolar d-glycerate and, usually, [3H]sucrose as an internal space indicator. The dilution of the vesicles into a medium that contained 0.4 millimolar [14C]d-glycerate resulted in a rapid accumulation of labeled glycerate, followed by a much slower loss of [14C]d-glycerate from the vesicles. This behavior is characteristic of counterflow. The accumulation of [14C]d-glycerate was strongly inhibited by HgCl2, which blocks glycolate/glycerate transport in intact chloroplasts. In the absence of proton ionophores, the extent of [14C]glycolate accumulation under similar conditions was much greater than that of [14C]d-glycerate. External glycolate inhibited d-glycerate counterflow and external d-glycerate inhibited glycolate counterflow. The external pH dependence of the efflux of [14C]d-glycerate accumulated in vesicles by counterflow and its inhibition by external l-mandelate are characteristics displayed by glycolate transport in intact chloroplasts. Partial purification of the transporter was achieved by glycerol gradient centrifugation. The solubilized glycolate and glycerate counterflow activities, assayed by reconstitution into vesicles, were found to sediment similarly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clement N. R., Gould J. M. Pyranine (8-hydroxy-1,3,6-pyrenetrisulfonate) as a probe of internal aqueous hydrogen ion concentration in phospholipid vesicles. Biochemistry. 1981 Mar 17;20(6):1534–1538. doi: 10.1021/bi00509a019. [DOI] [PubMed] [Google Scholar]
- Fliege R., Flügge U. I., Werdan K., Heldt H. W. Specific transport of inorganic phosphate, 3-phosphoglycerate and triosephosphates across the inner membrane of the envelope in spinach chloroplasts. Biochim Biophys Acta. 1978 May 10;502(2):232–247. doi: 10.1016/0005-2728(78)90045-2. [DOI] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Garcia M. L., Viitanen P., Foster D. L., Kaback H. R. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 1. Effect of pH and imposed membrane potential on efflux, exchange, and counterflow. Biochemistry. 1983 May 10;22(10):2524–2531. doi: 10.1021/bi00279a033. [DOI] [PubMed] [Google Scholar]
- Hjelmeland L. M. Solubilization of native membrane proteins. Methods Enzymol. 1990;182:253–264. doi: 10.1016/0076-6879(90)82021-s. [DOI] [PubMed] [Google Scholar]
- Howitz K. T., McCarty R. E. Measurement of proton-linked transport activities in pyranine loaded chloroplast inner envelope vesicles. Plant Physiol. 1988 Apr;86(4):999–1001. doi: 10.1104/pp.86.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz K. T., McCarty R. E. d-Glycerate Transport by the Pea Chloroplast Glycolate Carrier : Studies on [1-C]d-Glycerate Uptake and d-Glycerate Dependent O(2) Evolution. Plant Physiol. 1986 Feb;80(2):390–395. doi: 10.1104/pp.80.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification and reconstitution of functional lactose carrier from Escherichia coli. J Biol Chem. 1981 Nov 25;256(22):11804–11808. [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Pick U. Liposomes with a large trapping capacity prepared by freezing and thawing of sonicated phospholipid mixtures. Arch Biochem Biophys. 1981 Nov;212(1):186–194. doi: 10.1016/0003-9861(81)90358-1. [DOI] [PubMed] [Google Scholar]
- Robinson S. P. Lack of ATP requirement for light stimulation of glycerate transport into intact isolated chloroplasts. Plant Physiol. 1984 Jun;75(2):425–430. doi: 10.1104/pp.75.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P. Transport of Glycerate across the Envelope Membrane of Isolated Spinach Chloroplasts. Plant Physiol. 1982 Oct;70(4):1032–1038. doi: 10.1104/pp.70.4.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Kagawa Y. Reconstitution of vesicles capable of energy transformation from phospholipids and adenosine triphosphatase of a thermophilic bacterium. J Biochem. 1977 Feb;81(2):519–528. doi: 10.1093/oxfordjournals.jbchem.a131485. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yu C., Claybrook D. L., Huang A. H. Transport of glycine, serine, and proline into spinach leaf mitochondria. Arch Biochem Biophys. 1983 Nov;227(1):180–187. doi: 10.1016/0003-9861(83)90361-2. [DOI] [PubMed] [Google Scholar]