Abstract
Dementia, with homocysteine (Hcy) as an important risk factor, is a severe public health problem in the aging society. Betaine serves as a methyl donor and plays an important role in reducing Hcy. However, the effects and mechanisms of betaine on Hcy-induced cognitive impairment remain unclear. Firstly, SD rats were injected with Hcy (400 μg/kg) through vena caudalis, and betaine (2.5 % w/v) was supplemented via drinking water for 14 days. Betaine supplementation could attenuate Hcy-induced cognitive impairment in the Y maze and novel object recognition tests by repairing brain injury. Meanwhile, microglial activation was observed to be inhibited by betaine supplementation using immunofluorescence and sholl analysis. Secondly, HMC3 cells were treated with betaine, which was found to decrease the ROS level, ameliorate cell membrane rupture, reduce the release of LDH, IL-18 and IL-1β, and attenuate the damage of microglia to neurons. Mechanistically, betaine alleviates cognitive impairment by inhibiting microglial pyroptosis via reducing the expressions of NLRP3, ASC, pro-caspase-1, cleaved-caspase-1, GSDMD, GSDMD-N, IL-18 and IL-1β. Betaine treatment can increase SAM/SAH ratio, confirming its enhancement on methylation capacity. Furthermore, betaine treatment was found to enhance N6-methyladenosine (m6A) modification of NLRP3 mRNA, and reduced the NLRP3 mRNA stability through increasing the expression of the m6A reader YTH N6-methyladenosine RNA binding protein 2 (YTHDF2). Finally, silencing YTHDF2 could reverse the inhibitory effect of betaine on pyroptosis. Our data demonstrated that betaine attenuated Hcy-induced cognitive impairment by suppressing microglia pyroptosis via inhibiting the NLRP3/caspase-1/GSDMD pathway in an m6A-YTHDF2-dependent manner.
Keywords: Betaine, Homocysteine, Microglia, Pyroptosis, NLRP3, YTHDF2
Graphical abstract
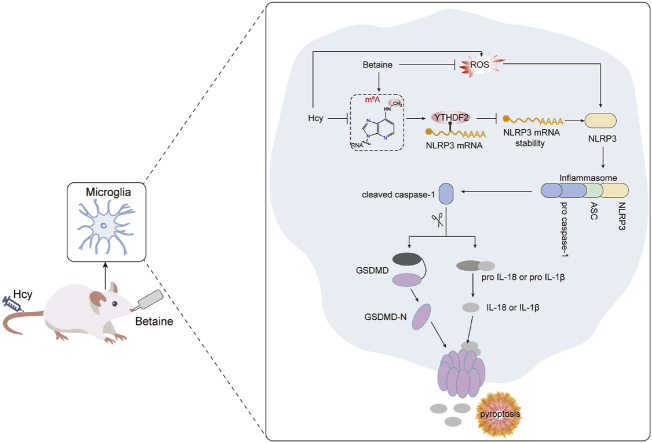
Betaine attenuates Hcy-induced microglia pyroptosis by inhibiting NLRP3/caspase-1/GSDMD signaling pathway in an m6A-YTHDF2-dependent manner, thereby exerting neuroprotective effects.
Highlights
-
•
Betaine alleviated cognitive impairment induced by homocysteine.
-
•
Betaine attenuated microglial activation and pyroptosis induced by homocysteine.
-
•
Betaine inhibited microglia pyroptosis through inhibiting NLRP3/caspase-1/GSDMD pathway in an m6A-YTHDF2-dependent manner.
1. Introduction
Dementia is a major global public health problem, imposing a substantial social and economic burden on governments, communities, families and individuals [1,2]. The global prevalence of dementia exceeds 55 million people, projected to reach 78 million by 2030 [3]. Precise prevention and control of important risk factors may be beneficial in preventing or mitigating the progression of dementia [4]. Several potential risk factors for cognitive decline or dementia have been identified, such as age, head trauma, genes, depression, obesity, hypertension and physical inactivity [[5], [6], [7]]. Moreover, epidemiological evidence has found that elevated plasma total homocysteine (Hcy) is a modifiable risk factor for dementia in older adults [[8], [9], [10]]. A recent meta-analysis demonstrated a 9 % increase in the risk of dementia for each 5 μmol/L increase in plasma Hcy levels, respectively [11]. Thus, reducing plasma Hcy levels and clarifying its pathogenic mechanisms may be a promising strategy for preventing and managing dementia.
The pathogenesis of dementia is complex and diverse, among which microglial activation is an early event in dementia [12]. Recent studies demonstrated that Hcy could induce microglial activation and the expression of pro-inflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor α (TNF-α) and inducible nitric oxide synthase (iNOS) [13]. Microglial activation and release inflammatory cytokines to mediate inflammatory responses [14,15]. Pyroptosis is an inflammatory programmed cell death mediated by the caspase family, resulting in the massive expression of inflammatory factors, which are released into the surrounding environment [16]. The assembly of the NLR family pyrin domain containing 3 (NLRP3) inflammasome can be induced by reactive oxygen species (ROS), and then mediates cysteinyl aspartate specific proteinase 1 (caspase-1) activation, which subsequently promotes the maturation and release of interleukin-1β (IL-1β) and interleukin-18 (IL-18), and triggers gasdermin D (GSDMD)-mediated pyroptosis [17,18]. Studies have found that inhibiting NLRP3-mediated pyroptosis could ameliorate lipopolysaccharide (LPS)-induced cognitive impairment by suppressing the NLRP3/GSDMD pathway [19]. Therefore, inhibiting NLRP3-mediated microglial pyroptosis may be a potential mechanism for ameliorating Hcy-induced cognitive impairment.
The expression regulation of NLRP3 is affected by N6-methyladenosine (m6A). m6A is the most prevalent internal mRNA modification [20,21]. S-adenosyl methionine (SAM) acts as a methyl donor, regulating m6A levels and influencing gene expression [22,23]. The fate and functions of m6A methylated RNAs are mainly mediated by m6A “readers” proteins, such as YT521-B homology domain family (YTHDF) proteins and the insulin like growth factor 2 mRNA binding proteins (IGF2BP) [24]. Studies have found that YTHDF1 could enhance the translation of NLRP3 and promote inflammatory responses [25]. Furthermore, IGF2BP2 negatively feedback regulated the activation of the NLRP3 inflammasome in microglia [26]. However, the underlying mechanism of m6A modification in Hcy-induced cognitive impairment has not been investigated.
Betaine (N, N, N-trimethylglycine) is abundant in various sources, such as sugar beet, spinach and seafood [27], and is transported to the brain by the betaine-GABA transporter (BGT-1) [28,29]. The main physiological action of betaine is serving as a methyl donor (transmethylation), which leads to an increase in SAM levels [30]. Substantial evidence indicated that betaine supplementation could effectively decrease plasma Hcy levels [31,32]. Additionally, betaine treatment for AD patients could improve brain cognition and functionality after 1 month, and the levels of Hcy, inflammatory factors IL-1β and TNF-α in blood were significantly reduced [33]. However, whether and how betaine exerts neuroprotective effects through m6A modification to mitigate Hcy-induced cognitive impairment remains unclear.
This study aimed to investigate the effect and mechanism of betaine on attenuating cognitive impairment induced by Hcy. Initially, cognitive function was assessed through Y maze and novel object recognition tests. Then, histological damage was evaluated using Nissl staining and H&E staining. Moreover, microglial activation and pyroptosis were assessed by immunofluorescence and sholl analysis. Pyroptosis was also observed using transmission electron microscopy in vitro. Furthermore, qRT-PCR and Western blot were performed to investigate mechanisms. Besides, the levels of SAM and SAH were detected via HPLC-MS/MS. Additionally, the m6A level was measure by m6A Dot blot, and the NLRP3 methylation level was assess using MeRIP-qPCR. Finally, it was further explored that YTHDF2 was a key factor in the action of betaine by silencing YTHDF2.
2. Materials and methods
2.1. Materials
Homocysteine (H4628), betaine (B2629) and methylene blue (M4159) were purchased from Sigma-Aldrich Company (St. Louis, Missouri, USA).
2.2. Animals and treatments
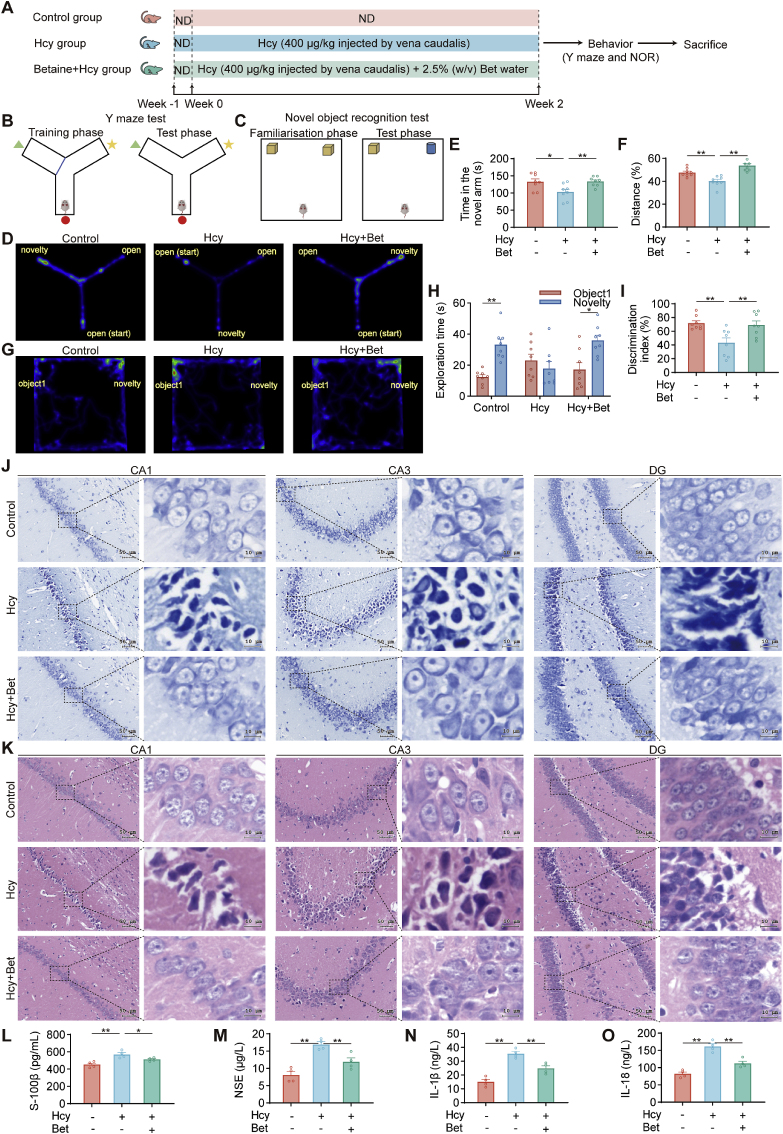
Nine-week-old male Sprague-Dawley rats were purchased from Zhuhai BesTest Bio-Tech Co., Ltd. (Zhuhai, China). The rats were divided into three groups, each consisting of eight animals. The groups were as follows: (1) Control group: the rats were injected with saline (0.9 %) through the vena caudalis for 14 days; (2) Hcy group: the rats were injected with Hcy (400 μg/kg/d) through the vena caudalis for 14 days [34]; (3) Hcy + Bet groups: the rats were injected with Hcy (400 μg/kg/d) through the vena caudalis and simultaneously supplemented betaine (2.5 % w/v) via drinking water for 14 days (Fig. 1A). The SD rats were housed with 4 per cage, maintained under controlled conditions of humidity (50–60 %), temperature (25 ± 2 °C) and a 12:12 h reversed light-dark cycle, with feed-free access to food and water. The animal experiments and protocols were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University.
Fig. 1.
Betaine attenuated cognitive impairment and brain injury induced by Hcy. (A) Schematic outline of the experimental design. (B–C) Schematic diagram of the Y maze test and NOR test. (D) Heat maps of the Y maze test. (E) Impacts of different groups on time in the novel arm (n = 8). (F) The percentage of movement distance in the novel arm (n = 8). (G) The heat maps of the NOR test. (H) The exploration time for the familiar and novel objects in the NOR test (n = 8). (I) The discrimination index analysis in the NOR test (n = 8). (J) Representative Nissl staining of hippocampus CA1, CA3 and DG area in different groups (n = 4). Representative photomicrographs were shown at 40× magnification, scale bar = 50 or 10 μm. (K) Representative H&E staining of the hippocampal regions in different groups (n = 4), scale bar = 50 or 10 μm, 40× magnification. (L–O) ELISA analysis of S-100β, NSE, IL-1β and IL-18 levels in the serum among three groups (n = 4). Data were analyzed by one-way analysis of variance followed by multiple comparisons or two-tailed paired t-test. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01.
2.3. Behavioral tests
2.3.1. Y maze test
The Y maze test is used to assess hippocampal-dependent short-term spatial memory in rodents, based on their innate tendency to explore new environments, and the test was performed as previously described [35]. The Y maze is composed of three arms (120° angles, arm length × height × width of 60 × 20 × 10 cm). The novel arm was blocked in the training phase, while the two other arms were opened. The rats were placed facing the wall of one of the open arms (start arm) and allowed to explore the maze for 10 min. One hour after the training phase, each animal was placed back in the start arm and allowed to explore all three open arms for 5 min. The position of both the start and novel arm was randomized among the rats. During each trial, the Y maze was cleaned with 75 % alcohol to eliminate possible bias due to smell left by the previous rat. The behaviors of the rats were video tracked and analyzed by TopScan 3.0 software (CleverSys, Inc.). The performance in the Y maze was expressed as the time spent in the novel arm (Fig. 1E).
2.3.2. Novel object recognition (NOR) test
NOR test is commonly employed to study learning and memory in rodents [36], as previously reported [37]. During the habituation phase, the rats were placed in the center of a chamber (90 × 90 × 60 cm) without objects and allowed to explore the chamber for 10 min. During the familiarization phase, the rats were positioned in the center of the chamber facing the opposite wall and allowed to explore the two similar objects for 10 min. One hour later (test phase), the rats were returned to the chamber and allowed to explore for 5 min, with one of the familiar objects and a novel object present. Between trials, the objects and chamber were cleaned with 75 % alcohol to minimize odor-based cues. Behaviors were recorded via video tracked and analyzed using TopScan 3.0 software (CleverSys, Inc.). Analysis was conducted based on the objects toward which they oriented their noses, within a 2 cm range. The discrimination index was calculated by dividing the time spent exploring the novel object by the total time spent exploring both objects (Fig. 1F).
2.4. Immunofluorescence
Frozen brain sections were subjected to antigen retrieval using sodium citrate, then permeabilized with 0.25 % Triton-X100 for 15 min. After blocking with 5 % donkey serum for 1 h at room temperature, the sections were incubated overnight at 4 °C with primary antibodies: goat anti-Iba1 (1:500, 011–27991, Wako, Japan), rabbit anti-CD68 (1:100, ab283654, Abcam), rabbit anti-NLRP3 (1:100, 27458-1-AP, Proteintech, Wuhan, China), rabbit anti-GSDMD (1:100, 20770-1-AP, Proteintech, Wuhan, China). After being washed three times with PBS, the slices were incubated with Alexa FluorTM Plus 488 antibody (donkey anti-goat IgG (H + L), 1:500, A32814, Thermo Fisher Scientific, Waltham, MA, USA) and Alexa FluorTM Plus 647 antibody (donkey anti-rabbit IgG (H + L), 1:500, A-31573, Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C for 2 h, followed by another three washes with PBS. Subsequently, the slices were counterstained with DAPI (Beyotime, China). Finally, the sections were observed, and fluorescence images were captured using an LSM 900 confocal microscope (Carl Zeiss, Germany). Microglia in the hippocampus were quantified using ImageJ software (National Institutes of Health, USA). Cell density was calculated as the number of microglia cells divided by the total fluorescent area of the outlined area [38].
2.5. Sholl analysis
The morphology of microglia was assessed using Imaris v10.0.0 software (Bitplane, Switzerland) for 3D reconstruction of Z-scan confocal images and sholl analysis, as described in previous publications [39,40]. For sholl analysis, concentric circles were drawn from the soma, and measurements including soma volume, the number of terminal points, total process length and the number of intersections per radius (increased by 5 μm) were calculated.
2.6. Bioinformatics analysis
Hcy-related targets were searched using the Comparative Toxicogenomics Database (http://ctdbase.org/) with “Homocysteine” as the search term [41]. The targets associated with Alzheimer's disease (AD) were obtained by searching the GeneCards database (http://www.genecards.org/) using the term “Alzheimer's disease” [42]. Overlapping genes between Hcy and AD were identified using the Venny 2.1.0 database (https://bioinfogp.cnb.csic.es/tools/venny/). Bubble plots depicting the top 10 relevant KEGG pathways were generated using the “ClusterProfiler (4.6.0)” software package in R (version 4.2.2).
The gene expression microarray dataset of human hippocampus regions in AD was collected from the Gene Expression Omnibus (GSE36980). The GSE36980 dataset included 7 hippocampus samples from AD patients and 10 hippocampus samples from healthy control individuals. The dataset was analyzed using the R (version 4.2.2) software package “limma (3.54.1)”.
2.7. m6A dot blot assay
The experiments were conducted as previously reported [43]. The mRNA samples of 50, 100 and 200 ng were denatured via heating at 65 °C for 5 min. Subsequently, 2 μL of denatured mRNA samples were spotted onto an Amersham Hybond N+ membrane (GE Healthcare, USA). The membranes were crosslinked using UV for 5 min, followed by blocking with 5 % BSA for 1 h. They were then incubated overnight at 4 °C with rabbit anti-m6A antibody (1:1000, Abcam, ab284130). Then, the membranes were incubated with HRP‐conjugated goat anti‐rabbit IgG (1:10000) for 1 h at room temperature. The blots were visualized using enhanced chemiluminescence. Additionally, another membrane was stained with a solution of 0.02 % methylene blue in 0.3 M sodium acetate (pH 5.2) for 1 h to ensure consistency among different groups.
2.8. Methylated RNA immunoprecipitation (MeRIP) qPCR assay
MeRIP-qPCR assay was performed to detect m6A modifications in individual gene transcripts using the BersinBioTM Methylated RNA Immunoprecipitation (MeRIP) Kit (BersinBio, #Bes5203), as per the manufacturer’s recommendation. Total RNAs were fragmented and then incubated with m6A antibody for 2 h at 4 °C with continuous rotation. Subsequently, they were incubated with prewashed Protein A/G Magnetic Beads for 1 h at 4 °C. Following immunoprecipitation, the enrichment of mRNA containing m6A modifications was examined using qRT-PCR.
2.9. mRNA stability assays
The stability of NLRP3 mRNA was evaluated by treating HMC3 cells with actinomycin-D (10 μg/mL, Sigma, SBR00013) for 0, 2, 4, 6, or 8 h, following the previously described method [44]. Total RNAs were extracted and examined by qRT-PCR assay, with normalization to the values of the 0-h group.
2.10. Lentivirus construction and transfection
Lentiviral short hairpin RNA (shRNA) was used to stably silence the expression of human YTHDF2, which was designed and purchased from Genechem Co., Ltd. (Shanghai, China). The HMC3 cells were transfected by lentivirus particles according to the manufacturer’s instructions. Subsequently, HMC3 cells were selected with medium containing 2 μg/mL of puromycin. The transduced cells were validated by qRT-PCR and western blotting.
2.11. Statistical analysis
Data were expressed as mean ± standard error of mean (SEM). All statistical analyses were performed using SPSS 25.0 software (SPSS Inc., Chicago, IL, USA). The two-tailed paired t-test was used to compare the means of two groups, and a one-way analysis of variance was used for multiple comparisons among different groups. Statistical significance was considered as follows: *p < 0.05, **p < 0.01.
More methods were described in the Supplementary information.
3. Results
3.1. Betaine alleviated cognitive impairment induced by Hcy
To investigate the effect of betaine on cognitive function, we evaluated learning and memory abilities by the Y maze (Fig. 1B) and NOR test (Fig. 1C). The heatmaps revealed differences in exploratory behavior among the groups during the Y maze test (Fig. 1D). The Hcy group exhibited a significantly reduced time and percentage of movement distance in the novel arm, as opposed to the control group. Conversely, the betaine group showed a significant and definite tendency toward exploring the novel arm compared with the Hcy group, as evidenced by increased in both time and distance of movement of the novel arm (Fig. 1E–F). Additionally, the heat map showed the preference for familiar and novel objects during the test phase (Fig. 1G). Hcy group significantly decreased in the time spent exploring the novel object and discrimination index compared with the control group. In contrast, betaine supplementation showed a significant preference for the novel object (Fig. 1H–I). These results indicated that betaine could ameliorate cognitive impairment induced by Hcy.
3.2. Betaine attenuated brain injury
To assess the effect of betaine on neuronal damage, we further observed the pathological changes in the hippocampal CA1, CA3 and DG regions by Nissl staining and H&E staining. Neurons in the control group exhibited tightly arranged morphology with intact structures, clear nucleoli, and no noticeable loss of neurons. In the Hcy group, a decrease in the number of neurons was observed, accompanied by a sparse arrangement, cellular shrinkage, cell membrane rupture, and intensified cytoplasmic staining. Betaine exhibited neuroprotective effects, resulting in a rebound in the number of neurons and improving their arrangement and hierarchy (Fig. 1J). The structure and shape of CA1, CA3 and DG regions in the control group exhibited a normal appearance, characterized by well-arranged neuronal cells and clearly visible nuclei. In the Hcy group, an increase in intercellular spaces was observed, accompanied by disordered cell morphology, vacuolization and edema of cells, and irregular, shriveled, and condensed nuclei. Betaine could ameliorate Hcy-induced neuronal damage. The cell morphology was basically regular, and the number of neurons was increased (Fig. 1K). These results demonstrated that betaine ameliorated the pathological damage caused by Hcy.
Then, the severity of brain injury was further assessed by measuring the levels of S-100β and NSE. The Hcy group exhibited a significant increase in S-100β and NSE levels compared with the control group, while the betaine group showed a decrease in S-100β and NSE levels (Fig. 1L-M). Additionally, after the administration, betaine treatment significantly reduced the IL-1β and IL-18 levels (Fig. 1N-O). These results indicated that betaine had the potential to attenuate brain injury induced by Hcy.
3.3. Betaine ameliorated microglial activation
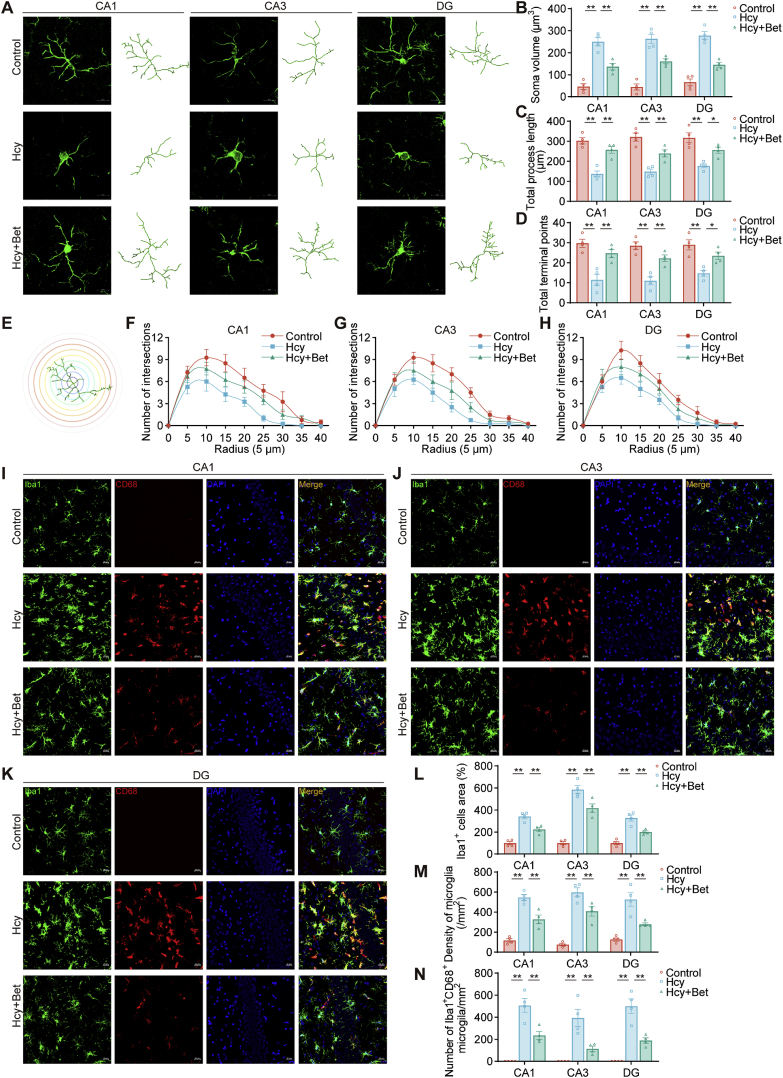
Microglia constantly monitor the microenvironment and rapidly activate in response to changes in brain environment. Consequently, our specific focus was to investigate the potential of betaine in mitigating microglial activation induced by Hcy. As shown in Fig. 2A, microglia in the hippocampus experienced morphological alterations in the Hcy group, characterized by shorter, thicker and sparsely branched processes, and enlarged soma. These changes were ameliorated by betaine. Betaine also exhibited a tendency to reduce soma volume compared with the Hcy group (Fig. 2B). Moreover, sholl analysis results revealed that betaine effectively increased total process length (Fig. 2C), total terminal points (Fig. 2D) and number of intersections (Fig. 2E–H).
Fig. 2.
Betaine alleviated microglial activation. (A) Representative images of Iba1 show the skeleton of microglia in the hippocampus among three groups, scale bar = 20 μm, 20× magnification (n = 4). (B–D) Quantitative analysis of soma volume, total process length, and total terminal points (n = 4). (E–H) Quantification of the number of intersections of projections in the sholl analysis (n = 4). (I–K) Representative confocal images of Iba1 and CD68 in the hippocampus among three groups, scale bar = 20 μm, 20× magnification (n = 4). (L–M) Quantitative analysis of Iba1+ cells area and density in the three groups (n = 4). (N) Quantification of Iba1+CD68+ cell numbers among the different groups (n = 4). Data were analyzed one-way analysis of variance followed for multiple comparisons. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01.
Additionally, activated microglia were identified through the co-localization of Iba1 and CD68, a phagocytic marker strongly expressed in activated microglia [45]. Immunofluorescent double staining was performed to detect the expression of CD68 in activated microglia in the hippocampus region (Fig. 2I–K). The results demonstrated that betaine inhibited the increase in Iba1+ cells area (Fig. 2L) and microglia density induced by Hcy (Fig. 2M), while also reducing the number of Iba1+CD68+ cells (Fig. 2N). These findings showed that betaine suppressed microglial activation induced by Hcy.
3.4. Betaine alleviated microglial pyroptosis by suppressing the NLRP3/caspase-1/GSDMD pathway
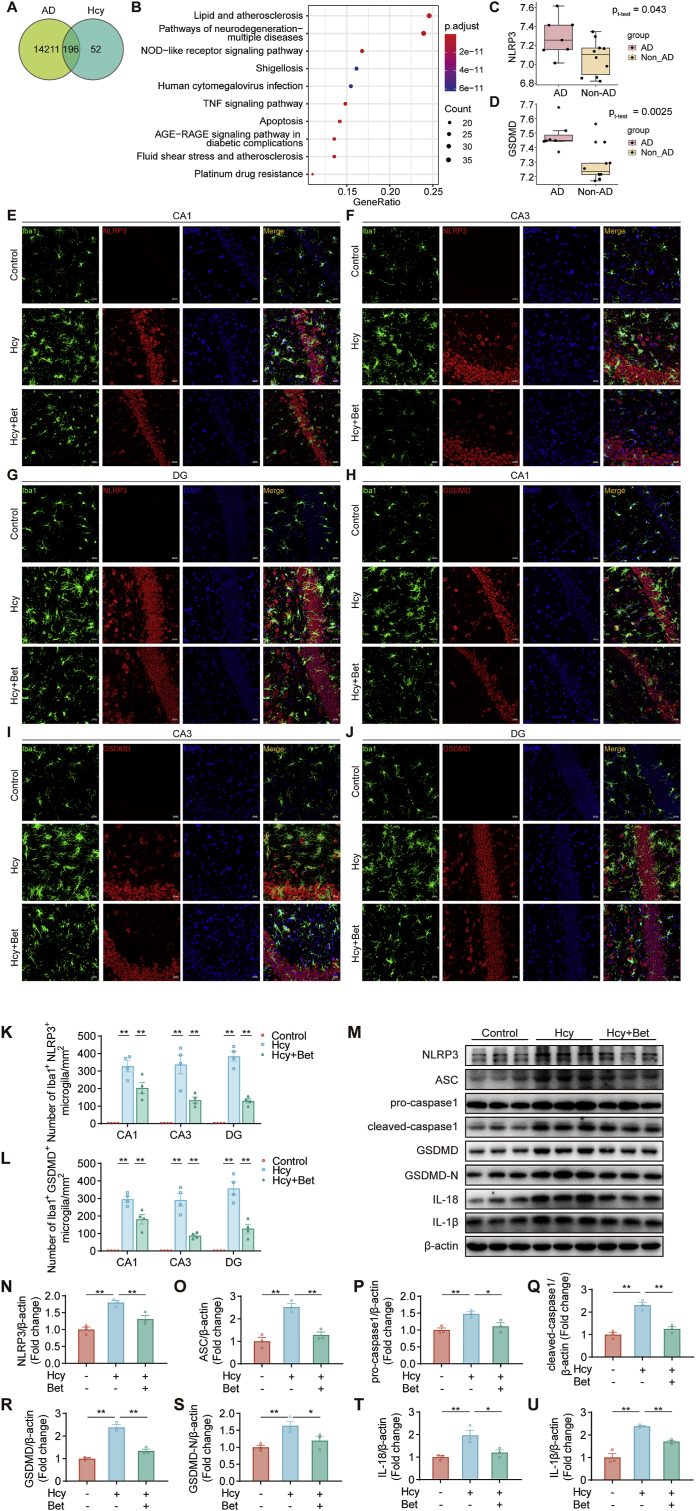
To predict the pathways by which Hcy causes cognitive impairment, we identified 196 target genes associated with both Hcy and AD (Tables S1–3) (Fig. 3A). KEGG analysis indicated that the NOD-like receptor signaling pathway plays an important role in Hcy-induced AD (Fig. 3B). NOD-like receptors (including NLRP3) are regarded as critical regulatory targets in inflammatory diseases [46,47]. Furthermore, our analysis revealed that the expression of NLRP3 was upregulated in the hippocampus of AD patients from the GSE36980 dataset (Fig. 3C). These findings suggested a close association between NLRP3 upregulation and cognitive impairment induced by Hcy.
Fig. 3.
Betaine inhibited microglial pyroptosis in the hippocampus region. (A) The overlapping targets of Hcy-related genes in AD. (B) KEGG enrichment analysis of those overlapping genes. (C–D) The expression of NLRP3 and GSDMD in the hippocampus of AD patients from GSE36980 datasets (AD patients, n = 7; non-AD patients, n = 10). (E–J) Representative confocal images of Iba1 and NLRP3, Iba1 and GSDMD in the hippocampus (CA1, CA3 and DG region) among three groups, scale bar = 20 μm, 20× magnification (n = 4). (K–L) Quantification of Iba1+NLRP3+ or Iba1+GSDMD+cell numbers in the hippocampus (CA1, CA3 and DG) among the different groups (n = 4). (M − U) The protein expression of NLRP3, ASC, pro-caspase-1, cleaved-caspase-1, GSDMD, GSDMD-N, IL-18 and IL-1β in the hippocampus among three groups was detected by western blot (n = 3). Data were analyzed by two-tailed paired t-test or one-way analysis of variance followed for multiple comparisons. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01.
NLRP3 is predominantly expressed in microglia [48]. The NLRP3 inflammasome-activated caspase-1 cleaves GSDMD, generating gasdermin D N-terminal (GSDMD-N), which serves as the effector molecule in pyroptosis [49]. In the hippocampus of AD patients (GSE36980), there was a significant increase in the level of GSDMD (Fig. 3D). Immunofluorescence staining revealed that betaine treatment mitigated microglial pyroptosis in the CA1, CA3, and DG regions induced by Hcy (Fig. 3E–J), as evidenced by a reduction in the number of Iba1+NLRP3+ double-labeled cells (Fig. 3K) and Iba1+GSDMD+ double-labeled cells (Fig. 3L). Consistent with the immunostaining results, betaine treatment suppressed the expression of NLRP3, ASC, pro-caspase-1, cleaved-caspase-1, GSDMD, GSDMD-N, IL-18, and IL-1β proteins (Fig. 3M − U). The results indicated that betaine might inhibit Hcy-induced microglial pyroptosis by inhibiting the NLRP3/caspase-1/GSDMD signaling pathway.
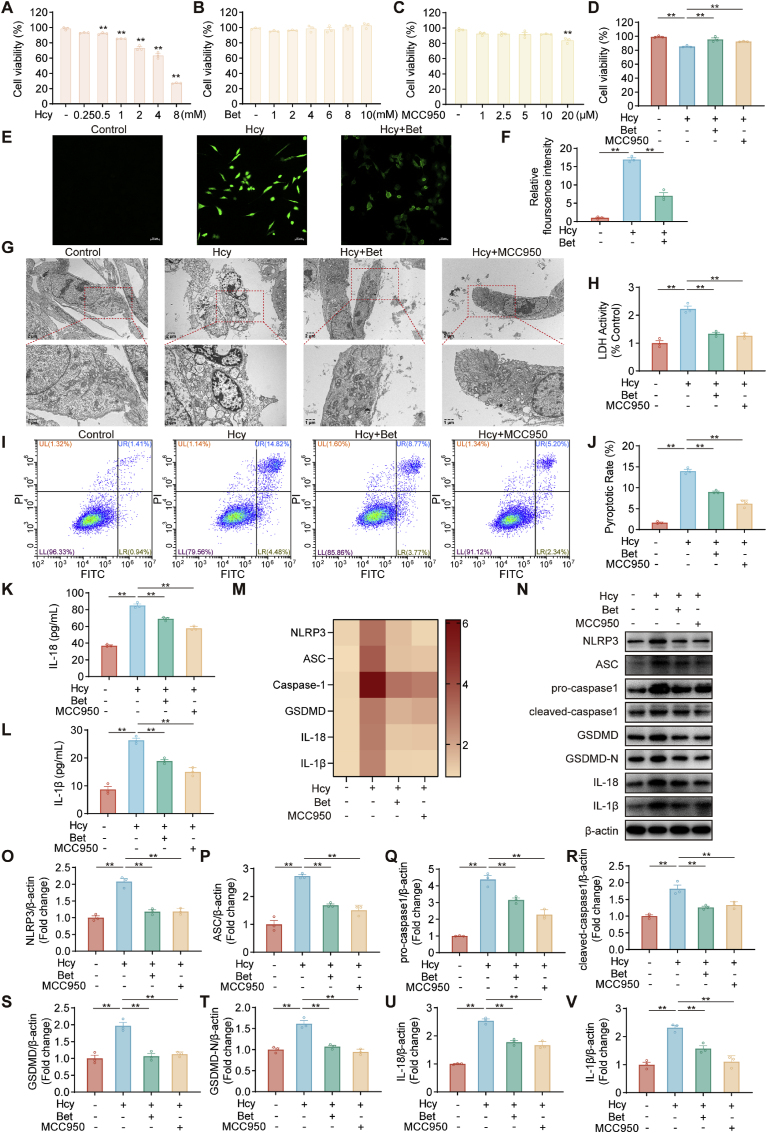
Further confirmation was obtained regarding the inhibitory effect of betaine on Hcy-induced microglial pyroptosis. We used MCC950, which specifically inhibits NLRP3 activation [50]. As shown in Fig. 4A–C, subsequent experiments were designed as follows: HMC3 cells were pretreated with betaine (10 mM) or MCC950 (10 μM) for 4 h, followed by Hcy (2 mM) treatment for 24 h. Pretreatment with betaine increased cell viability in Hcy-induced HMC3 cells (Fig. 4D). Pretreatment with betaine resulted in a reduction in the ROS level compared to the Hcy group (Fig. 4E–F). TEM results revealed that betaine treatment effectively mitigated the typical plasma membrane bubbles and membrane ruptures associated with pyroptosis induced by Hcy (Fig. 4G). Betaine pretreatment reversed the significant increase in the levels of LDH release in the culture supernatant (Fig. 4H), indicating inhibition of Hcy-induced plasma membrane rupture and leakage. Betaine also could inhibit the rate of pyroptotic cell pyroptosis induced by Hcy (Fig. 4I–J). Meanwhile, betaine reduced the inflammatory cytokines IL‐1β and IL-18 in the culture supernatant (Fig. 4K-L). Moreover, qRT-PCR and western blot results showed that betaine treatment inhibited the NLRP3/caspase-1/GSDMD pathway (Fig. 4M–V). Meanwhile, pretreatment with MCC950 reversed all of these effects and suppressed pyroptosis induced by Hcy. Therefore, betaine inhibited Hcy-induced microglial pyroptosis by suppressing the NLRP3/caspase-1/GSDMD pathway.
Fig. 4.
Betaine suppressed microglial pyroptosis in HMC3 cells. (A–D) HMC3 cells were treated with different concentrations Hcy, betaine or MCC950 and examined for cell viability. (E–F) Representative fluorescence images and intensity of ROS in HMC3 cells under different treatment groups, scale bar = 50 μm, 10× magnification. (G) Representative TEM images showing morphological changes of HMC3 cells under different treatments. (H) Release of LDH in HMC3 cells under different treatments. (I–J) Flow cytometry analysis of HMC3 cells pyroptosis in different treatment groups, stained by Annexin V-FITC and PI. (K–L) ELISA analysis of IL-18 and IL-1β levels in HMC3 cells. (M) The heat map showed the mRNA changes of NLRP3, ASC, pro-caspase-1, cleaved-caspase-1, GSDMD, GSDMD-N, IL-18 and IL-1β in HMC3 cells. (N–V) Western blot analysis of protein levels for NLRP3, ASC, pro-caspase-1, cleaved-caspase-1, GSDMD, GSDMD-N, IL-18 and IL-1β in HMC3 cells. Data were analyzed by one-way analysis of variance followed by multiple comparisons. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01.
3.5. Betaine protected neurons from microglia-mediated damage
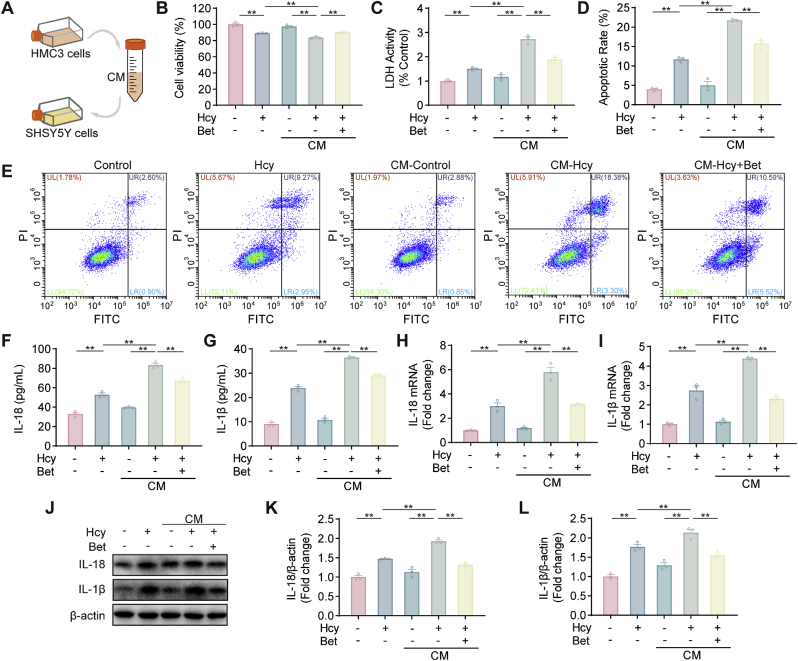
It has been reported that microglia activation-mediated inflammatory responses lead to neuronal damage and neurodegenerative diseases [51]. Therefore, we explored whether the protective effect of betaine on neurons was mediated by inhibiting pro-inflammatory cytokines secretion from microglia (Fig. 5A). Treatment with Hcy-CM significantly reduced SH-SY5Y cells viability compared with Hcy alone or the supernatant of unstimulated HMC3 cells. In contrast, the Hcy-betaine-CM group exhibited higher cell viability than the Hcy-CM group (Fig. 5B). The results of the LDH release were similar to those of cell viability (Fig. 5C). The apoptosis rate of SH-SY5Y cells treated with Hcy-CM was significantly increased, while the Hcy-betaine-CM group showed a lower percentage of apoptotic cells compared with the Hcy-CM group (Fig. 5D–E). Additionally, betaine treatment reduced the levels of IL‐1β and IL‐18 in the culture supernatant (Fig. 5F–G). Furthermore, both mRNA and protein levels of IL-18 and IL-1β expression were increased in the Hcy and Hcy-CM groups compared with the control group, and these effects were further ameliorated after the administration of Hcy-betaine-CM (Fig. 5H-L). Taken together, our results suggested that betaine could attenuate neuronal injury by inhibiting the release of toxic mediators during microglial activation.
Fig. 5.
Betaine attenuated microglia-mediated neurotoxicity. (A) Schematic diagram of the microglia-conditioned medium (CM). (B) SH-SY5Y cells were treated with CM and examined for cell viability. (C) Release of LDH in SH-SY5Y cells under different treatments. (D–E) The cell apoptosis of different treatment groups was examined using Flow cytometry in SH-SY5Y cells. (F–G) ELISA analysis of IL-18 and IL-1β levels in SH-SY5Y cells. (H–I) The mRNA changes of IL-18 and IL-1β in SH-SY5Y cells. (J–L) The protein expression of IL-18 and IL-1β was measured by western blot assay. Data were analyzed by one-way analysis of variance followed by multiple comparisons. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01.
3.6. Betaine increased NLRP3 mRNA m6A modification and reduced NLRP3 mRNA stability
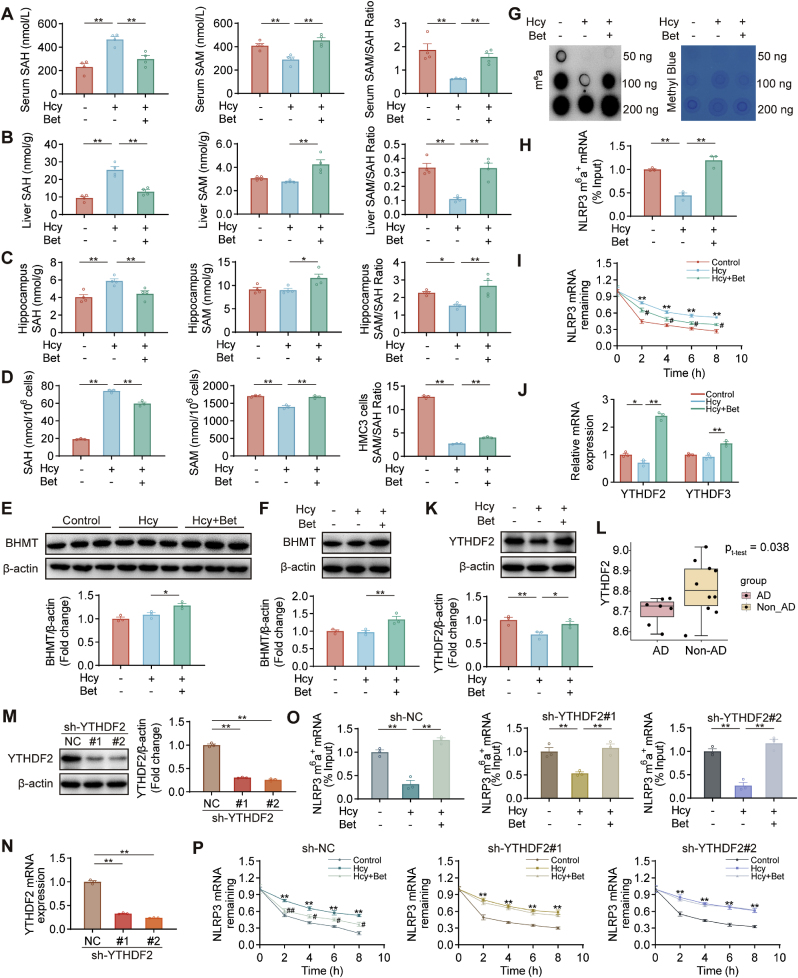
Next, we explored the effect of betaine on methylation capacity. The results revealed that the levels of SAH in serum, liver and hippocampus were significantly increased in the Hcy group, whereas the levels of SAM and the methylation capacity index (SAM/SAH ratio) were significantly decreased. Administration of betaine decreased the levels of SAH and increased SAM levels and the SAM/SAH ratio (Fig. 6A–C). Furthermore, betaine supplementation restored the SAM levels and the SAM/SAH ratio in Hcy-induced HMC3 cells (Fig. 6D). This effect might be attributed to the promotion of betaine homocysteine methyltransferase (BHMT) expression by betaine in SD rats and HMC3 cells, which remethylates Hcy (Fig. 6E–F). These findings suggested that betaine could promote methylation.
Fig. 6.
Betaine reduced the NLRP3 mRNA stability in an m6A-YTHDF2-dependent manner. (A–D) The levels of SAH, SAM and SAM/SAH ratio in serum, liver, hippocampus and HMC3 cells were measured by HPLC-MS/MS (n = 4). (E–F) The protein expression of BHMT was measured by western blot assay in the hippocampus and HMC3 cells (n = 3). (G) The m6A level of total RNA in HMC3 cells of different treatment groups was determined by m6A dot blot assay, and methylene blue staining blot processed parallelly (loading control). (H) MeRIP-qPCR assays showed the relative percentage of NLRP3 mRNA with methylation under different treatments. (I) The degradation rate of NLRP3 in HMC3 cells treated with actinomycin D over time via qRT-PCR assay. (J) qRT-PCR analysis of mRNA levels for YTHDF2 and YTHDF3 in HMC3 cells. (K) Western blot analysis of protein levels for YTHDF2 in HMC3 cells. (L) The expression of YTHDF2 in the hippocampus of AD patients from GSE36980 datasets (AD patients, n = 7; non-AD patients, n = 10). (M − N) Results of Western blot and qRT-PCR assays showed YTHDF2 expression in HMC3 cells transfected with two different short shRNA-expressing lentiviruses for YTHDF2 (sh-YTHDF2#1 and (sh-YTHDF2#2) or negative controls (sh-NC). (O) MeRIP-qPCR assays showed the relative percentage of NLRP3 mRNA with methylation in sh-NC, sh-YTHDF2#1 and sh-YTHDF2#2 HMC3 cells. (P) The degradation rate of NLRP3 in sh-NC, sh-YTHDF2#1 and sh-YTHDF2#2 HMC3 cells treated with actinomycin D over time via qRT-PCR assay (* Control vs Hcy group; # Hcy vs Betaine group). Data were analyzed by two-tailed paired t-test or one-way analysis of variance followed by multiple comparisons. Data are presented as mean ± SEM. *,#p < 0.05, **,##p < 0.01.
Then, we assessed the global m6A levels. Dot blot analysis showed a significant decrease in m6A levels in the Hcy group compared with the control group, which were restored after betaine pretreatment (Fig. 6G). Subsequently, MeRIP-qPCR assays were conducted to examine the m6A level of NLRP3. The m6A level of NLRP3 was reduced in Hcy group, this reduction was reversed by betaine pretreatment (Fig. 6H). Furthermore, we explored the impact of m6A modification on NLRP3 stability. To block new RNA synthesis in HMC3 cells, we treated them with the transcriptional inhibitor actinomycin D and measured the stability of NLRP3 mRNA. The degradation rate of NLRP3 was higher in the betaine group compared with the Hcy group, suggesting that betaine could reduce the stability of NLRP3 mRNA (Fig. 6I).
3.7. Betaine reduced the NLRP3 mRNA stability in an m6A-YTHDF2-dependent manner
Considering that betaine increased the m6A modification of NLRP3 while reduced the stability of NLRP3 mRNA, we hypothesized that betaine might exert its protective effect through YTHDF2 or YTHDF3. Our results showed that betaine pretreatment significantly elevated the mRNA level of YTHDF2 (Fig. 6J). Additionally, the protein expression of YTHDF2 was up-regulated in the betaine group (Fig. 6K). Interestingly, we observed a significant decrease in YTHDF2 expression in the hippocampus of AD patients (GSE36980) (Fig. 6L).
Furthermore, we aimed to investigate whether betaine regulated the NLRP3 mRNA stability through YTHDF2. We employed shRNA-expressing lentiviruses to silence YTHDF2 (sh-YTHDF2) or shRNA negative controls (sh-NC) in HMC3 cells (Fig. 6M − N). Our data indicated that betaine treatment could still increase the m6A methylation level of NLRP3 compared with Hcy treatment in HMC3 cells transfected with sh-NC and sh-YTHDF2 (#1 and #2) (Fig. 6O). Moreover, analyzing NLRP3 mRNA levels in the presence of betaine pretreatment revealed that betaine promoted the degradation of NLRP3 in sh-NC transfected HMC3 cells, but when YTHDF2 was silenced, the promoting effect of betaine on the NLRP3 degradation was inhibited (Fig. 6P). Taken together, these results demonstrated that betaine attenuated NLRP3 mRNA stability in a YTHDF2-dependent manner.
3.8. Silencing of YTHDF2 abolished the inhibitory effect of betaine on pyroptosis
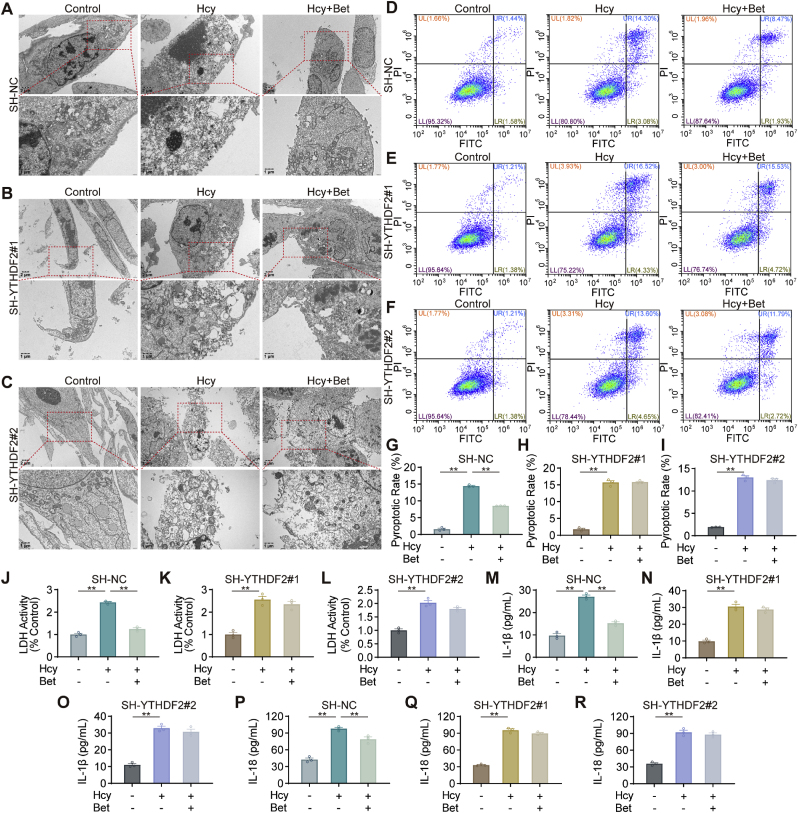
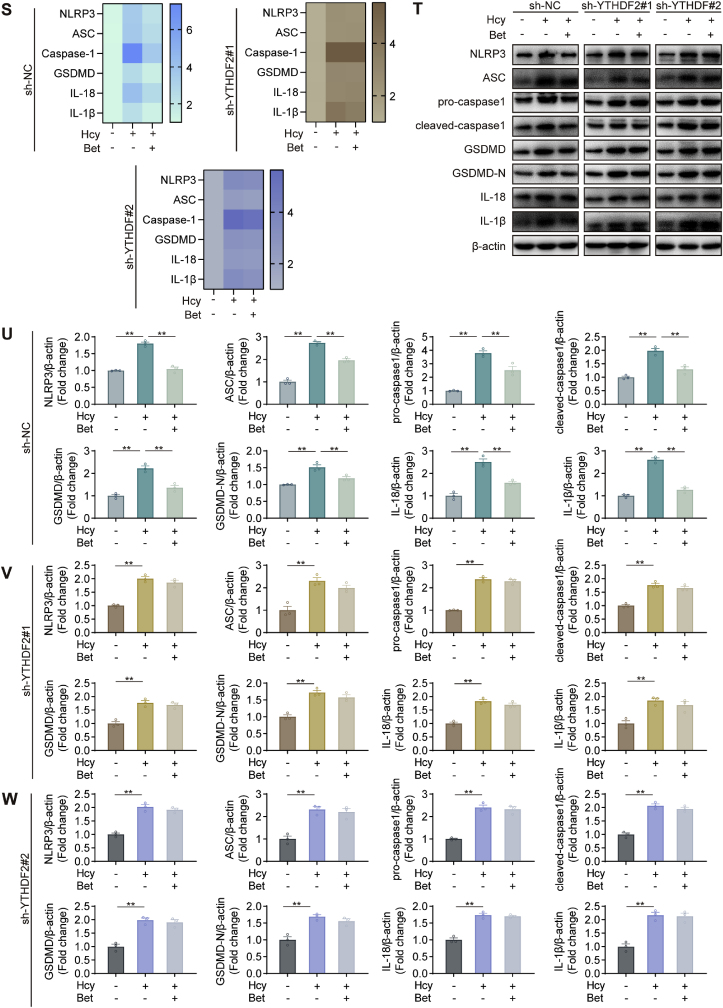
Moreover, we investigated whether silencing YTHDF2 affected the effects of betaine in alleviating pyroptosis. Silencing YTHDF2 did not improve the formation of plasma membrane bubbles and membrane ruptures after betaine treatment (Fig. 7A–C). Moreover, silencing YTHDF2 had no significant inhibitory effect on pyroptosis in the betaine group (Fig. 7D–I). The accumulation of LDH was observed in the betaine group after silencing YTHDF2 (Fig. 7J-L). Furthermore, after silencing YTHDF2, the levels of IL-18 and IL-1β in the culture supernatant were not significantly reduced in the betaine group (Fig. 7M-R). Moreover, pretreatment with betaine did not reduce the mRNA and protein levels of NLRP3, ASC, pro-caspase-1, cleaved caspase-1, GSDMD, IL-18 and IL-1β, which were targets involved in pyroptosis, after silencing YTHDF2 (Fig. 7S-W). Transfection with sh-NC did not affect the effect of betaine on inhibiting Hcy-induced pyroptosis. These results suggested that betaine inhibited Hcy-induced pyroptosis through YTHDF2-mediated m6A methylation.
Fig. 7.
Betaine inhibited NLRP3-mediated microglial pyroptosis in an m6A-YTHDF2-dependent manner. (A–C) Representative TEM images showing morphological changes in sh-NC, sh-YTHDF2#1 and sh-YTHDF2#2 HMC3 cells. (D–I) Cell pyroptosis in sh-NC, sh-YTHDF2#1 and sh-YTHDF2#2 HMC3 cells, stained by Annexin V-FITC and PI. (J–L) Release of LDH in sh-NC, sh-YTHDF2#1 and sh-YTHDF2#2 HMC3 cells. (M–R) ELISA analysis of IL-18 and IL-1β levels in sh-NC, sh-YTHDF2#1 and sh-YTHDF2#2 HMC3 cells. (S) The heat map showed the mRNA changes of NLRP3, ASC, pro-caspase-1, cleaved-caspase-1, GSDMD, GSDMD-N, IL-18 and IL-1β in sh-NC, sh-YTHDF2#1 and sh-YTHDF2#2 HMC3 cells. (T–W) Western blot analysis of protein levels for NLRP3, ASC, pro-caspase-1, cleaved-caspase-1, GSDMD, GSDMD-N, IL-18 and IL-1β in sh-NC, sh-YTHDF2#1 and sh-YTHDF2#2 HMC3 cells. Data were analyzed by one-way analysis of variance followed by multiple comparisons. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01.
4. Discussion
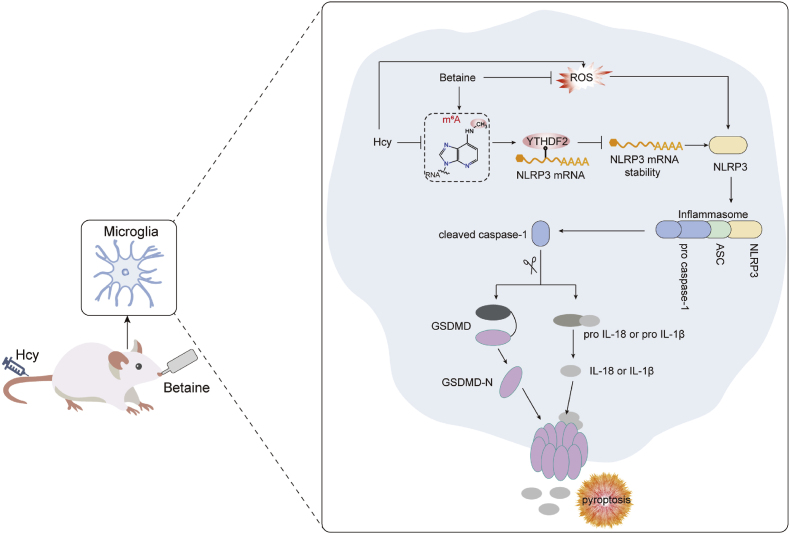
Hcy is widely acknowledged as a risk factor for dementia [8]. Lowering Hcy levels represents a promising strategy for preventing and alleviating dementia. Although betaine has been shown to reduce Hcy levels, the effects and mechanisms underlying its protective effects against Hcy-induced cognitive impairment remain largely unexplored. This study revealed that betaine effectively attenuated Hcy-induced cognitive impairment by inhibiting microglial pyroptosis through m6A modification (Fig. 8). In this study, betaine concentration was 2.5 % (w/v) and the intervention time was 14 days, which was derived from published literature [52,53]. The dosage of betaine was approximately 2000–2500 mg/kg body weight. Our findings suggested that betaine supplementation could be an effective approach to alleviate cognitive impairment induced by Hcy.
Fig. 8.
Schematic illustration showing the protective effects and underlying mechanisms of betaine on cognitive impairment induced by Hcy. Betaine could inhibit microglial pyroptosis by suppressing NLRP3/caspase-1/GSDMD pathway in an m6A-YTHDF2-dependent manner and ultimately alleviate cognitive impairment induced by Hcy.
Microglia are immune cells of the central nervous system [54], and their activation is characterized by morphological changes, such as soma enlargement and process retraction [[55], [56], [57]]. Microglial activation plays a crucial role in cognitive impairment induced by Hcy. For example, microglia exhibited a hyper-ramified morphology after Hcy treatment, and the levels of Iba1 mRNA and protein were increased in the cortex and hippocampus of the Hcy group, suggesting that microglia were activated [13]. Additionally, Hcy increased IL-1β and TNF-α expression in the hippocampus, accompanied by evident microglial activation in CBS−/- mice [58]. Our study demonstrated that betaine treatment significantly reduced microglial volume and increased microglial branching and complexity in the hippocampal region. Furthermore, betaine also decreased the Iba1 area, microglial density, and the phagocytic lysosomal marker CD68 expression, indicating betaine could suppress microglial activation.
Microglial activation triggers the release of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-18 and IL-6. These cytokines result in neuronal damage and potentially lead to brain injury [59,60]. Consequently, our investigation aimed to determine whether betaine could suppress microglial inflammation induced by Hcy. Pyroptosis, an inflammatory form of programmed cell death, has been implicated in the pathogenesis of various neurological diseases, such as AD, epilepsy and stroke [61]. The typical pyroptosis pathway is mediated by inflammasomes, such as NLRP3 [62]. Oxidative stress is the main driver of inflammation, and ROS can induce the activation of the NLRP3 inflammasome [63]. NLRP3 recruits the inflammasome adapter protein ASC, which subsequently activates caspase-1, leading to the maturation of IL-1β and IL-18 and the cleavage of GSDMD [64,65]. GSDMD-N binds to the inner leaflet of the cell membrane and oligomerizes, forming pores with 10–33 nm diameter. This process results in cell swelling, membrane rupture and discharge of inflammatory factors. Ultimately, it triggers immune cell recruitment and amplifies the cascade of inflammatory responses [66,67]. Microglia can strongly exhibit a pyroptotic response associated with NRLP3 inflammasome activation [68].
In this study, we conducted a bioinformatics analysis and identified NLRP3 and GSDMD as highly expressed genes in cognitive impairment induced by Hcy. Meanwhile, it was found that NLRP3 inflammasome is a crucial target in microglia [68]. Furthermore, double immunofluorescence staining showed the expression of both NLRP3 and GSDMD in microglia induced by Hcy. Moreover, the Hcy group exhibited high ROS level, membrane rupture, up-regulated expression of NLRP3, ASC, pro-caspase-1, cleaved caspase-1, GSDMD, GSDMD-N, IL-18 and IL-1β, along with elevated IL-18, IL-1β and LDH release. However, these effects were mitigated by betaine treatment. Our findings are consistent with previous studies that the inhibitory effect of betaine on pyroptosis induced by acute severe ulcerative colitis [69]. Additionally, our conditioned medium experiments revealed that betaine could further attenuate the neuronal damage caused by microglia-mediated inflammatory responses, alleviate neuronal apoptosis and inhibit the expression of inflammatory factors. This study demonstrated that betaine prevented Hcy-induced microglial pyroptosis and protected neurons from microglia-mediated damage.
Betaine contains three chemically reactive methyl groups, and its methyl group is transferred to Hcy by BHMT to form methionine. Methionine is subsequently converted into SAM, which plays a crucial role in the methionine cycle [27]. Studies have identified the expression of BHMT in the brain [70,71]. The SAM/SAH ratio serves as an indicator of transmethylation potential, and its decrease predicts a decline in cellular methylation potential [72,73]. High Hcy levels led to increased SAH and decreased SAM/SAH ratio [74], which is consistent with our findings in vivo and in vitro. Aberrant methylation affects cognitive performance. In the brain of stress-induced cognitive decline SD rats, Hcy disturbed DNA methylation, resulting in DNA hypermethylation of the BDNF promoter and downregulation of BDNF levels [75]. Hcy-induced DNA hypomethylation leads to irreversible DNA damage and cytotoxicity in microglia [76]. The present study showed that betaine supplementation significantly increased SAM level, decreased SAH level, and reduced the SAM/SAH ratio compared with the Hcy group.
In recent years, RNA m6A modification, a hot topic in epigenetic regulation, has been involved in multiple cellular processes, such as the maturation or degradation of mRNA and protein translation [77]. Recent studies have revealed that m6A is more abundant in the central nervous system than in other organs [78]. In addition, m6A modification plays a pivotal role in neurological diseases, such as AD, Parkinson’s disease and depression [79,80]. Previous reports indicated that lead exposure resulted in a decline in learning and memory ability in SD rats, accompanied by a decrease in m6A level in the hippocampus. Furthermore, learning memory and m6A levels were improved after folic acid intervention [81]. LPS-induced microglial activation and inflammatory responses were potentially mediated by enhanced m6A methylation and stability of Gbp11 and Cp mRNA through IGF2BP1 [82]. m6A modification may be a significant regulator in microglial pyroptosis.
Notably, our findings demonstrated that betaine significantly enhanced overall m6A levels. Accumulating evidence indicates a link between m6A modification and NLRP3. In LPS-induced human astrocytoma 1321N1 cells, emodin reduced the expression of NLRP3 by regulating METTL3-mediated m6A methylation, and further relieved inflammation and pyroptosis [83]. WTAP promoted NLRP3-dependent inflammation and pyroptosis by enhancing m6A methylation of NLRP3 mRNA in human renal tubular epithelial cells [84]. The present study found that betaine effectively increased the m6A methylation level of NLRP3 mRNA and reduced the stability of NLRP3 mRNA, which possibly was regulated by the m6A reader YTHDF2.
The m6A reader is the executor of the m6A signal, and YTHDF2 directly recognizes the m6A site through its YTH domain to regulate mRNA stability [85,86]. In our study, we observed that betaine could promote the expression of YTHDF2. Subsequent silencing YTHDF2 showed that the effect of betaine on NLRP3 mRNA degradation was weakened, resulting in attenuated inhibition of Hcy-induced pyroptosis. Consistent with our findings, recent studies have shown that silencing YTHDF2 increased the expression of IL-6, TNF-α, IL-1β and IL-12, enhanced the stability of MAP2K4 and MAP4K4 and activated MAPK and NF-κB signaling pathways, thereby exacerbating the inflammatory response in RAW 264.7 cells stimulated with LPS [87]. In human hepatocellular carcinoma cell lines SMMC7721 and MHCC97H, silencing YTHDF2 led to severe inflammation [88]. Therefore, we speculate that betaine alleviated Hcy-induced inflammation and pyroptosis in an m6A-YTHDF2-dependent manner.
5. Conclusions
In summary, our study demonstrated that betaine inhibited the NLRP3/caspase-1/GSDMD signaling pathway in an m6A-YTHDF2-dependent manner, further inhibiting microglial pyroptosis, ultimately alleviating Hcy-induced cognitive impairment. Betaine supplementation may open a new therapeutic window for Hcy-induced cognitive impairment. This study provides basis for betaine in development and application of functional food/pharmaceuticals for improving cognitive ability.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81973016), National Natural Science Youth Foundation of China (No. 82204020), China Postdoctoral Science Foundation (No. 2022M713556).
CRediT authorship contribution statement
Zhi-Jun Yang: Conceptualization, Investigation, Methodology, Writing – original draft. Si-Yu Huang: Methodology. Kai-Yi Zhong: Investigation, Methodology. Wen-Ge Huang: Methodology. Zi-Hui Huang: Investigation. Tong-Tong He: Investigation. Meng-Tao Yang: Investigation. Maierhaba Wusiman: Investigation. Dan-Dan Zhou: Methodology. Si Chen: Funding acquisition, Resources. Bi-Xia Huang: Resources. Xiao-Lin Luo: Methodology. Hua-Bin Li: Writing – review & editing. Hui-Lian Zhu: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors declare no conflict of interest.
Abbreviations
- AD
Alzheimer’s disease
- ASC
PYD and CARD domain containing
- BDNF
brain derived neurotrophic factor
- caspase-1
cysteinyl aspartate specific proteinase 1
- BHMT
betaine homocysteine methyltransferase
- CCK‐8
cell counting kit-8
- CM
conditioned medium
- GSDMD
gasdermin D
- GSDMD-N
gasdermin D N-terminal
- LDH
lactate dehydrogenase
- Hcy
homocysteine
- H&E staining
hematoxylin and Eosin staining
- HPLC-MS/MS
high-performance liquid chromatography-tandem mass spectrometry
- IGF2
insulin growth factor II
- IGF2BP1
insulin like growth factor 2 mRNA binding protein 1
- iNOS
inducible nitric oxide synthase
- IL-1β
interleukin-1β
- IL-18
interleukin-18
- IL-6
interleukin-6
- LPS
lipopolysaccharides
- m6a
N6-methyladenosine
- MAPK
mitogen-activated protein kinase
- MeRIP
methylated RNA immunoprecipitation
- METTL3
methyltransferase 3
- MyD88
myeloid differentiation primary response 88
- NLRP3
NLR family pyrin domain containing 3
- NF-κB
nuclear factor kappa-B
- NOR
novel object recognition
- NSE
neuron-specific enolase
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- qRT-PCR
quantitative reverse transcription-polymerase chain reaction
- ROS
reactive oxygen species
- S-100β
S100 calcium-binding protein 201 B
- TEM
transmission electron microscopy
- SAM
S-adenosyl methionine
- SAH
S-adenosyl-l-homocysteine
- TNF-α
tumor necrosis factor α
- WTAP
WT1 associated protein
- YTHDF2
YTH N6-methyladenosine RNA binding protein 2
Footnotes
Some of the research results in our manuscript were presented through oral and poster presentations, as well as in the form of meeting abstracts, at the 14th Asian Congress of Nutrition held from September 14–17th, 2023.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103026.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D., Ballard C., Banerjee S., Burns A., Cohen-Mansfield J., Cooper C., Fox N., Gitlin L.N., Howard R., Kales H.C., Larson E.B., Ritchie K., Rockwood K., Sampson E.L., Samus Q., Schneider L.S., Selbaek G., Teri L., Mukadam N. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 2.Yang Z., Zhou D.D., Huang S.Y., Fang A.P., Li H.B., Zhu H.L. Effects and mechanisms of natural products on Alzheimer's disease. Crit. Rev. Food Sci. Nutr. 2023;63:3168–3188. doi: 10.1080/10408398.2021.1985428. [DOI] [PubMed] [Google Scholar]
- 3.Gauthier S., Rosa-Neto P., Morais J., Webster C. Alzheimer’s Disease International; 2021. World Alzheimer Report 2021: Journey through the Diagnosis of Dementia. [Google Scholar]
- 4.Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S., Brayne C., Burns A., Cohen-Mansfield J., Cooper C., Costafreda S.G., Dias A., Fox N., Gitlin L.N., Howard R., Kales H.C., Kivimaki M., Larson E.B., Ogunniyi A., Orgeta V., Ritchie K., Rockwood K., Sampson E.L., Samus Q., Schneider L.S., Selbaek G., Teri L., Mukadam N. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . WHO guidelines; 2019. Risk Reduction of Cognitive Decline and Dementia. [PubMed] [Google Scholar]
- 6.Sonnen J.A., Montine K.S., Quinn J.F., Kaye J.A., Breitner J.C., Montine T.J. Biomarkers for cognitive impairment and dementia in elderly people. Lancet Neurol. 2008;7:704–714. doi: 10.1016/S1474-4422(08)70162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larson E.B., Kukull W.A., Katzman R.L. Cognitive impairment: dementia and Alzheimer's disease. Annu. Rev. Publ. Health. 1992;13:431–449. doi: 10.1146/annurev.pu.13.050192.002243. [DOI] [PubMed] [Google Scholar]
- 8.Seshadri S., Beiser A., Selhub J., Jacques P.F., Rosenberg I.H., D'Agostino R.B., Wilson P.W., Wolf P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N. Engl. J. Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 9.Smith A.D., Refsum H., Bottiglieri T., Fenech M., Hooshmand B., McCaddon A., Miller J.W., Rosenberg I.H., Obeid R. Homocysteine and dementia: an international consensus statement. J. Alzheimers Dis. 2018;62:561–570. doi: 10.3233/JAD-171042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris M.S. Homocysteine and Alzheimer's disease. Lancet Neurol. 2003;2:425–428. doi: 10.1016/s1474-4422(03)00438-1. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q., Zhao J., Chang H., Liu X., Zhu R. Homocysteine and folic acid: risk factors for Alzheimer's disease-an updated meta-analysis. Front. Aging Neurosci. 2021;13:665114. doi: 10.3389/fnagi.2021.665114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cagnin A., Brooks D.J., Kennedy A.M., Gunn R.N., Myers R., Turkheimer F.E., Jones T., Banati R.B. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- 13.Kumar M., Sandhir R. Hydrogen sulfide suppresses homocysteine-induced glial activation and inflammatory response. Nitric Oxide. 2019;90:15–28. doi: 10.1016/j.niox.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Xu L., He D., Bai Y. Microglia-mediated inflammation and neurodegenerative disease. Mol. Neurobiol. 2016;53:6709–6715. doi: 10.1007/s12035-015-9593-4. [DOI] [PubMed] [Google Scholar]
- 15.Prinz M., Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 16.Bergsbaken T., Fink S.L., Cookson B.T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tschopp J., Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 19.Li D.D., Fan H.X., Yang R., Li Y.Y., Zhang F., Shi J.S., Dendrobium Nobile Lindl Alkaloid suppresses NLRP3-mediated pyroptosis to alleviate LPS-induced neurotoxicity. Front. Pharmacol. 2022;13:846541. doi: 10.3389/fphar.2022.846541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulias K., Greer E.L. Biological roles of adenine methylation in RNA. Nat. Rev. Genet. 2023;24:143–160. doi: 10.1038/s41576-022-00534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W., Chen T.Q., Fang K., Zeng Z.C., Ye H., Chen Y.Q. N6-methyladenosine methyltransferases: functions, regulation, and clinical potential. J. Hematol. Oncol. 2021;14:117. doi: 10.1186/s13045-021-01129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang P., Doxtader K.A., Nam Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol. Cell. 2016;63:306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X.Y., Zhang J., Zhu J.S. The role of m(6)A RNA methylation in human cancer. Mol. Cancer. 2019;18:103. doi: 10.1186/s12943-019-1033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao W.Y., Lou Y., Hu G.Y., Qian C.Y., Liang W.R., Zhao J., Wang X.H. RNA m6A reader YTHDF1 facilitates inflammation via enhancing NLRP3 translation. Biochem. Biophys. Res. Commun. 2022;616:76–81. doi: 10.1016/j.bbrc.2022.05.076. [DOI] [PubMed] [Google Scholar]
- 26.Ge P., Duan H., Tao C., Niu S., Hu Y., Duan R., Shen A., Sun Y., Sun W. TMAO promotes NLRP3 inflammasome activation of microglia aggravating neurological injury in ischemic stroke through FTO/IGF2BP2. J. Inflamm. Res. 2023;16:3699–3714. doi: 10.2147/JIR.S399480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craig S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004;80:539–549. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 28.Takanaga H., Ohtsuki S., Hosoya K., Terasaki T. GAT2/BGT-1 as a system responsible for the transport of gamma-aminobutyric acid at the mouse blood-brain barrier. J. Cerebr. Blood Flow Metabol. 2001;21:1232–1239. doi: 10.1097/00004647-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Knight L.S., Piibe Q., Lambie I., Perkins C., Yancey P.H. Betaine in the brain: characterization of betaine uptake, its influence on other osmolytes and its potential role in neuroprotection from osmotic stress. Neurochem. Res. 2017;42:3490–3503. doi: 10.1007/s11064-017-2397-3. [DOI] [PubMed] [Google Scholar]
- 30.Ueland P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011;34:3–15. doi: 10.1007/s10545-010-9088-4. [DOI] [PubMed] [Google Scholar]
- 31.Atkinson W., Elmslie J., Lever M., Chambers S.T., George P.M. Dietary and supplementary betaine: acute effects on plasma betaine and homocysteine concentrations under standard and postmethionine load conditions in healthy male subjects. Am. J. Clin. Nutr. 2008;87:577–585. doi: 10.1093/ajcn/87.3.577. [DOI] [PubMed] [Google Scholar]
- 32.Steenge G.R., Verhoef P., Katan M.B. Betaine supplementation lowers plasma homocysteine in healthy men and women. J. Nutr. 2003;133:1291–1295. doi: 10.1093/jn/133.5.1291. [DOI] [PubMed] [Google Scholar]
- 33.Sun J., Wen S., Zhou J., Ding S. Association between malnutrition and hyperhomocysteine in Alzheimer's disease patients and diet intervention of betaine. J. Clin. Lab. Anal. 2017;31 doi: 10.1002/jcla.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C.E., Wei W., Liu Y.H., Peng J.H., Tian Q., Liu G.P., Zhang Y., Wang J.Z. Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain. Am. J. Pathol. 2009;174:1481–1491. doi: 10.2353/ajpath.2009.081036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Notaras M., Hill R., Gogos J.A., van den Buuse M. BDNF Val66Met genotype determines hippocampus-dependent behavior via sensitivity to glucocorticoid signaling. Mol. Psychiatr. 2016;21:730–732. doi: 10.1038/mp.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lueptow L.M. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 2017:55718. doi: 10.3791/55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang S., Zhang S., Tang W., Fang S., Zhang H., Zheng J., Liu X., Zhang Y., Zhao L., Huang L., Li B. Enriched environment prevents surgery-induced persistent neural inhibition and cognitive dysfunction. Front. Aging Neurosci. 2021;13:744719. doi: 10.3389/fnagi.2021.744719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Favuzzi E., Huang S., Saldi G.A., Binan L., Ibrahim L.A., Fernandez-Otero M., Cao Y., Zeine A., Sefah A., Zheng K., Xu Q., Khlestova E., Farhi S.L., Bonneau R., Datta S.R., Stevens B., Fishell G. GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell. 2021;184:5686. doi: 10.1016/j.cell.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J., Wang H., Liu D., Li X., He L., Pan J., Shen Q., Peng Y. CB2R activation ameliorates late adolescent chronic alcohol exposure-induced anxiety-like behaviors during withdrawal by preventing morphological changes and suppressing NLRP3 inflammasome activation in prefrontal cortex microglia in mice. Brain Behav. Immun. 2023;110:60–79. doi: 10.1016/j.bbi.2023.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Xavier S., Soch A., Younesi S., Malik S., Spencer S.J., Sominsky L. Maternal diet before and during pregnancy modulates microglial activation and neurogenesis in the postpartum rat brain. Brain Behav. Immun. 2021;98:185–197. doi: 10.1016/j.bbi.2021.08.223. [DOI] [PubMed] [Google Scholar]
- 41.Davis A.P., Wiegers T.C., Johnson R.J., Sciaky D., Wiegers J., Mattingly C.J. Comparative Toxicogenomics database (CTD): update 2023. Nucleic Acids Res. 2023;51:D1257–D1262. doi: 10.1093/nar/gkac833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stelzer G., Rosen N., Plaschkes I., Zimmerman S., Twik M., Fishilevich S., Stein T.I., Nudel R., Lieder I., Mazor Y., Kaplan S., Dahary D., Warshawsky D., Guan-Golan Y., Kohn A., Rappaport N., Safran M., Lancet D. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 2016;54 doi: 10.1002/cpbi.5. 1 30 1-1 30 33. [DOI] [PubMed] [Google Scholar]
- 43.Chen J., Xu C., Yang K., Gao R., Cao Y., Liang L., Chen S., Xu S., Rong R., Wang J., Zhu T. Inhibition of ALKBH5 attenuates I/R-induced renal injury in male mice by promoting Ccl28 m6A modification and increasing Treg recruitment. Nat. Commun. 2023;14:1161. doi: 10.1038/s41467-023-36747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X., Yin H., Zhang X., Jiang X., Liu Y., Zhang H., Peng Y., Li D., Yu Y., Zhang J., Cheng S., Yang A., Zhang R. N6-methyladenosine modification governs liver glycogenesis by stabilizing the glycogen synthase 2 mRNA. Nat. Commun. 2022;13:7038. doi: 10.1038/s41467-022-34808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z., Jiang J., He Y., Cai J., Xie J., Wu M., Xing M., Zhang Z., Chang H., Yu P., Chen S., Yang Y., Shi Z., Liu Q., Sun H., He B., Zeng J., Huang J., Chen J., Li H., Li Y., Lin W.J., Tang Y. Pregabalin mitigates microglial activation and neuronal injury by inhibiting HMGB1 signaling pathway in radiation-induced brain injury. J. Neuroinflammation. 2022;19:231. doi: 10.1186/s12974-022-02596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanneganti T.D., Lamkanfi M., Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Geddes K., Magalhaes J.G., Girardin S.E. Unleashing the therapeutic potential of NOD-like receptors. Nat. Rev. Drug Discov. 2009;8:465–479. doi: 10.1038/nrd2783. [DOI] [PubMed] [Google Scholar]
- 48.Hou Y., Wei Y., Lautrup S., Yang B., Wang Y., Cordonnier S., Mattson M.P., Croteau D.L., Bohr V.A. NAD(+) supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer's disease via cGAS-STING. Proc. Natl. Acad. Sci. U.S.A. 2021;118 doi: 10.1073/pnas.2011226118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 50.Coll R.C., Robertson A.A., Chae J.J., Higgins S.C., Munoz-Planillo R., Inserra M.C., Vetter I., Dungan L.S., Monks B.G., Stutz A., Croker D.E., Butler M.S., Haneklaus M., Sutton C.E., Nunez G., Latz E., Kastner D.L., Mills K.H., Masters S.L., Schroder K., Cooper M.A., O'Neill L.A. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Block M.L., Zecca L., Hong J.S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 52.Ohnishi T., Balan S., Toyoshima M., Maekawa M., Ohba H., Watanabe A., Iwayama Y., Fujita Y., Tan Y., Hisano Y., Shimamoto-Mitsuyama C., Nozaki Y., Esaki K., Nagaoka A., Matsumoto J., Hino M., Mataga N., Hayashi-Takagi A., Hashimoto K., Kunii Y., Kakita A., Yabe H., Yoshikawa T. Investigation of betaine as a novel psychotherapeutic for schizophrenia. EBioMedicine. 2019;45:432–446. doi: 10.1016/j.ebiom.2019.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shedid S.M., Abdel-Magied N., Saada H.N. Role of betaine in liver injury induced by the exposure to ionizing radiation. Environ. Toxicol. 2019;34:123–130. doi: 10.1002/tox.22664. [DOI] [PubMed] [Google Scholar]
- 54.Prinz M., Jung S., Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179:292–311. doi: 10.1016/j.cell.2019.08.053. [DOI] [PubMed] [Google Scholar]
- 55.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 56.Walker F.R., Beynon S.B., Jones K.A., Zhao Z., Kongsui R., Cairns M., Nilsson M. Dynamic structural remodelling of microglia in health and disease: a review of the models, the signals and the mechanisms. Brain Behav. Immun. 2014;37:1–14. doi: 10.1016/j.bbi.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Wolf S.A., Boddeke H.W., Kettenmann H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017;79:619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- 58.Elsherbiny N.M., Sharma I., Kira D., Alhusban S., Samra Y.A., Jadeja R., Martin P., Al-Shabrawey M., Tawfik A. Homocysteine induces inflammation in retina and brain. Biomolecules. 2020;10:393. doi: 10.3390/biom10030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leng F., Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 2021;17:157–172. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- 60.Ransohoff R.M. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 61.McKenzie B.A., Dixit V.M., Power C. Fiery cell death: pyroptosis in the central nervous system. Trends Neurosci. 2020;43:55–73. doi: 10.1016/j.tins.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 62.Xue Y., Enosi Tuipulotu D., Tan W.H., Kay C., Man S.M. Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol. 2019;40:1035–1052. doi: 10.1016/j.it.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Schroder K., Zhou R., Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 64.Fu J., Wu H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu. Rev. Immunol. 2023;41:301–316. doi: 10.1146/annurev-immunol-081022-021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu P., Zhang X., Liu N., Tang L., Peng C., Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct. Targeted Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V.G., Wu H., Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Man S.M., Karki R., Kanneganti T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walsh J.G., Muruve D.A., Power C. Inflammasomes in the CNS. Nat. Rev. Neurosci. 2014;15:84–97. doi: 10.1038/nrn3638. [DOI] [PubMed] [Google Scholar]
- 69.Chen L., Liu D., Mao M., Liu W., Wang Y., Liang Y., Cao W., Zhong X. Betaine ameliorates acute sever ulcerative colitis by inhibiting oxidative stress induced inflammatory pyroptosis. Mol. Nutr. Food Res. 2022;66 doi: 10.1002/mnfr.202200341. [DOI] [PubMed] [Google Scholar]
- 70.Velazquez R., Ferreira E., Winslow W., Dave N., Piras I.S., Naymik M., Huentelman M.J., Tran A., Caccamo A., Oddo S. Maternal choline supplementation ameliorates Alzheimer's disease pathology by reducing brain homocysteine levels across multiple generations. Mol. Psychiatr. 2020;25:2620–2629. doi: 10.1038/s41380-018-0322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singhal N.K., Sternbach S., Fleming S., Alkhayer K., Shelestak J., Popescu D., Weaver A., Clements R., Wasek B., Bottiglieri T., Freeman E.J., McDonough J. Betaine restores epigenetic control and supports neuronal mitochondria in the cuprizone mouse model of multiple sclerosis. Epigenetics. 2020;15:871–886. doi: 10.1080/15592294.2020.1735075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caudill M.A., Wang J.C., Melnyk S., Pogribny I.P., Jernigan S., Collins M.D., Santos-Guzman J., Swendseid M.E., Cogger E.A., James S.J. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J. Nutr. 2001;131:2811–2818. doi: 10.1093/jn/131.11.2811. [DOI] [PubMed] [Google Scholar]
- 73.Hoffman D.R., Marion D.W., Cornatzer W.E., Duerre J.A. S-Adenosylmethionine and S-adenosylhomocystein metabolism in isolated rat liver. Effects of L-methionine, L-homocystein, and adenosine. J. Biol. Chem. 1980;255:10822–10827. [PubMed] [Google Scholar]
- 74.James S.J., Melnyk S., Pogribna M., Pogribny I.P., Caudill M.A. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J. Nutr. 2002;132:2361S–2366S. doi: 10.1093/jn/132.8.2361S. [DOI] [PubMed] [Google Scholar]
- 75.Wang S.D., Wang X., Zhao Y., Xue B.H., Wang X.T., Chen Y.X., Zhang Z.Q., Tian Y.R., Xie F., Qian L.J. Homocysteine-induced disturbances in DNA methylation contribute to development of stress-associated cognitive decline in rats. Neurosci. Bull. 2022;38:887–900. doi: 10.1007/s12264-022-00852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu C.C., Ho W.Y., Leu K.L., Tsai H.M., Yang T.H. Effects of S-adenosylhomocysteine and homocysteine on DNA damage and cell cytotoxicity in murine hepatic and microglia cell lines. J. Biochem. Mol. Toxicol. 2009;23:349–356. doi: 10.1002/jbt.20298. [DOI] [PubMed] [Google Scholar]
- 77.Fu Y., Dominissini D., Rechavi G., He C. Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat. Rev. Genet. 2014;15:293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- 78.Jiang X., Liu B., Nie Z., Duan L., Xiong Q., Jin Z., Yang C., Chen Y. The role of m6A modification in the biological functions and diseases. Signal Transduct. Targeted Ther. 2021;6:74. doi: 10.1038/s41392-020-00450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shafik A.M., Zhang F., Guo Z., Dai Q., Pajdzik K., Li Y., Kang Y., Yao B., Wu H., He C., Allen E.G., Duan R., Jin P. N6-methyladenosine dynamics in neurodevelopment and aging, and its potential role in Alzheimer's disease. Genome Biol. 2021;22:17. doi: 10.1186/s13059-020-02249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang N., Ding C., Zuo Y., Peng Y., Zuo L. N6-methyladenosine and neurological diseases. Mol. Neurobiol. 2022;59:1925–1937. doi: 10.1007/s12035-022-02739-0. [DOI] [PubMed] [Google Scholar]
- 81.Li N., Zhang D., Cao S., Qiao M.W., Zhang P.G., Zhao Q.Y., Shen Y., Huang X.Q., Song L.J. The effects of folic acid on RNA m6A methylation in hippocampus as well as learning and memory ability of rats with acute lead exposure. J. Funct.Foods. 2021;76 doi: 10.1016/j.jff.2020.104276. [DOI] [Google Scholar]
- 82.Ding L., Wu H., Wang Y., Li Y., Liang Z., Xia X., Zheng J.C. m6A reader Igf2bp1 regulates the inflammatory responses of microglia by stabilizing Gbp11 and Cp mRNAs. Front. Immunol. 2022;13:872252. doi: 10.3389/fimmu.2022.872252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang B., Liu Y., Jiang R., Liu Z., Gao H., Chen F., Mei J. Emodin relieves the inflammation and pyroptosis of lipopolysaccharide-treated 1321N1 cells by regulating methyltransferase-like 3 -mediated NLR family pyrin domain containing 3 expression. Bioengineered. 2022;13:6740–6749. doi: 10.1080/21655979.2022.2045836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lan J., Xu B., Shi X., Pan Q., Tao Q. WTAP-mediated N(6)-methyladenosine modification of NLRP3 mRNA in kidney injury of diabetic nephropathy. Cell. Mol. Biol. Lett. 2022;27:51. doi: 10.1186/s11658-022-00350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X., Lu Z., Gomez A., Hon G.C., Yue Y., Han D., Fu Y., Parisien M., Dai Q., Jia G., Ren B., Pan T., He C. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deng X., Qing Y., Horne D., Huang H., Chen J. The roles and implications of RNA m(6)A modification in cancer. Nat. Rev. Clin. Oncol. 2023;20:507–526. doi: 10.1038/s41571-023-00774-x. [DOI] [PubMed] [Google Scholar]
- 87.Yu R., Li Q., Feng Z., Cai L., Xu Q. m6A reader YTHDF2 regulates LPS-induced inflammatory response. Int. J. Mol. Sci. 2019;20:1323. doi: 10.3390/ijms20061323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hou J., Zhang H., Liu J., Zhao Z., Wang J., Lu Z., Hu B., Zhou J., Zhao Z., Feng M., Zhang H., Shen B., Huang X., Sun B., Smyth M.J., He C., Xia Q. YTHDF2 reduction fuels inflammation and vascular abnormalization in hepatocellular carcinoma. Mol. Cancer. 2019;18:163. doi: 10.1186/s12943-019-1082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.