Summary
Glioblastoma (GBM) is one of the most lethal central nervous systems (CNS) tumours in adults. As supplements to standard of care (SOC), various immunotherapies improve the therapeutic effect in other cancers. Among them, tumour vaccines can serve as complementary monotherapy or boost the clinical efficacy with other immunotherapies, such as immune checkpoint blockade (ICB) and chimeric antigen receptor T cells (CAR-T) therapy. Previous studies in GBM therapeutic vaccines have suggested that few neoantigens could be targeted in GBM due to low mutation burden, and single-peptide therapeutic vaccination had limited efficacy in tumour control as monotherapy. Combining diverse antigens, including neoantigens, tumour-associated antigens (TAAs), and pathogen-derived antigens, and optimizing vaccine design or vaccination strategy may help with clinical efficacy improvement. In this review, we discussed current GBM therapeutic vaccine platforms, evaluated and potential antigenic targets, current challenges, and perspective opportunities for efficacy improvement.
Keywords: Glioblastoma, Vaccine platform, Tumour antigen, Vaccine efficacy, Vaccine perspective
Introduction
Glioblastoma (GBM) is one of the most aggressive malignancies of the central nervous system (CNS) tumours. Standard of care (SOC) therapy for GBM includes maximum safe surgical resection followed by adjuvant radiotherapy, temozolomide (TMZ) chemotherapy, and tumour-treating fields (TTF). These treatments (with the exception of TTF) have remained unchanged for almost 20 years without vital progress and median overall survival (OS) from diagnosis of roughly 15 months.1, 2, 3 Most GBM patients experience tumour recurrence.4, 5, 6 The high recurrence rate can be attributed to unresectable sites of quiescent tumour seeding cells distantly located from the initial tumour at the early stage of tumorigenesis7 or tumour-normal brain boundary-infiltrated tumour cells.8 The most recent World Health Organization (WHO) criteria classifies adult malignant gliomas as astrocytoma, IDH-mutant (grades II, III, or IV), Oligodendroglioma, IDH-mutant, 1p/19q-codeleted (grades II, III), and Glioblastoma, IDH-wildtype (grade IV). Important to note, non-GBM gliomas can also recur as more malignant and lethal higher-grade gliomas.9 Insufficient treatment allows these residual tumour cells to form a virtual nuclear and cytoplasmic continuum with adjacent normal cells,10 whereby they introduce genetic elements and abnormal proteins to change normal cell phenotype by directly forming gap junctions11 or releasing tumour-derived extracellular vesicles,12 as well as rescue damaged tumour cells. Overall, new supplementary therapies are necessary to further decrease the unobservable tumour load and attenuate tumour progression. Provoking host immunity using immunotherapy has long been thought promising to achieve a better clinical outcome in GBM management. Integrating immunotherapy into SOC of GBM is a feasible next step to improve treatment efficacy for GBM patients.13
There are several modes of immunotherapy. Prior work has initiated such type of distinction for the domain of neuro-oncology14 including the concepts of 1) restorative immunotherapy (e.g., cytokine stimulations), 2) adoptive cell transfer immunotherapy (e.g. chimeric antigen receptor T cells (CAR-T) and T cell receptor-engineered T cells (TCR-T)), 3) passive immunotherapy (antibodies), 4) active specific immunotherapy (vaccines), 5) modulatory immunotherapy (amongst others the checkpoint inhibitors), and 6) immunogenic cell death (ICD) therapy (e.g. tumour-treating fields, modulated electrohyperthermia, oncolytic virus therapy).15,16 Other concepts related to immunotherapy include those not associated with a concrete patient phenotype (such as ICD therapy), those personalized in a manner that an antigen present on that patient’s tumour is the target, or individualized immunotherapies that account for the presence of a target and the patient’s immune identity such as autologous DC vaccines or adoptive cell transfer of tumour-infiltrating leukocyte therapy (TIL therapy). While these definitions have been evolving, the basis remains the same.
According to antigen dependency, immunotherapies can be separated as antigen-independent therapies, such as indoleamine 2,3-dioxygenase 1 (IDO) inhibitor, oncolytic viral therapy, and immune checkpoint blockade (ICB), and antigen-specific approaches, including CAR-T, TCR-T, and vaccines.17 The primary distinction between these antigen dependencies is not whether tumour antigens are required but rather whether a known antigen or set of antigens is being targeted. Antigen-independent therapies often aim to block the immunosuppressive mechanism within the tumour microenvironment (TME) to assist T cells to escape exhaustion, activate antitumor immunity, or facilitate tumour antigen release. To date, no results from clinical trials of GBM IDO inhibitor treatment have been reported (NCT02052648, NCT04047706, and NCT02502708). Despite dramatic successes against a variety of solid tumours,18, 19, 20 several phase III clinical trials on GBM ICB therapies have reported no improvement in OS.21, 22, 23 The efficacy of neoadjuvant ICB immunotherapy in patients with GBM is controversial. Some researchers have reported that neoadjuvant ICB immunotherapy promotes OS in patients with recurrent GBM24; however, no significant change in cytotoxic T-cell function or immune checkpoint expression was reported in others,25,26 contributing to an unsatisfactory survival benefit in GBM patients. Overall, the limited efficacy of ICB as a monotherapy supplement to SOC has been attributed to the ‘cold’ (immunologically inert) TME,27 GBM-induced tumour-supportive phenotypic modifications,10 and low tumour mutation burden (TMB) of GBM,28,29 which has been recently reported to be derived from the abnormal epigenome.30 High mutational load is typically essential for successful ICB treatments in some types of cancers,31,32 however, a recent study reported that very low TMB in recurrent GBM patients is associated with longer survival after ICB treatment.33 Furthermore, oncolytic virus pre-treatment can effectively promote ICB treatment efficacy by increasing antigen exposure.34, 35, 36 Therefore, enrolment of antigen-specific immunotherapy in a patient treatment regimen can improve clinical outcomes.
In this review, we broadly discuss antigen-specific immunotherapies for brain tumours, with a focus on therapeutic vaccine types and platforms for GBM vaccination. We also outline current challenges, ongoing strategies, and perspectives for improving GBM immunotherapy in the clinic.
Antigen-specific therapies
Recently, antigen-targeting therapy, using mRNA vaccines or TCR-T showed a favourable effect in promoting the regression of pancreatic cancers that acquired resistance to ICB.37,38 More than just targeting designed antigens, some patients treated with these antigen-targeting strategies exhibit antigen spreading to other non-targeting antigens.39,40 These studies provide a foundation for antigen-targeted therapies for checkpoint-refractory tumours.
Cancer vaccines aim to generate de novo tumour antigen-specific T cells through professional antigen-presenting cell (APC) presentation. Prophylactic (preventative) anti-viral vaccines to mediate cancer protection have been extremely successful.41, 42, 43 Furthermore, therapeutic vaccines against cervical intraepithelial neoplasia (CIN) associated with the high-risk human papillomavirus (HPV) subtype, HPV-16 and HPV-18, can significantly increase the rate of histopathological regression by around 20%44 and even induce a complete response.45 Although no vaccines have been reported to prevent non-viral cancer, some therapeutic vaccines show promise for regressing non-viral tumours and improving outcomes. Triple-negative breast cancer (TNBC) is the most aggressive form of breast cancer worldwide. Treating TNBC patients with the HER2 peptide AE37 vaccine demonstrated the clinical benefit potential of tumour vaccination.46 For GBM patients, the therapeutic benefit of single peptide vaccines performed poorly in phase III trials and remains controversial,47, 48, 49, 50 but some early-phase multi-peptide vaccines show favourable clinical efficacy as a single supplementary therapy for SOC.51, 52, 53, 54, 55 For cellular antigen-specific therapies, CAR-T cell clinical trials targeting GBM antigens (epidermal growth factor receptor variant III (EGFRvIII),56 human epidermal growth factor receptor 2 (HER2),57 and IL-13 receptor α2 (IL-13Rα2)58,59) demonstrated the feasibility, safety, and potential efficacy of this approach in GBM treatment.
In addition to promoting GBM regression as monotherapy, vaccination can augment the therapeutic activity of other immunotherapies, including both antigen-dependent and antigen-independent approaches.60 For example, amphiphile-ligand with conjugated tumour vaccine peptide61 is a novel antigen supplementary strategy to enhance CAR-T cell tumour regression efficacy in the pre-clinical murine EGFRvIII GBM model.62 It partitions into the membrane of resident APCs after trafficking from the blood to lymph nodes through injection, then robustly primes endogenous CD4+ and CD8+ T cell responses and further induces antigen spreading. The above evidence suggests that antigen-specific therapies, including vaccines, which are the focus of this review, may enhance antitumor immune responses and prolong survival after SOC. Next, we discuss vaccine platforms, antigen types, remaining challenges, and opportunities for therapeutic GBM vaccination.
Vaccine platforms
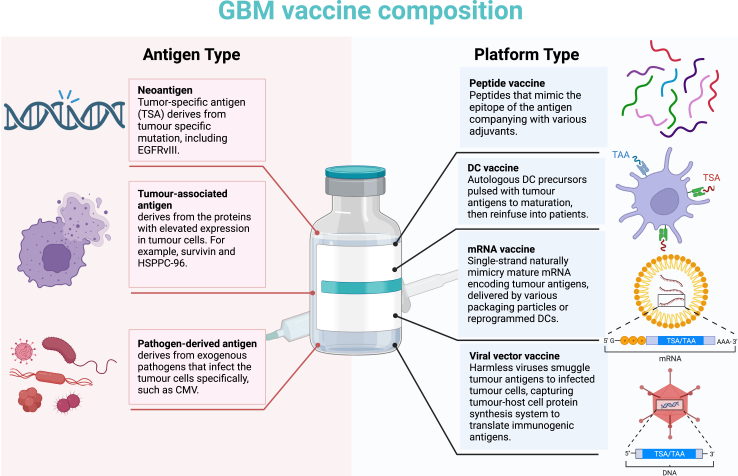
The general elements for vaccine design include an antigen, a danger signal, and a vehicle to transport the antigen. The combination of these three contributes make the potency and spectrum of the vaccine. Next, we discussed four vaccine platforms (Fig. 1), which incorporate these elements, that have been applied in clinical trials in vaccines for GBM: peptide vaccines, dendritic cell (DC) vaccines, mRNA vaccines, and viral vector vaccines.
Fig. 1.
GBM vaccine composition. Left, the antigen type for the GBM vaccine. Right, the vaccine platform used in GBM vaccine delivery.
Peptide vaccines
Compared to cell-based vaccines, peptide vaccines can be produced faster and with fewer resources. These utilize tumour-specific, or tumour-highly-expressed peptide sequences synthesized chemically in vitro to immunize patients.63 There are two main types of therapeutic cancer peptide vaccines, minimal peptide epitopes (∼8–11 amino acids, AAs) or synthetic long peptides (SLPs, ∼25–30 AAs).64 The minimal peptide epitope could directly bind to the major histocompatibility complex (MHC) I-binding groove without an internalization process. However, it simultaneously raises the risk of binding to non-APC nucleated cells that express MHC I molecules but lack costimulatory molecules, which may lead to insufficient T-cell activation and even peptide tolerance.65 In contrast to the minimal peptide epitopes, SLPs require APC processing. Its long length allows APC to efficiently present both MHC I- (by cross-presentation) and MHC II-restricted epitopes.66 After in vivo injection, SLPs are engulfed and presented by APCs to autologous CD4+ and CD8+ T cells, and then these primed T cells circulate to the tumour and perform cytotoxicity. Owing to the lack of capability to activate the innate immune system, peptide vaccines need to be combined with additional immune adjuvants. Assist from adjuvants ensure sufficient costimulatory signals from APCs to elicit a robust T-cell response.67 The popular GBM vaccines are mainly peptide vaccines, such as rindopepimut (EGFRvIII) and SurVax (survivin).68 More details on current GBM peptide vaccines will be introduced in a later section.
Dendritic cell (DC) vaccines
DCs are the most potent professional APCs to provoke adaptive immune responses. Before antigen uptake, DCs mainly existed during the immature stage. Antigen capture promotes DC maturation, with the secretion of various cytokines and upregulation of surface MHC and costimulatory molecules and cytokine receptors.69 DCs play pivotal roles in presenting tumour antigens in the lymph nodes to elicit T-cell priming and provoke distant antitumor response.70 Providing DCs with tumour antigens in (peptide vaccines) or ex vivo (DC vaccine) is of vital importance for inducing tumour-specific effector T cells. DC vaccines are generated ex vivo by culturing hematopoietic progenitor cells or monocytes with a combination of cytokines to induce mature DC differentiation. Then loading the selected tumour antigen with these DCs.70 In addition to priming CD4+ T cells by peptide-MHC II complex, these tumour antigen-loaded DCs also elicit effective CD8+ T-cell anti-tumour response through cross-priming, a process in which DC presents the exogenous peptide by MHC I molecules to prime CD8+ T cells.71 Compared to the whole protein directly coming from autologous tumour lysates, the synthetic peptides are more rapidly and efficiently processed by APCs, thus enhancing CD8+ T-cell activation.66 To guarantee an effective antitumor response, the existence of a DC co-stimulatory signal is an essential parameter. The lack of the necessary DC co-stimulation signals in the TME hampers DC maturation. Immature DC can present self-antigens to T cells, which leads to immune tolerance either through T cell deletion or immunosuppressive phenotype transformation.72 The additional inflammatory stimulus can help DC maturation and home to cervical lymph nodes73 and T cell immunostimulatory phenotype shift,74 improving vaccination efficacy. Compared to the peptide vaccine, the DC vaccine ensures DC maturation and high antigen-presenting efficiency with sufficient exogenous costimulatory signals, whereas DC maturation may be impeded by insufficient costimulatory signals in patients with a weak primary immune status when vaccinated with peptides. Recently, a phase III trial on DC vaccine addition to SOC, DCVax-L,75 revealed a significant extension of OS in both primary and recurrent GBM patients which was well-tolerated. Meanwhile, the phase I clinical trial of the DC vaccine targeting GBM stem-like cells (GSC) in both newly diagnosed and recurrent GBM patients76 reported encouraging results for stem-like cell targeting strategy, consistent with results of a previous DC vaccine phase II study targeting GSC marker, CD133.77 Beyond the standard mature DCs, an early-phase clinical trial revealed that α-type 1 polarized DC (αDC1) is another feasible DC type that can induce prolonged type-1 T-cell response more efficiently in GBM patients.78 Different from incubating selected tumour antigens with autologous DCs ex vivo, a Sweden research group induced a type 1 conventional DC (cDC1) phenotype in healthy human somatic cells79 and various human tumour cell lines80 to improve tumour heterogeneous antigen presentation, including GBM cell lines with favourable reprogramming efficiency.80 The reprogrammed cDC1-like cells engulfed tumour antigen from the TME, upregulated both MHC I complexes and costimulatory molecules, and secreted proinflammatory cytokine, such as IL-12 and IFN-γ, reverting immunostimulatory response due to tumour MHC I degradation.81 These tumour-APCs boost self-immunogenicity and CTL-killing susceptibility by presenting more endogenous and exogenous tumour antigens, driving anti-tumour immunity in vitro and in vivo. This strategy solves the human leukocyte antigen (HLA) restriction problem in vaccine design.
mRNA vaccines
mRNA vaccines transduce mRNA into cells, especially APCs, to present translated peptides. It possesses the advantages of high potency, versatility, ease of development, and low manufacturing costs. mRNA can encode peptides that may be difficult to synthesize. Additionally, mRNA can induce type I IFN secretion as an intrinsic adjuvant, recognized by TLR3, 7, 8, and cytosolic RNA receptors.64 To mimic an mRNA with a naturally mature structure, the mRNA vaccine was designed as a single strand with a 5′ cap and 3′ poly(A) tail. The open reading frame (ORF) of the antigen-coding region is equipped with start and stop codons, flanked by untranslated regions (UTRs).82,83 It is either delivered directly using various routes, such as cationic liposomes, mRNA-packaging nanotechnologies,83 and viruses,84 or indirectly by in vitro reprogramming cells.82,83 Once uptake through endocytosis, the escaped mRNA from ubiquitous RNase uses the host’s protein synthesis system to translate tumour antigen proteins instantly.83 These proteins are either further trimmed by immunoproteasomes and presented by MHC I molecules, or secreted outside the cells and taken up by APCs to be presented by MHC II molecules or cross-presented in MHC I molecules.82,83 The mRNA vaccine is only transiently active and completely degrades. In GBM patients, mRNA either induces the host immune system by RNA-pulsed DC or is delivered directly by nanoparticles.85 Pre-clinical86 and early phase clinical trials86 showed that the mRNA has the potency to induce a favourable anti-tumour response in GBM animal models or patients and effectively prolongs overall survival. Clinical trials of mRNA vaccine for GBM are ongoing (NCT04573140 and NCT04573140) and no results have yet been reported. Nevertheless, one phase I/II nonrandomized trial in stage IV renal cell cancer patients vaccinated with mRNA that codes the same tumour antigens for GBM patients, including MAGE-1, Survivin, and HER-2, reported favourable clinical benefits.87
Viral vector vaccines
There are diverse recombinant viral vectors that have been used to deliver antigens clinically.88,89 Unlike oncolytic viruses lysing tumour cells, viral vectors are genetically modified to insert antigen-encoding sequences and lose their toxicity to suit clinically safe applications. These virus vectors can infect the target cells and translate antigen peptides by the host protein synthesis system. Due to their capability to deliver multiple antigens and induce a robust adaptive immune response mimicking a natural infection, no exogenous adjuvants are needed.88 In addition to inducing a potent immune response through delivered antigens, the recipient’s immune system is also augmented by the viral pathogen-associated molecular patterns (PAMPs). However, long-term usage of viral vectors can induce anti-viral humoral immunity.34 No clinical trial results have been reported; however, clinical trials on viral vector vaccines targeting human cytomegalovirus peptide in GBM are ongoing (NCT03382977).
GBM antigen classification
Optimal antigen selection is key to the efficacy of tumour vaccination. An ideal tumour antigen should possess high immunogenicity and be expressed specifically by almost all tumour cells. During maturation, T cells have to pass the negative selection in the thymic medulla, which will delete the potential self-reactive T cell with high-affinity TCR for the self-antigen presented on DCs or thymic epithelial cells (TECs).90 Only non-self-antigens distinguished from peptides present in normal peripheral tissues can elicit an effective T cell response. Furthermore, an ideal tumour antigen should be expressed abundantly and stably during tumour progression, or tumour cells can escape from exogenous tumour antigen-induced immunosurveillance due to antigen loss. Not all tumour-derived peptides exhibit antigenicity. Impacted by MHC restriction, only peptides that are at fitted length and have matched anchor residues allow being presented by MHC molecules. A peptide with high affinity to MHC molecules correlates with stronger T-cell anti-tumour activity.91 Likewise, the peptides’ immunogenicity is also influenced by MHC restriction that TCR recognizes both antigenic peptide and MHC molecules, which have been discovered with more than 25,000 alleles.92 Thus, tumour antigen candidates should meet the MHC limitation, possess both antigenicity and immunogenicity, and have tumour specificity and stable abundance. Tumour antigens can be divided into endogenous and exogenous types. Endogenous antigens are antigens that originate from tumour cell intracellular proteins, including neoantigens (tumour-specific antigen, TSA) and tumour-associated antigen (TAA). Exogenous antigens have recently gained attention for use in tumour vaccine therapy. These antigens are derived from the tumour cells that preferentially infect pathogens and are presented by tumour MHC to activate the T cell response.93,94 Of note, GBM bulk tumour-derived antigen sources including tumour lysate vaccines, such as GBM6-AD95 and AV-GBM-1,96 or tumour-mRNA vaccines,86 putatively include these categories of antigens and still not recognized classes of antigens. In the following section, we discuss each tumour antigen type in GBM in more detail (Fig. 1).
Neoantigen in GBM
Neoantigens come from somatic mutations whose clones only exist in the tumour cells. Due to GBM’s low TMB and high heterogeneity of GBM, only one neoantigen, EGFRvIII, has been identified and evaluated as an off-the-shelf neoantigen approach in clinical trials related to GBM. Rindopepimut is a peptide vaccine designed from the EGFRvIII deletion mutation that occurs in roughly 20–30% of all GBM.48,97 Although its phase II clinical trial reported a promising beneficial potency,48 the phase III randomized multicentre clinical trial on rindopepimut failed to show the clinical benefits of the EGFRvIII vaccine.47 Roughly 60% of patients after treatment lose the EGFRvIII expression at recurrence,47,56,98 potentially resulting from the cancer immunoediting effects,99 however this has not been definitively determined. Nevertheless, the EGFRvIII vaccine can be used as a complementary therapy to other immunotherapies. Recently, a study on EGFRvIII vaccine-boosted CAR T therapy reported an enhanced response despite the antigen loss in the murine EGFRvIII GBM model.62 The adjuvant EGFRvIII vaccine induced antigen spreading39 and promoted endogenous DC to engulf more tumour antigens to reinforce T-cell activation.
Future neoantigen detection
Current GBM neoantigens are mainly selected based on single nucleotide mutations, which have only one amino acid difference in the mutated peptide.55,100 Despite their high privacy and heterogeneity, GBM neoantigens from structural variation (SV), frameshifting, gene fusion, and cancer-associated chromosomal abnormalities101 are actively being explored.
Tumour-associated antigens (TAAs) in GBM
TAAs include 1) developmental antigens, expressed during embryonic development and decrease or are silent after birth, 2) cancer-testis antigens (CTAs), elevated in tumour cells and germ cells (e.g. testis) relative to other tissues and contributing to meiosis, abnormal chromosome segregation, and aneuploidy,102 and 3) tumour overexpressed antigens, proteins expressed at a very high level in tumours compared with healthy organs. Increased CTA expression in GBM cells has been shown to promote CD8+ T cell activation and cytotoxicity,103 and immunized GBM patients with TAAs can extend overall survival,104 indicating TAAs’ potential in GBM vaccination. A recent clinical trial on newly diagnosed GBM patients54 revealed that both neoantigens and TAAs resulted in effective immunotherapies, especially for tumours with low TMB. TAA usage solves the antigen universality problem caused by preexisting antigenic heterogeneity, and these antigens can be shared among various patients. Several clinical trials reported that GBM TAAs, including MAGE-1, HER-2, gp100, AIM-2, TRP-2, EphA2,105 survivin50, IL13Rα2,58 and heat-shock peptide protein complex-96 (HSPPC-96),106,107 possess favourable anti-tumour potency51, 52, 53 (Table 1). In contrast to GBM neoantigen vaccines, GBM TAA vaccines tend to be in the form of combinations of peptides, such as SL-701,51 ICT-107,52 and IMA950,53 for clinical trial usage to compensate for their lower immunogenicity compared to neoantigens.108 Telomerase (TERT) is a major oncogene responsible for primary brain tumours, and its promoter mutation is found in approximately 80% of primary GBM cases, making TERT-derived peptides an ideal target for GBM neoantigen vaccines. Phase II clinical trials immunizing GBM patients with TERT mRNA-transfected DC (NCT03548571) or TERT-derived helper peptides (NCT04280848) as supplements to SOC are ongoing. GSCs play a pivotal role in tumour initiation, progression, and therapy resistance.109,110 It shows similar heterogeneity111 to different single-cell subtypes.112 GSCs highly express TAAs, mainly CTAs113 and MHC molecules,114,115 which strongly activate CD4+ and CD8+ T cells. TAAs, such as CD133,77 are ideal targets for potent immune therapy to reduce the GSC load. Recently, Wilms’ tumour 1 protein (WT1), a development-specific transcription factor that contributes to oncogenesis116 and used to be a TAA of other tumours,117,118 has been evaluated in several clinical trials on GBM patients (NCT03149003, NCT02649582, NCT01291420), suggesting that more GBM TAAs can be explored from existing TAAs of other tumours. However, one main limitation of TAA clinical usage is HLA restriction, which has over 25,000 variants in humans.92 Clinical trials for testing GBM TAAs are mainly restricted to the HLA-A2 allotype,51, 52, 53 and may lose function in patients with different HLA haplotypes. In addition to these existing TAAs, there is potential for multiple undefined TAA candidates worth exploring, such as alternative splicing or transposable elements.
Table 1.
Current phase II/III clinical trials of GBM vaccines.
| Antigen Target | Antigen Platform | Antigen Classification | Phase | Status | NCT Identifier | |
|---|---|---|---|---|---|---|
| Autologous GSC lysate | Autologous glioma stem-like cell antigens | DC | Personalized antigen | I/II | Completed | NCT00846456 |
| Autologous tumour and GSC lysate | DC | TSA + TAA | II/III | Unknown | NCT01759810 | |
| Autologous tumour lysate | Autologous tumour antigens | Uncertain | Personalized antigen | II | Completed | NCT00014573 |
| Autologous tumour lysate | Peptide | Personalized antigen | II/III | Not yet recruiting | NCT05685004 | |
| Autologous tumour lysate | DC | Personalized antigen | I/II | Recruiting | NCT03879512 | |
| Autologous tumour lysate | DC | Personalized antigen | I/II | Recruiting | NCT04801147 | |
| Autologous tumour lysate | DC | Personalized antigen | II | Active | NCT01204684 | |
| Autologous tumour lysate | DC | Personalized antigen | II | Active | NCT03400917 | |
| Autologous tumour lysate | DC | Personalized antigen | II | Completed | NCT00323115 | |
| Autologous tumour lysate | DC | Personalized antigen | II | Completed | NCT01213407 | |
| Autologous tumour lysate | DC | Personalized antigen | II | Completed | NCT00576537 | |
| Autologous tumour lysate | DC | Personalized antigen | II | Completed | NCT01006044 | |
| Autologous tumour lysate | DC | Personalized antigen | II | Recruiting | NCT04523688 | |
| Autologous tumour lysate | DC | Personalized antigen | II | Recruiting | NCT04115761 | |
| Autologous tumour lysate | DC | Personalized antigen | II | Recruiting | NCT03395587 | |
| Autologous tumour lysate | DC | Personalized antigen | II | Unknown | NCT02772094 | |
| Autologous tumour lysate | DC | Personalized antigen | II | Withdrawn | NCT03014804 | |
| Autologous tumour lysate | DC | Personalized antigen | III | Active | NCT00045968 | |
| Autologous tumour lysate | DC | Personalized antigen | III | Not yet recruiting | NCT05100641 | |
| Autologous tumour lysate | DC | Personalized antigen | III | Unknown | NCT04277221 | |
| EGFRvIII | EGFRvIII | Peptide | TSA | II | Completed | NCT00643097 |
| EGFRvIII | Peptide | TSA | II | Completed | NCT00458601 | |
| EGFRvIII | Peptide | TSA | III | Completed | NCT01480479 | |
| EGFRvIII, IL13Rα2, EphA2, Her2/neu, YKL-40 | Peptide | TSA + TAA | II | Withdrawn | NCT02754362 | |
| GSC Antigen | GSC antigens | DC | TSA + TAA | II | Recruiting | NCT04888611 |
| GSC antigens | DC | TSA + TAA | II | Unknown | NCT01567202 | |
| GSC antigens, survivin and hTERT | DC | TSA + TAA | II/III | Active | NCT03548571 | |
| Survivin | SurVaxM (survivin) | Peptide | TAA | II | Active | NCT02455557 |
| SurVaxM (survivin) | Peptide | TAA | II | Recruiting | NCT05163080 | |
| WT1 | Wilms’ tumour protein 1 (WT1) | Peptide | TAA | III | Completed | NCT03149003 |
| Wilms’ tumour protein 1 (WT1) | DC | TAA | I/II | Completed | NCT01291420 | |
| Wilms’ tumor protein 1 (WT1) | DC | TAA | I/II | Recruiting | NCT02649582 | |
| HSPPC-96 | HSPPC-96 | Peptide | TAA | I/II | Completed | NCT00293423 |
| HSPPC-96 | Peptide | TAA | I/II | Recruiting | NCT03650257 | |
| HSPPC-96 | Peptide | TAA | II | Completed | NCT00905060 | |
| HSPPC-96 | Peptide | TAA | II | Completed | NCT03018288 | |
| HSPPC-96 | Peptide | TAA | II | Terminated | NCT01814813 | |
| Combined TAA | ICT-107 (MAGE-1, HER-2, AIM-2, TRP-2, gp100, IL13Rα2) | DC | TAA | II | Completed | NCT01280552 |
| IMA950 (BCAN, CSPG4, FABP7, IGF2BP3, NRCAM, NLGN4X, PTPRZ1, TNC, survivin, c-met) | Peptide | TAA | I/II | Active | NCT03665545 | |
| IMA950 (BCAN, CSPG4, FABP7, IGF2BP3, NRCAM, NLGN4X, PTPRZ2, TNC, survivin, c-met) | Peptide | TAA | I/II | Completed | NCT01920191 | |
| SL-701 (survivin, IL-13Rα2, and EphA2) | Peptide | TAA | I/II | Completed | NCT02078648 | |
| CMV | CMV (gB and pp65) | Virus vector | Pathogen-derived antigen | I/II | Recruiting | NCT03382977 |
| CMV (pp65) | DC | Pathogen-derived antigen | II | Active | NCT02465268 | |
| CMV (pp65) | DC | Pathogen-derived antigen | II | Active | NCT03688178 | |
| CMV (pp65) | DC | Pathogen-derived antigen | II | Completed | NCT02366728 | |
| CMV (pp65) | DC | Pathogen-derived antigen | II | Terminated | NCT03927222 | |
| Other | Dendritic and Glioma Cells Fusion vaccine | DC | Personalized antigen | I/II | Recruiting | NCT04388033 |
| Telomerase (TERT)-derived helper peptides | Peptide | TAA | II | Active | NCT04280848 | |
| VXM01 (VEGFR2) | Attenuated Salmonella bacteria | TAA | I/II | Active | NCT03750071 | |
| EO2401 (IL13Rα2, BIRC5, FOXM1) | Peptide | TAA | I/II | Active | NCT04116658 |
TAAs from alternative splicing
Alternative splicing occurs in approximately 92%–95% of multi-exon genes in humans,119 whose changes reshape cancer-associated phenotypes by promoting angiogenesis,120 inducing cell proliferation,121 and avoiding apoptosis.122 Alternative splicing changes can be another tumorigenesis driver that is mutually exclusive of the mutation driver.123 Changes in the expression of core or auxiliary splicing factors in tumour cells generate oncogenic splicing variants, and a relatively small change in splicing factors or their downstream transcript abundance is sufficient to trigger oncogenic effects in cells.124 By aberrant alternative splicing events, tumour cells can generate abnormal proteins with gained/loss domains to remodel the intracellular protein–protein interaction (PPI) network, and these newly formed peptide sequences, especially located at the splicing junction, may be ideal candidates for alternative splicing-derived antigen vaccines.
TAAs from transposable elements
Epigenetic dysregulation in tumours lead to de-repression of transposable elements (TEs).125 As a result, the non-coding genome from TEs in normal adult somatic cells aberrantly translates into peptides in GBM cells.126 These tumour-associated peptides with potential immunogenicity can be further presented by MHC I molecules127 and may function as GBM TAA targets.
Pathogen-derived antigen in GBM
Viral-derived antigen
Human cytomegalovirus (CMV) nucleic acids and proteins are initially distinctively detected in GBM tissues, but not in the surrounding normal brain, from >90% of patients.128,129 However, the presence of CMV in GBM cells is still under significant debate because some rigorously performed studies have failed to detect CMV proteins or DNAs in GBM clinical samples.130, 131, 132 Some of these failures may be due to sample preparation methodologies and detection strategies or fragmented/discontinuous viral genomes in the bulk short-read sequencing data generated from next-generation sequencing (NGS),133,134 which may be improved by optimizing the protocol134,135 or using long-read sequencing technology.136 Despite the debated CMV existence, some clinical trials have reported that targeting pp65 CMV immunodominant epitope with one injection of autologous tumour lysate-pulsed DC immediately invoked a CMV-specific CD8+ T cell response in GBM patients,93,94 revealing CMV viral antigen as an attractive exogenous immunotherapeutic target.73 Clinical trials conducted by the Khanna group in Australia reported that autologous CMV-specific T cell transduction is a safe adjuvant immunotherapy to improve the clinical outcomes of patients with primary137 and recurrent GBM.138 In addition, lysed CMV-infected tumour cells expose more tumour antigens to the TME,138 allowing APCs to present various antigens, thereby promoting antigen spreading.39 Despite the increasing expansion of primed CD8+ T cells, some issues related to the anti-CMV T cell strategy remain to be resolved before conducting further clinical trials, such as immune dysfunction and loss of susceptibility.139
Intratumoural bacteria
Intratumoural bacteria potentially induce strong immunogenic antitumor efficacies and have significantly extended survival rates with effective immunological memory in various murine cancer models.140,141 Bacteria-derived peptide-recognized memory T cells, which are pre-stimulated by bacteria-derived or tumour-derived antigens, exist in both peripheral blood and tumours.142 As a result of molecular mimicry,143 cross-reactivity between human tumour antigens and bacterial antigens allows tumour antigen recognition by CD4+ T cells142 and CD8+ cytotoxic T cells.144 These bacteria-derived peptides can be used in combination with other tumour vaccines. GBM contains distinct microbial communities compared with adjacent normal tissues.145 Both GBM tissues and cell lines present bacteria-specific peptides via MHC II molecules. After bacterial peptide stimulation, tumour-infiltrating lymphocytes secrete more pro-inflammatory cytokines and recruit CD8+ T cells to promote tumour-killing.99 For patients that hardly detect intratumoural bacterial nucleic acids or peptides, injecting tumour antigen-engineered commensal bacteria subcutaneously to elicit distant anti-tumour immune reactions is a novel and safe approach,141 and is worth exploring for GBM treatment. By lysing subcutaneously engineered bacteria, epidermal Langerhans cells further present tumour peptides in MHC molecules for T-cell priming, and then distant primed CD8+ T cells enter the circulation and infiltrate into tumours for cytotoxicity. This vaccination strategy avoids HLA restriction and tumour desertification,146 in which tumour cells disturb antigen uptake by DCs via various immunosuppressive mechanisms.
Challenges for the GBM vaccine
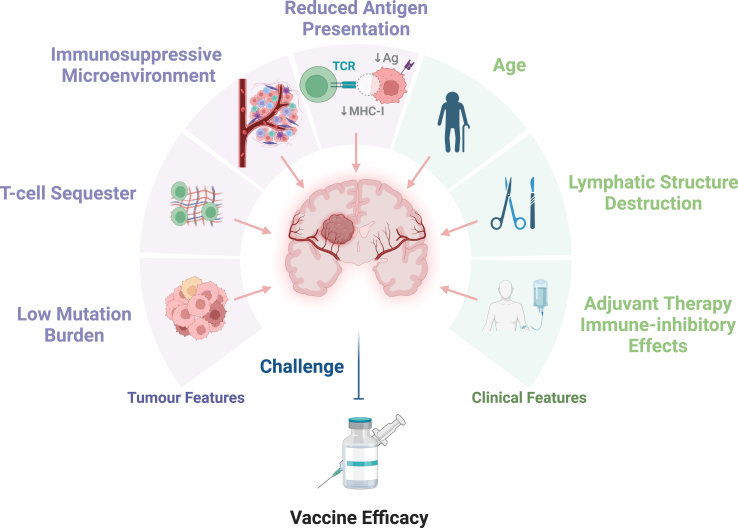
To identify additional GBM antigens, many studies have attempted to predict GBM neoantigens147,148 and TAAs126,149 from tumour lysates, peptides isolated from purified tumour MHC molecules,150 and next-generation sequencing analysis. Because existing GBM neoantigen predictions are mainly based on single nucleotide mutations, there are still plenty of GBM neoantigens derived from structural variants, frameshifts, and changes in chromosome structure waiting to be explored. Immunopeptidome applications help detect HLA-presented peptides on the tumour surface, accelerating GBM antigen prediction progression and increasing reliability.113,150 Apart from the lack of valid GBM antigens, other challenges need to be considered to improve the clinical outcome of vaccination (Fig. 2).
Fig. 2.
Challenges that hamper GBM vaccine efficacy. The purple means the challenges related to GBM tumour features. The green represents the challenges caused by the patient’s clinical features.
Challenges from GBM tumour features
Low tumour mutation burden
CNS tumours, including GBM, which may initiate mainly from epigenetic lesions,30 have regularly low TMB.28,29 Because neoantigen presentation is a probabilistic process29 dependent on neoantigen abundance, few mutations can be present in GBM cells as neoepitopes54 for efficient autologous T-cell recognition. Although some neoantigens occasionally possess immunogenicity and are presented by APCs, only a minority of mutations are processed to MHC-presented neoepitopes that can be targeted by T cells.151, 152, 153 Among the presented neoantigens, most arise from passenger mutations and have limited potency because tumour subclones without these highly antigenic tumour-specific mutant peptides escape from T-cell-dependent immunoselection.154 Additionally, antigen spreading, a critical mechanism of immunotherapy response, occurs after therapeutic tumour vaccination.39 It is induced by increased tumour antigen exposure, which is attributed to the initial vaccine-mediated tumour lysis. A smaller antigen pool due to low TMB allows fewer immunogenic neoantigens to be exposed following vaccination, which may reduce the benefits from antigen spreading after immunotherapy.
T-cell sequester
In GBM patients, including treatment-naïve patients, it has been reported that the majority of T cells, including both CD4+ and CD8+ T cells, have been sequestered in the bone marrow due to tumour-imposed loss of S1P1,155 leading to insufficient T cell homing to secondary lymph organs or circulating in the peripheral blood, resulting in less T cell infiltration into GBM. Although high microvessel density correlates with glioma grade, these dysfunctional vessels are poorly perfused.156 Dysfunctional glioma vasculature hampers T cell recruitment and tertiary lymphoid structure (TLS) neogenesis,157,158 resulting in a hypoxic and immunosuppressive TME that aggravates with tumour grade. With increasing intratumoural hypoxia levels, more CD8+ cytotoxic T cells will be entrapped in pseudopalisading hypoxic zones formed by hypoxic GBM cells in immunocompetent hosts, limiting inflammatory spread.159 Sequestered CD8+ cytotoxic T cells will transform to immunosuppressive phenotype by experiencing hypoxia and interacting with trapped immunosuppressive tumour-associated macrophages (TAMs) that mainly migrate from peripheral blood. Hypoxia-associated T-cell sequesters may counteract the clinical benefits of bevacizumab therapy.160 Thus, before performing efficacy studies, when feasible, early-phase trials should evaluate antigen-targeted T-cell infiltration as it is an essential step in achieving a response. Poor infiltration of T-cells in many GBM remains a barrier to progress and future studies are warranted to understand and improve the infiltration and biodistribution of immune cells throughout GBM.
Immunosuppressive TME
In GBM, effector T cells gain exhaustion features that are attributed to various factors in the TME.10 IL-10 plays a crucial role in conferring immunosuppressive TME in GBM161 by inhibiting APCs, hampering T-cell proliferation, and inducing the activity of regulatory T (Treg) cells.162 It induces tolerogenic DC maturation, thereby promoting Treg differentiation.70 Previous studies have revealed that glioma patients exhibit an increased fraction of CD4+ Treg cells in both the blood163 and tumours.164 High levels of Treg cells decrease the T cell antitumor response by upregulating immune checkpoint molecules or secreting immunosuppressive cytokines,54,165 and treating mice with neutralizing antibodies against CD25 to eliminate the suppressive function of Tregs166 can rescue the inhibited cytotoxic T cell antitumor functions. Moreover, intratumoural Tregs have a distinct TCR repertoire that specifically recognizes tumour neoantigens167 or TAAs,168 suggesting that the tumour antigens may lead to tumour antigen-recognized Treg cell clonal expansion. Depleting Treg clones that recognize tumour antigens will promote anti-tumour responses of effector/memory T cells targeting identical tumour antigens.168 Tumour-associated myeloid cells (TAMC), consisting of tumour-associated macrophages (TAM) and myeloid-derived suppressor cells (MDSC), are another critical component in the formation of an immunosuppressive TME in GBM. TAMCs account for 30%–50% of the mass in GBM169,170 and are the major source of PD-L1 expression in GBM TME to hamper adaptive immune response.171 Targeting PD-L1 on GBM TAMCs can profoundly reverse their immune inhibitory effects, reactivate CD8+ T cells, and prolong OS in GBM mouse models.172 Approximately 85% of TAMs originate from peripheral blood (bone marrow-derived macrophage/monocyte, BMDM) and are recruited to perivascular areas in GBM with the remaining resident TAMs (microglia) located surrounding the GBM tumour mass.173 TAMs from BMDMs (macrophages) preferentially exhibit nonpolarized (undifferentiated) or immunosuppressive phenotypes,174 and their abundance in the GBM TME may be associated with ICB resistance and poor T-cell infiltration.175 Resident TAMs (microglia) can promote tumour immune evasion by mTOR signalling to hinder effector T cell infiltration and proliferation within the GBM TME.174 Co-culture of human CD14+ monocytes from healthy donors with GBM cells induces MDSC-like properties in these monocytes.176 A previous study depicted MDSC accumulation in the peripheral blood of GBM patients.176,177 As a result of the upregulated GM-CSF expression in the GBM TME, GBM-infiltrated myeloid cells increase their Interleukin-4 receptor-α (IL-4Rα) expression, which mediates IL-13-induced production of arginase, thereby suppressing T-cell proliferation and function.178 The inhibition of T-cell function can be significantly restored after removing MDSCs from peripheral blood.177 Additionally, for patients who accept tumour vaccination, T cells persistently accumulate at the vaccination site owing to the antigen concentration gradient. Constant interaction with the vaccine for at least five days drives T cell anergy mediated by IFN-γ and MDSCs.179 Although short-term IFN-γ secretion can promote MHC I complex expression in GBM cells,55 accumulation of IFN-γ induces T cell anergy and upregulates the expression of various immune checkpoint genes,179 promoting a profoundly exhausted phenotype of T cells during long-term vaccination courses.55
Antigen loss and reduced antigen processing and presentation
In addition to the overexpression of checkpoint inhibitor molecules,180 antigen loss, resulting from the downregulation of antigen-originated proteins or antigen presentation,181 and degradation of MHC molecules,81 are other key challenges for successful immunotherapy. GBM is one of the refractory tumours with high heterogeneity,182,183 especially at the molecular level.112,184 Apart from diverse bulk184,185 and single-cell112,186 transcriptional subtypes, the tumour exhibits high plasticity that enables its dynamic phenotype to change to adjust to variable pressures,29,187 leading to most GBM antigens being highly changeable private antigens, even in different regions of the same tumour.188 The currently evaluated GBM neoantigen vaccine targeting EGFRvIII face the risk of antigen loss, and the phase III clinical trial of rindopepimut reported that approximately 60% of GBM patients lost EGFRvIII expression during recurrence.47 The disappearance of clonal or subclonal mutations during GBM clonal evolution98 mediated by chromosomal deletions and loss of heterozygosity (LOH),189 abnormal chromosomal structure,190,191 and epigenetic modifications contribute to GBM antigen loss, thereby decreasing its immunogenic antigenic protein expression. Furthermore, during post-surgery recurrence, the immunoediting effect99 and the balance between immune supervision function and tumorigenesis consistently sculpts the tumour antigen pool. During the escape phase, the loss of abundant immunogenic antigens192 and the dominance of immunosuppressive features challenge immunotherapy efficacy. Of note, the kinetics of antigenic change over time with therapy pose challenges in vaccine design. One group is now trying to solve this problem by using immunogenic cell death-induced serum-derived antigenic extracellular microvesicles and apoptotic bodies, which results in an actualized antigenic profile at the time of effective vaccination.15 Mutation, downregulation, or release of MHC molecules is another immune evasion mechanism in GBM.150,193,194 Loss-of-function mutations or LOH195 of MHC-I and beta-2-microglobulin (B2M) are frequently enriched across tumours181,196 and the adaptive immune system promotes evolutionary selection by killing tumour cells that present immunogenic antigens. During the proposed equilibrium and escape phase of the GBM “immunoediting” process,99 the tumour cells that lost immunogenic antigen presentation survived under adaptive immune system supervision. As evidence for the importance of MHC expression, upregulating glioma-derived exosomal MHC-I expression can assist CD8+ T cells in recognizing tumour cells, interrupting glioma cells from evading immunosurveillance.197 Cooperatively, a large scale of stochastic mutation induced by TMZ29,98 will accelerate this selection progression to promote surface immunogenic MHC-antigen complex loss. Thus, clonal evolution,98 subtype switching,198 and LOH in MHC and B2M genes199 after treatment potentially alter the GBM antigen repertoire longitudinally, resulting in antigen loss for vaccine therapy.47
Challenges from GBM patient clinical features
Age
GBM frequently occurs in the older population (median age:64 years), with the highest occurrence between 75 and 84 years,200,201 and patients at younger ages receive more benefits from tumour vaccination.50 Natural changes in the immune system with age can negatively affect the efficacy of tumour vaccination. Memory T cells, especially virtual memory T cells (Tvm),202 which have a memory phenotype in the absence of foreign antigens, accumulate with age, erode the naïve CD8+ T cell compartment, and restrict the T-cell receptor (TCR) pool and neoplastic antigen recognition in older patients. This naïve:memory ratio imbalance of T cells has been attributed to thymus involution.203 During age-related thymus involution, thymocytes decrease and are replaced by adipocytes, resulting in diminished peripheral T-cell repertoire diversity and immunosenescence,204 compromising the detection and response to new stimulants. Deep cervical lymph nodes (DcLNs), considered the main secondary lymph organ response to CNS tumours, also experience age-related atrophy,205 thereby further shrinking the T cell compartment. Dural lymph vessel cerebrospinal fluid (CSF) drainage function decreases with age. Old age is associated with dysmorphology, increased lymphatic channel thickness,205 and dysfunction of dural lymph vessels.206 These alterations impair metabolic waste clearance from the CNS,207 and hamper the circulation of GBM antigens through the lymph as well.208 Simultaneously, age-related inflammageing condition209 may further inhibit antigen-specific immunity and reduce the vaccination effectiveness.210 In a phase I/II trial of oncolytic DNX-2401 virotherapy plus ICB in recurrent GBM, patients with complete response (CR) were under 30 years old suggesting age as a potential beneficial factor.34 Consequently, measures to enhance the basal immune reactivity of elderly GBM patients may be needed to render tumour vaccine therapy more efficacious. The Thymus Regeneration, Immunorestoration, and Insulin Mitigation (TRIIM) clinical trial results211 point to a future direction to overcome age-associated unfavourable factors for enhancing anti-tumour immunity. It succeeded in causing thymic regeneration and an expansion of the pool of naive T cells by a combination of growth hormone, metformin, and dehydroepiandrosterone in nine healthy 51–65-year-old men for one year. This may be a potential method for enhancing the immune response following vaccination in GBM patients.
Postsurgical lymphatic structure destruction
The brain was considered devoid of lymphatics until the exact dural lymphatic vessels were observed in 2015.212 The discovery of structural lymphatic vessels206,212 and the glymphatic system213 in the brain has helped researchers understand the accurate process of tumour antigen presentation from parenchymal to dcLNs.205,212,214 Surgery is the primary treatment option for GBM patients. For visual exposure, GBM resection involves incision and peeling of the membranes above the tumour, perturbing the proximal structures around the tumour. The procedure may irreversibly destroy the dural lymph vessel206,212 and the subarachnoid lymphatic-like membrane (SLYM)215 proximal to the primary site, where APCs, particularly DCs, localize or migrate for immune surveillance. Due to lymph structure damage, tumour antigens from the post-resection remaining tumour that invade the surrounding brain parenchyma may not adequately reach the secondary lymph nodes. This is supported by data demonstrating that CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature.214 Meanwhile, the postoperative inflammageing condition of patients may exacerbate immunosuppression.209,210 More work is warranted to identify the precise mechanism of brain tumour antigen drainage into the lymph nodes.
Adjuvant therapies decrease T cell clone diversity and function
Apart from age-induced immunosenescence, multiple antineoplastic therapies, such as radiotherapy and chemotherapy, deplete lymphoid precursors, induce thymocyte apoptosis,216 and accelerate thymus involution,217 thereby damaging the normal T cell development process and leading to prolonged deficiency in the adaptive immune system.218 Antigen-induced clonal expansion in lymph nodes cannot resolve this impairment.219 TMZ is the primary alkylating agent used for GBM adjuvant chemotherapy. Despite significant survival-prolonged benefits,1,220 TMZ treatment promotes GBM gain by more than 10 times mutations stochastically at recurrence (mainly C>T transitions),29,98 including the DNA mismatch repair (MMR)-encoding genes (MSH2, MSH5, MSH6, MHS4, MLH1, MLH2, PMS1, and PMS2),221 whose deficiency contributes to post-treatment hypermutation222 and TMZ resistance.223 By increasing genome instability within GBM, TMZ treatment limits the effectiveness of antigen-targeting therapies.224 Genes containing TMZ-induced hypermutated loci are more highly expressed in recurrent GBM, but the increased neoantigen load fails to improve the effects of immunotherapy.222 Additionally, standard TMZ eradicates both proliferating CD4+ and CD8+ T cells and promotes CD8+ T cell exhaustion, thereby hindering immunotherapy effectiveness.224 Edema is a common complication of GBM. To limit the progression of peritumoral edema, physicians often choose corticosteroids. Theoretically, corticosteroid mediated depletion of immune cells may counteract the anti-tumour effects of tumour vaccines,55,225 however, there is no phase III clinical trial data yet definitely reporting the adverse effect on GBM patients’ survival. Alternatives to steroids should also be considered in conjunction with GBM vaccines. Bevacizumab performs well in controlling brain edema.226 Combining GBM vaccine therapy with bevacizumab is a feasible strategy to control edema as a steroid alternative.227
Perspective: future direction and opportunities to improve GBM tumour vaccine efficacy
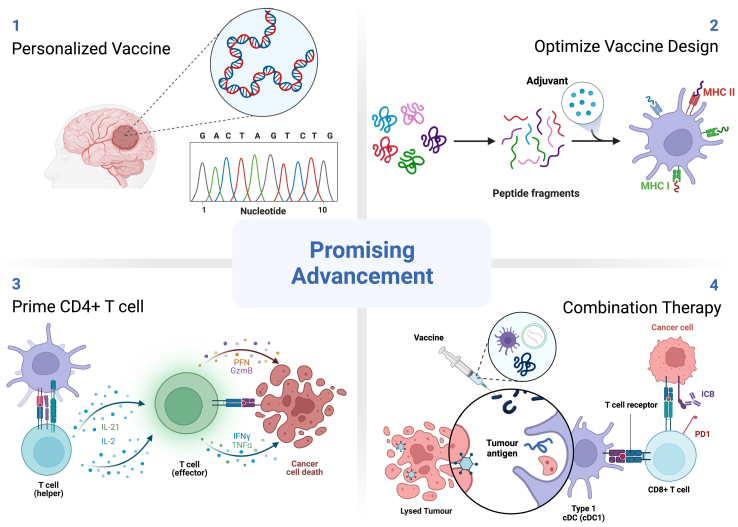
GBM vaccine is a feasible approach to improve patients’ clinical outcomes with SOC. Although the previously mentioned challenges can hamper the efficacy of current GBM vaccines, there are many promising opportunities to improve their therapeutic effects (Fig. 3).
Fig. 3.
Promising advancements for GBM vaccine efficacy.
Personalized multi-peptide vaccine
Due to interpatient heterogeneity, except for some common driver mutations, such as EGFRvIII mutation, among GBM patients vary from each other, resulting in the majority of neoantigens derived from these mutations being seldom shared. Testing of personalized vaccines for GBM patients began over 20 years ago, which harvested tumour lysates, primed T cells from the peripheral blood of GBM patient, stimulated and expanded them in vitro, and then infused tumour antigen-target T cells back into the patient.228 This clinical trial reported an increased antitumor immune response but no survival benefit in the test cohort (median OS:12 months). Compared to priming T cells with all tumour lysate proteins unselectively, personalized neoantigen design from bioinformatic prediction shows potential for improving GBM patient clinical vaccine efficacy.55 Meanwhile, by integrating diverse private tumour antigens, a large proportion of tumour cells that present different antigens will be recognized by primed T cells, thereby effectively reducing the tumour load, and counteracting the antigen loss risk. The multi-peptide combination strategy has been evaluated to improve the prognosis of patients with newly diagnosed GBM.54,55 Generally, neoantigens are expected to be highly immunogenic, but tend to be private (most mutations are unique to individual tumours; exceptions include some well-known driver mutations), whereas TAAs are widely applicable with less immunogenicity.108 Combining both neoantigens and TAAs in GBM patient vaccination can boost the vaccine effect compared with the monotype vaccine. Personalized multi-peptide vaccine design containing both neoantigens and TAAs detected by exome sequencing, transcriptome sequencing, and HLA immunopeptidomes may yield a favourable CD8 cytotoxic T cell intratumoural infiltration and survival extension.54 In two small-scale clinical trials of the GBM personalized vaccine, two study groups tested the efficacy of personalized antigens with/without TAAs in aged cohorts (median age:52.5, 65 years) of newly diagnosed GBM.54,55 Even with more mutations, the cohort receiving a personalized vaccine composed of neoantigens showed shorter OS than the other cohort being vaccinated by both neoantigens and TAAs (median OS:29 months vs. 16.8 months), indicating TAA supplementation in the personalized multi-peptide design can further extend patients’ survival. Additionally, a therapeutic vaccine prepared via GSC mRNA-transfected DC can significantly prolong PFS in GBM patients,229 which offers an additional option for personalized multi-peptide vaccine design.
Optimize vaccine peptide design
Long-length peptide designs can induce minimal T-cell sequestration at the vaccination site, attenuate T-cell exhaustion, and enhance anti-tumour responses after tumour vaccination.179 On the one hand, long peptides require cleavage in vivo by APCs, preventing overwhelming peptide presentation by various cells, including B cells, thus trapping fewer T cells at the vaccination site. On the other hand, it allows injected peptides to be trimmed into various short-length peptides to fit the binding grooves of multiple MHC allotypes, including MHC I (through cross-presentation) and MHC II molecules.49 In addition to direct tumour cell killing mediated by MHC-I-peptide primed CD8+ T cells, MHC–II–peptide primed CD4+ T cells can provide sufficient co-stimulatory signals to CD8+ T cells to enhance their cytotoxicity.230,231
Short-lived vaccine’s fast degradation of the vaccine peptides, attributed to the lack of persistent peptide-holding adjuvants, decreases the probability of T-cell sequestration at the vaccination site and T-cell apoptosis mediated by vaccine-induced high levels of IFN-γ.179 Continued tumour antigen exposure impairs T-cell effector function and drives T-cell epigenetic remodelling to a dysfunctional state.232 Short-lived vaccines can promote T cell localization to tumours, prevent T cell dysfunction, and attenuate T cell apoptosis at the expense of reduced T cell priming efficiency.179 However, this shortcoming can be rescued by combination with immunostimulatory supplements.
Vaccine adjuvant and neoadjuvant
Vaccine adjuvants refer to immunostimulatory components, given in addition to the antigen, to enhance the durability and magnitude of the immune response. Owing to the low immunogenicity of cancer peptide vaccines, combination with adjuvants is likely to improve the induction of effective antigen-targeting immune responses.233,234 After in vivo injection with tumour antigens, the adjuvant typically activates the innate immune system by introducing pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), inducing cytokine production, and recruiting innate immune cells to enhance adaptive immune responses.235 Insoluble aluminium salt (alum) is the most commonly used adjuvant in humans and in infectious disease vaccines such as hepatitis B236 and malaria.237 Alum mainly promotes the Th2-type response,235 making it less suitable as a tumour vaccine complement, in which a proinflammatory response is the typical goal. For tumour vaccines, incomplete Freund’s adjuvant (IFA) is widely used in clinical research on various cancers, which can protect against antigen degradation and release antigens slowly to APCs.238, 239, 240 However, it can cause T-cell sequestration at the vaccination site and destroy long-lived CD8+ T-cell immunity.179 Adjuvants on the market used in prophylactic vaccines are typically thought to not effectively induce a CD8+ T-cell response.235 AS04, an aluminium salt absorbing 3-O-desacyl-4′-monophosphoryl lipid A (MPL), which is a detoxified form of lipopolysaccharide (LPS) extracted from Salmonella Minnesota, can provoke a prominent Th1-type response, suggesting the added value of the Toll-like receptor (TLR) agonist MPL (TLR4 agonist) as a human cancer vaccine adjuvant.241 Targeting TLRs has great potential as tumour vaccine adjuvants. In mice, delivering antigens with TLRs ligands can induce antigen-specific CD8+ T-cell responses.242,243 A phase I clinical trial in GBM patients reported significantly increased T cell chemoattraction after complementing CpG-oligodeoxynucleotides (CpG-ODN), a TLR9 ligand, with a peptide vaccine from hTERT.244 Polyinosinic–polycytidylic acid (poly (I:C)), a TLR3 agonist, potently induces antitumor CD8+ T cytotoxic responses.245 Stabilizing this synthetic double-stranded RNA (poly (I:C)) with poly-L-lysine forms its RNase-resistant analog, ploy-ICLC, which can be a reliable and authentic microbial mimic for upregulating interferon and inflammasome signals in innate immune cells.246 Poly-ICLC is often used in GBM vaccine clinical trials (NCT03665545, NCT05557240, NCT02078648, NCT02510950, NCT01920191) with or without granulocyte-macrophage colony-stimulating factor (GM-CSF) (NCT02149225), a widely used adjuvant,247 to advance APC function and boost antitumor response.248,249 The stimulator of interferon genes (STING) pathway is a sensor of central cellular cytosolic double-stranded DNA (dsDNA), which can be activated by intrinsic self-dsDNA release from cancer cells. Its activation attenuates the early progression of tumour cells by mediating immune cell infiltration, such as T cells and natural killer (NK) cells, and upregulating proinflammatory cytokines, such as type I IFNs and chemokine secretion.250 STING agonists are another potential adjuvant for tumour vaccine activation.250 Its usage significantly enhances T-cell infiltration and inhibits tumour size in a dose-dependent manner compared with GM-CSF alone.251 In addition to the available adjuvants mentioned above, novel adjuvants for tissue damage, cell death, metabolic modulation, and epigenetic modulation are worth further exploration.235
Prime CD4 T cells
The CD4+ helper T cells, are important for tumour antigen specific CD8+ T cell population maintenance. After presenting tumour antigens, DCs prime CD4+ T cells, resulting in IL-2 production, which facilitates T cell proliferation and expansion.70 CD8+ T cells primed by interacting with both DCs and activated CD4+ T cells will gain a robust primary induction, including long-term maintenance and higher proliferative ability when suffering a secondary challenge, compared to those primed without CD4+ T cells.252 Primed CD4+ T cells promote CD8+ T cells to gain a cytotoxic phenotype by IL-21 secretion against differentiation to exhaustion subclones.253,254 A recent study identified a cellular triad associated with antitumor efficacy.255 The intratumoural cellular triad consists of DCs, CD4+ T cells, and CD8+ T cells. The triads of spatially attracted CD4+ T cells and DCs to the periphery of CD8+ T cells promote local CD8+ T cell differentiation into effector T cells and enhance CD8+ T cell cytotoxic potency. Antigen-induced antitumor responses require both tumour-antigen-primed CD4+ and CD8+ T cells.230 CD4+ T cell priming guarantees effective tumour rejection during vaccination.254,256 Disregarding CD4+ T cells, the level of CD8+ T cell infiltration alone is not a significant prognostic factor in glioma patients,257 revealing the importance of CD4+ T cells which has been underestimated in previous glioma antigen studies. GBM vaccine expanded both CD4+ and CD8+ T cells. Traditionally, TAAs have been designed to elicit sustained responses of effector and central memory CD8+ T cells. A recent study targeting a neoantigen, IDH1 R132H,49,258 was developed to induce CD4+ T cells of T helper Type 1.54 Thus, the CD4+ T cell response is vital to neoantigen-induced antitumor immunity, whose expanded TCR clone can react to IDH R132H.49 In addition to supporting CD8+ T cell cytotoxicity as a helper or regulator, the primary antitumor immunity response of CD4+ T cells is also non-negligible.259,260 CD4+ T cells effectively eliminated MHC-deficient tumour cells. For example, CD8+ T cells are unable to kill MHC-I-deficient tumour cells, whereas CD4+ T cells can eradicate MHC-I-downregulated tumour cells in cooperation with the innate immune system,259,261 or mediate CD8+ T cell anti-tumour response through CD40L signalling to kill tumour cells that lack MHC II molecule expression.256,262 Additionally, CD4+ T cells can elicit cytotoxicity to kill tumour cells in an MHC II-dependence manner.263, 264, 265 After priming, cytotoxic CD4+ T cell clones expand and enrich within tumours with various inflammatory cytokine secretions, such as IFN-γ and TNF-α, functioning as cytotoxic T cells. Consequently, considering MHC I and MHC II molecules’ compatible peptides and their TCR avidity together when designing a GBM vaccine can improve future vaccination efficacy.
Combination therapy
Long-term vaccine usage upregulates immune checkpoint gene expression owing to the accumulation of IFN-γ. To combat this immunosuppressive mechanism, ICB is an ideal complement to vaccines. Combined ICB therapy augments vaccine anti-tumour efficacy in both a preclinical murine cancer model266 and a clinical trial,267 and was even able to induce a rapid and durable complete response in tumour patients with metastasis.268 ICB combination with neoantigen vaccine augments survival benefits in an ICB-resistant GBM murine model.269 A small-scale clinical trial on recurrent GBM (n = 3 patients/group) revealed that neoadjuvant ICB plus DC vaccine treatment prolonged the patient OS compared with treatment with DC vaccine without neoadjuvant ICB (median OS:15.3 vs 8 months) (NCT02529072). Another study reported on a GBM case with long-term combinational therapy consisting of autologous DC vaccines expressing mRNAs encoding both TAAs and neoantigens, with anti-PD-1 and poly I:C in which the patient exhibited 69 months of progression-free survival.270 Other clinical trials of ICB and vaccine combinations for GBM treatment are ongoing (NCT02287428, NCT04888611, NCT04201873, NCT03018288), and no results have been reported. Another issue for immunotherapy in GBM is the ‘cold’-immune excluded TME.271 Intratumoural injection of oncolytic virus, DNX-2401, can transform the ‘cold’ GBM TME to ‘hot’ TME in patients with recurrent GBM by lysing tumour cells and exposing tumour antigens,272 benefiting GBM regression even to a best response of CR in two patients when combined with intravenous ICB.34 A phase I/II clinical trial reported favourable survival extension and tumour regression after oncolytic virus treatment with expected blood–brain barrier (BBB) penetration in an intravenous delivery cohort.273 Despite the direct virus-mediated pro-antitumor effect, arming viruses with immunoregulatory genes can confer additional antitumor functions. The Yu group inserted an extra sequence that encodes anti-CD47 IgG1 or IgG4 antibodies into the oncolytic virus, resulting in anti-CD47 antibody secretion from infected GBM cells, which blocked the phagocytosis inhibitory immune checkpoint.274 In addition to directly lysing tumour cells, the oncolytic virus activates innate immune cells by translating anti-CD47 antibodies, thereby improving the antitumor response and survival. Other studies evaluating armed oncolytic viruses for GBM are ongoing.275 This technique offers the possibility of integrating tumour antigens with oncolytic viruses to enhance their antitumor properties. Additionally, a multiphase combined treatment approach may be a promising regimen complement after SOC.276 During chemotherapy and oncolytic virus treatment, the TME can transform from ‘cold’ to ‘hot’, with tumour-lysed antigens exposed in the TME. These tumour-lysed antigens are important sources of ICD-induced antigens. The efficacy of subsequent DC vaccine therapy will be boosted by diverse ICD-induced antigens in addition to its preload antigens.15,276
Conclusion
GBM tumour vaccination holds promise as a complementary therapy to SOC. Compared to single-peptide vaccination, personalized multi-peptide vaccines consisting of neoantigens and TAAs exhibit better tumour regression and stronger anti-tumour responses. Due to the unique epigenetic mechanism of gliomagenesis, neoantigens are likely to be limited in number for GBM immunotherapy, and TAAs and pathogen-derived antigens may be useful in increasing the arsenal of vaccine antigens. Patient age, immune fitness, and treatment regimens can affect the efficacy of GBM vaccines. The optimal combination of tumour vaccines and SOC will be the focus of future clinical applications of GBM tumour vaccines. Optimizing vaccine design (antigen and adjuvant selection), concentrating on the role of CD4+ T cells in tumour vaccines, and combination immunotherapy will become new keys to increasing the efficacy of GBM vaccines.
Outstanding questions
Tumour vaccine is a potent strategy to improve the therapeutic effect of patients with GBM after SOC. As a malignant tumour possibly derived from an abnormal epigenome, GBM has low TMB and few neoantigens, which may hamper the efficacy of immunotherapy. Various clinical trials on GBM vaccines reported unsatisfactory results for few clinical benefits. Optimizing vaccine design, including personalized antigen selection, multi-antigen targeting, and improving vaccine platform or adjuvant, will be future topics for improving GBM vaccine efficacy. Another challenge is activating robust anti-tumour response in patients against the immunosuppressive effects of GBM. How to strengthen a GBM patient’s immunity weakened by age, treatment, or tumour immune escape mechanism is worth considering when performing tumour vaccine therapy. Tumour vaccine works well when combining to other immunotherapies, such as ICB. Combinational treatment and immunizing premalignant LGG patients are novel aspects of improving vaccine treatment regime design. While there are numerous established and presumed advantages and disadvantages of each vaccine platform (mRNA vs. peptide, vs DC loaded, vs viral vector) and antigen source, ongoing and future trials should empirically determine the difference between each.
Search strategy and selection criteria.
Data for this Review were identified by searches of MEDLINE, PubMed, and references from relevant articles using the search terms “glioblastoma”, “vaccine”, “antigen”, “clinical trial”, and “immunotherapy”. Abstracts and reports from meetings were included only when they were related directly to previously published work. Only articles published in English between 1996 and 2023 were included.
Contributors
Z. X: Conceptualization, data curation, investigation, visualization, writing - original draft, writing - review & editing. I. R: Conceptualization, investigation, writing - review & editing. M. O: Conceptualization, writing - review & editing. H. O: Conceptualization, writing - review & editing. X. L: supervision, Conceptualization, writing - review & editing. G. K: Conceptualization, funding acquisition, project administration, resources, supervision, writing - review & editing. All authors read and approved the final version of the manuscript.
Declaration of interests
All authors have nothing to disclosure.
Acknowledgements
We acknowledge the Xiangya School of Medicine of Central South University for their support of Zujian Xiong’s program. Funders had no role in paper design, data collection, data analysis, interpretation, or writing of the paper. We acknowledge BioRender.com for the figures.
Contributor Information
Xuejun Li, Email: lxjneuro@csu.edu.cn.
Gary Kohanbash, Email: gary.kohanbash@pitt.edu.
References
- 1.Stupp R., Mason W.P., van den Bent M.J., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Weller M., van den Bent M., Hopkins K., et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15(9):e395–e403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R., Hegi M.E., Mason W.P., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Rapp M., Baernreuther J., Turowski B., Steiger H.J., Sabel M., Kamp M.A. Recurrence pattern analysis of primary glioblastoma. World Neurosurg. 2017;103:733–740. doi: 10.1016/j.wneu.2017.04.053. [DOI] [PubMed] [Google Scholar]
- 5.Jiang H., Yu K., Li M., et al. Classification of progression patterns in glioblastoma: analysis of predictive factors and clinical implications. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.590648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandes A.A., Tosoni A., Franceschi E., et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation with MGMT promoter methylation status. J Clin Oncol. 2009;27(8):1275–1279. doi: 10.1200/JCO.2008.19.4969. [DOI] [PubMed] [Google Scholar]
- 7.Johnson B.E., Mazor T., Hong C., et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darmanis S., Sloan S.A., Croote D., et al. Single-cell RNA-seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 2017;21(5):1399–1410. doi: 10.1016/j.celrep.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lombardi G., Barresi V., Castellano A., et al. Clinical management of diffuse low-grade gliomas. Cancers. 2020;12(10) doi: 10.3390/cancers12103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broekman M.L., Maas S.L.N., Abels E.R., Mempel T.R., Krichevsky A.M., Breakefield X.O. Multidimensional communication in the microenvirons of glioblastoma. Nat Rev Neurol. 2018;14(8):482–495. doi: 10.1038/s41582-018-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balça-Silva J., Matias D., Dubois L.G., et al. The expression of Connexins and SOX2 Reflects the plasticity of glioma stem-like cells. Transl Oncol. 2017;10(4):555–569. doi: 10.1016/j.tranon.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Asti E., Chennakrishnaiah S., Lee T.H., Rak J. Extracellular vesicles in brain tumour progression. Cell Mol Neurobiol. 2016;36(3):383–407. doi: 10.1007/s10571-015-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim M., Xia Y., Bettegowda C., Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. 2018;15(7):422–442. doi: 10.1038/s41571-018-0003-5. [DOI] [PubMed] [Google Scholar]
- 14.De Vleeschouwer S., Van Gool S.W., Van Calenbergh F. Immunotherapy for malignant gliomas: emphasis on strategies of active specific immunotherapy using autologous dendritic cells. Child’s Nerv Syst. 2005;21(1):7–18. doi: 10.1007/s00381-004-0994-3. [DOI] [PubMed] [Google Scholar]
- 15.Van Gool S.W., Makalowski J., Van de Vliet P., et al. Individualized multimodal immunotherapy for adults with IDH1 wild-type GBM: a single institute experience. Cancers. 2023;15(4):1194. doi: 10.3390/cancers15041194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroemer G., Galluzzi L., Kepp O., Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 17.Sampson J.H., Gunn M.D., Fecci P.E., Ashley D.M. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20(1):12–25. doi: 10.1038/s41568-019-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larkin J., Chiarion-Sileni V., Gonzalez R., et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motzer R.J., Escudier B., McDermott D.F., et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferris R.L., Blumenschein G., Jr., Fayette J., et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reardon D.A., Brandes A.A., Omuro A., et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omuro A., Brandes A.A., Carpentier A.F., et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: an international randomized phase III trial. Neuro Oncol. 2023;25(1):123–134. doi: 10.1093/neuonc/noac099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim M., Weller M., Idbaih A., et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol. 2022;24(11):1935–1949. doi: 10.1093/neuonc/noac116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cloughesy T.F., Mochizuki A.Y., Orpilla J.R., et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. doi: 10.1038/s41591-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schalper K.A., Rodriguez-Ruiz M.E., Diez-Valle R., et al. Neoadjuvant nivolumab modifies the tumour immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470–476. doi: 10.1038/s41591-018-0339-5. [DOI] [PubMed] [Google Scholar]
- 26.Lee A.H., Sun L., Mochizuki A.Y., et al. Neoadjuvant PD-1 blockade induces T cell and cDC1 activation but fails to overcome the immunosuppressive tumour associated macrophages in recurrent glioblastoma. Nat Commun. 2021;12(1):6938. doi: 10.1038/s41467-021-26940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutledge W.C., Kong J., Gao J., et al. Tumour-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res. 2013;19(18):4951–4960. doi: 10.1158/1078-0432.CCR-13-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexandrov L.B., Nik-Zainal S., Wedge D.C., et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barthel F.P., Johnson K.C., Varn F.S., et al. Longitudinal molecular trajectories of diffuse glioma in adults. Nature. 2019;576(7785):112–120. doi: 10.1038/s41586-019-1775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahme G.J., Javed N.M., Puorro K.L., et al. Modeling epigenetic lesions that cause gliomas. Cell. 2023;186(17):3674–3685.e14. doi: 10.1016/j.cell.2023.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder A., Makarov V., Merghoub T., et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarchoan M., Hopkins A., Jaffee E.M. Tumour mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gromeier M., Brown M.C., Zhang G., et al. Very low mutation burden is a feature of inflamed recurrent glioblastomas responsive to cancer immunotherapy. Nat Commun. 2021;12(1):352. doi: 10.1038/s41467-020-20469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nassiri F., Patil V., Yefet L.S., et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: a phase 1/2 trial. Nat Med. 2023;29(6):1370–1378. doi: 10.1038/s41591-023-02347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desjardins A., Gromeier M., Herndon J.E., 2nd, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150–161. doi: 10.1056/NEJMoa1716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribas A., Dummer R., Puzanov I., et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leidner R., Sanjuan Silva N., Huang H., et al. Neoantigen T-cell receptor gene therapy in pancreatic cancer. N Engl J Med. 2022;386(22):2112–2119. doi: 10.1056/NEJMoa2119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rojas L.A., Sethna Z., Soares K.C., et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature. 2023;618(7963):144–150. doi: 10.1038/s41586-023-06063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brossart P. The role of antigen spreading in the efficacy of immunotherapies. Clin Cancer Res. 2020;26(17):4442–4447. doi: 10.1158/1078-0432.CCR-20-0305. [DOI] [PubMed] [Google Scholar]
- 40.Awad M.M., Govindan R., Balogh K.N., et al. Personalized neoantigen vaccine NEO-PV-01 with chemotherapy and anti-PD-1 as first-line treatment for non-squamous non-small cell lung cancer. Cancer Cell. 2022;40(9):1010–1026.e11. doi: 10.1016/j.ccell.2022.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Roden R.B.S., Stern P.L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer. 2018;18(4):240–254. doi: 10.1038/nrc.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finn O.J. The dawn of vaccines for cancer prevention. Nat Rev Immunol. 2018;18(3):183–194. doi: 10.1038/nri.2017.140. [DOI] [PubMed] [Google Scholar]
- 43.Pol S. HBV vaccine—the first vaccine to prevent cancer. Nat Rev Gastroenterol Hepatol. 2015;12(4):190–191. doi: 10.1038/nrgastro.2015.28. [DOI] [PubMed] [Google Scholar]
- 44.Trimble C.L., Morrow M.P., Kraynyak K.A., et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386(10008):2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenter G.G., Welters M.J., Valentijn A.R., et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361(19):1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 46.Mittendorf E.A., Ardavanis A., Symanowski J., et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann Oncol. 2016;27(7):1241–1248. doi: 10.1093/annonc/mdw150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weller M., Butowski N., Tran D.D., et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. doi: 10.1016/S1470-2045(17)30517-X. [DOI] [PubMed] [Google Scholar]
- 48.Schuster J., Lai R.K., Recht L.D., et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol. 2015;17(6):854–861. doi: 10.1093/neuonc/nou348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Platten M., Bunse L., Wick A., et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature. 2021;592(7854):463–468. doi: 10.1038/s41586-021-03363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahluwalia M.S., Reardon D.A., Abad A.P., et al. Phase IIa study of SurVaxM plus adjuvant temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2023;41(7):1453–1465. doi: 10.1200/JCO.22.00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peereboom D., Lindsay R., Badruddoja M., et al. CTIM-29. Phase 2 study of a novel immunotherapy SL-701 in adults with recurrent GBM: identification of treatment-induced CD8+CD107A+ CD57+ PD-1- memory T-cells that are associated with increased survival. Neuro Oncol. 2022;24(Supplement_7) [Google Scholar]
- 52.Wen P.Y., Reardon D.A., Armstrong T.S., et al. A randomized double-blind placebo-controlled phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin Cancer Res. 2019;25(19):5799–5807. doi: 10.1158/1078-0432.CCR-19-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rampling R., Peoples S., Mulholland P.J., et al. A cancer research UK first time in human phase I trial of IMA950 (novel multipeptide therapeutic vaccine) in patients with newly diagnosed glioblastoma. Clin Cancer Res. 2016;22(19):4776–4785. doi: 10.1158/1078-0432.CCR-16-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hilf N., Kuttruff-Coqui S., Frenzel K., et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 55.Keskin D.B., Anandappa A.J., Sun J., et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Rourke D.M., Nasrallah M.P., Desai A., et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399) doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed N., Brawley V., Hegde M., et al. HER2-Specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3(8):1094–1101. doi: 10.1001/jamaoncol.2017.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown C.E., Badie B., Barish M.E., et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 2015;21(18):4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown C.E., Alizadeh D., Starr R., et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weller M., Roth P., Preusser M., et al. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat Rev Neurol. 2017;13(6):363–374. doi: 10.1038/nrneurol.2017.64. [DOI] [PubMed] [Google Scholar]
- 61.Ma L., Dichwalkar T., Chang J.Y.H., et al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science. 2019;365(6449):162–168. doi: 10.1126/science.aav8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma L., Hostetler A., Morgan D.M., et al. Vaccine-boosted CAR T crosstalk with host immunity to reject tumors with antigen heterogeneity. Cell. 2023;186(15):3148–3165.e20. doi: 10.1016/j.cell.2023.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He X., Abrams S.I., Lovell J.F. Peptide delivery systems for cancer vaccines. Adv Ther. 2018;1(5) [Google Scholar]
- 64.Rojas L.A., Balachandran V.P. In: Clinical immuno-oncology. Niederhuber J.E., editor. Elsevier; New Delhi: 2024. Chapter 9 - vaccines and active immunization against cancer; pp. 177–194.e3. [Google Scholar]
- 65.Bijker M.S., van den Eeden S.J., Franken K.L., Melief C.J., van der Burg S.H., Offringa R. Superior induction of anti-tumour CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol. 2008;38(4):1033–1042. doi: 10.1002/eji.200737995. [DOI] [PubMed] [Google Scholar]
- 66.Rosalia R.A., Quakkelaar E.D., Redeker A., et al. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and T-cell activation. Eur J Immunol. 2013;43(10):2554–2565. doi: 10.1002/eji.201343324. [DOI] [PubMed] [Google Scholar]
- 67.Calvo Tardón M., Allard M., Dutoit V., Dietrich P.-Y., Walker P.R. Peptides as cancer vaccines. Curr Opin Pharmacol. 2019;47:20–26. doi: 10.1016/j.coph.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Yang T., Shi Y., Liang T., et al. Peptide vaccine against glioblastoma: from bench to bedside. Holistic Integr Oncol. 2022;1(1):21. [Google Scholar]
- 69.Santos P.M., Butterfield L.H. Dendritic cell-based cancer vaccines. J Immunol. 2018;200(2):443–449. doi: 10.4049/jimmunol.1701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palucka K., Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andersen B.M., Ohlfest J.R. Increasing the efficacy of tumour cell vaccines by enhancing cross priming. Cancer Lett. 2012;325(2):155–164. doi: 10.1016/j.canlet.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinman R.M., Hawiger D., Nussenzweig M.C. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 73.Mitchell D.A., Batich K.A., Gunn M.D., et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519(7543):366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garg A.D., Vandenberk L., Koks C., et al. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci Transl Med. 2016;8(328) doi: 10.1126/scitranslmed.aae0105. [DOI] [PubMed] [Google Scholar]
- 75.Liau L.M., Ashkan K., Brem S., et al. Association of autologous tumour lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial. JAMA Oncol. 2023;9(1):112–121. doi: 10.1001/jamaoncol.2022.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu J.L., Omofoye O.A., Rudnick J.D., et al. A phase I study of autologous dendritic cell vaccine pulsed with allogeneic stem-like cell line lysate in patients with newly diagnosed or recurrent glioblastoma. Clin Cancer Res. 2022;28(4):689–696. doi: 10.1158/1078-0432.CCR-21-2867. [DOI] [PubMed] [Google Scholar]
- 77.Yao Y., Luo F., Tang C., et al. Molecular subgroups and B7-H4 expression levels predict responses to dendritic cell vaccines in glioblastoma: an exploratory randomized phase II clinical trial. Cancer Immunol Immunother. 2018;67(11):1777–1788. doi: 10.1007/s00262-018-2232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okada H., Kalinski P., Ueda R., et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosa F.F., Pires C.F., Kurochkin I., et al. Single-cell transcriptional profiling informs efficient reprogramming of human somatic cells to cross-presenting dendritic cells. Sci Immunol. 2022;7(69) doi: 10.1126/sciimmunol.abg5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zimmermannova O., Ferreira A.G., Ascic E., et al. Restoring tumour immunogenicity with dendritic cell reprogramming. Sci Immunol. 2023;8(85) doi: 10.1126/sciimmunol.add4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamamoto K., Venida A., Yano J., et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581(7806):100–105. doi: 10.1038/s41586-020-2229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 83.Liu C., Shi Q., Huang X., Koo S., Kong N., Tao W. mRNA-based cancer therapeutics. Nat Rev Cancer. 2023;23(8):526–543. doi: 10.1038/s41568-023-00586-2. [DOI] [PubMed] [Google Scholar]
- 84.Ji D., Zhang Y., Sun J., et al. An engineered influenza virus to deliver antigens for lung cancer vaccination. Nat Biotechnol. 2023 doi: 10.1038/s41587-023-01796-7. [DOI] [PubMed] [Google Scholar]
- 85.Melnick K., Dastmalchi F., Mitchell D., Rahman M., Sayour E.J. Contemporary RNA therapeutics for glioblastoma. NeuroMolecular Med. 2022;24(1):8–12. doi: 10.1007/s12017-021-08669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sayour E.J., Grippin A., De Leon G., et al. Personalized tumour RNA loaded lipid-nanoparticles prime the systemic and intratumoral milieu for response to cancer immunotherapy. Nano Lett. 2018;18(10):6195–6206. doi: 10.1021/acs.nanolett.8b02179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rittig S.M., Haentschel M., Weimer K.J., et al. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol Ther. 2011;19(5):990–999. doi: 10.1038/mt.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang S., Liang B., Wang W., et al. Viral vectored vaccines: design, development, preventive and therapeutic applications in human diseases. Signal Transduct Target Ther. 2023;8(1):149. doi: 10.1038/s41392-023-01408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Travieso T., Li J., Mahesh S., Mello J., Blasi M. The use of viral vectors in vaccine development. NPJ Vaccines. 2022;7(1):75. doi: 10.1038/s41541-022-00503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palmer E. Negative selection — clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3(5):383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 91.Liu T., Tan J., Wu M., et al. High-affinity neoantigens correlate with better prognosis and trigger potent antihepatocellular carcinoma (HCC) activity by activating CD39(+)CD8(+) T cells. Gut. 2021;70(10):1965–1977. doi: 10.1136/gutjnl-2020-322196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robinson J., Barker D.J., Georgiou X., Cooper M.A., Flicek P., Marsh S.G.E. IPD-IMGT/HLA database. Nucleic Acids Res. 2020;48(D1):D948–D955. doi: 10.1093/nar/gkz950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prins R.M., Cloughesy T.F., Liau L.M. Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. N Engl J Med. 2008;359(5):539–541. doi: 10.1056/NEJMc0804818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reap E.A., Suryadevara C.M., Batich K.A., et al. Dendritic cells enhance polyfunctionality of adoptively transferred T cells that target cytomegalovirus in glioblastoma. Cancer Res. 2018;78(1):256–264. doi: 10.1158/0008-5472.CAN-17-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Olin M.R., Low W., McKenna D.H., et al. Vaccination with dendritic cells loaded with allogeneic brain tumour cells for recurrent malignant brain tumors induces a CD4(+)IL17(+) response. J Immunother Cancer. 2014;2:4. doi: 10.1186/2051-1426-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bota D.A., Taylor T.H., Piccioni D.E., et al. Phase 2 study of AV-GBM-1 (a tumour-initiating cell targeted dendritic cell vaccine) in newly diagnosed Glioblastoma patients: safety and efficacy assessment. J Exp Clin Cancer Res. 2022;41(1):344. doi: 10.1186/s13046-022-02552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brennan C.W., Verhaak R.G., McKenna A., et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang J., Cazzato E., Ladewig E., et al. Clonal evolution of glioblastoma under therapy. Nat Genet. 2016;48(7):768–776. doi: 10.1038/ng.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 100.De Mattos-Arruda L., Vazquez M., Finotello F., et al. Neoantigen prediction and computational perspectives towards clinical benefit: recommendations from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31(8):978–990. doi: 10.1016/j.annonc.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ruiz Cuevas M.V., Hardy M.P., Hollý J., et al. Most non-canonical proteins uniquely populate the proteome or immunopeptidome. Cell Rep. 2021;34(10) doi: 10.1016/j.celrep.2021.108815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Simpson A.J., Caballero O.L., Jungbluth A., Chen Y.T., Old L.J. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5(8):615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 103.Ma R., Rei M., Woodhouse I., et al. Decitabine increases neoantigen and cancer testis antigen expression to enhance T-cell-mediated toxicity against glioblastoma. Neuro Oncol. 2022;24(12):2093–2106. doi: 10.1093/neuonc/noac107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Q.T., Nie Y., Sun S.N., et al. Tumour-associated antigen-based personalized dendritic cell vaccine in solid tumour patients. Cancer Immunol Immunother. 2020;69(7):1375–1387. doi: 10.1007/s00262-020-02496-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bielamowicz K., Fousek K., Byrd T.T., et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018;20(4):506–518. doi: 10.1093/neuonc/nox182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bloch O., Crane C.A., Fuks Y., et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro Oncol. 2014;16(2):274–279. doi: 10.1093/neuonc/not203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ji N., Zhang Y., Liu Y., et al. Heat shock protein peptide complex-96 vaccination for newly diagnosed glioblastoma: a phase I, single-arm trial. JCI insight. 2018;3(10) doi: 10.1172/jci.insight.99145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peri A., Salomon N., Wolf Y., Kreiter S., Diken M., Samuels Y. The landscape of T cell antigens for cancer immunotherapy. Nat Cancer. 2023;4(7):937–954. doi: 10.1038/s43018-023-00588-x. [DOI] [PubMed] [Google Scholar]
- 109.Bao S., Wu Q., McLendon R.E., et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 110.Chen J., Li Y., Yu T.S., et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang L., Babikir H., Müller S., et al. The phenotypes of proliferating glioblastoma cells reside on a single Axis of variation. Cancer Discov. 2019;9(12):1708–1719. doi: 10.1158/2159-8290.CD-19-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Neftel C., Laffy J., Filbin M.G., et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178(4):835–849.e21. doi: 10.1016/j.cell.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Neidert M.C., Kowalewski D.J., Silginer M., et al. The natural HLA ligandome of glioblastoma stem-like cells: antigen discovery for T cell-based immunotherapy. Acta Neuropathol. 2018;135(6):923–938. doi: 10.1007/s00401-018-1836-9. [DOI] [PubMed] [Google Scholar]
- 114.Xu Q., Liu G., Yuan X., et al. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cell. 2009;27(8):1734–1740. doi: 10.1002/stem.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Do A.S.S., Amano T., Edwards L.A., Zhang L., De Peralta-Venturina M., Yu J.S. CD133 mRNA-loaded dendritic cell vaccination abrogates glioma stem cell propagation in humanized glioblastoma mouse model. Mol Ther Oncolyt. 2020;18:295–303. doi: 10.1016/j.omto.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cheever M.A., Allison J.P., Ferris A.S., et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Origuchi M., Korth C., Li Z., Braendle E.E. A phase Ib/II, multicenter, open-label study of DSP-7888 dosing emulsion in combination with immune checkpoint inhibitors (CPI) nivolumab or pembrolizumab in adult patients (pts) with advanced solid tumors, including platinum-resistant ovarian cancer (PROC) J Clin Oncol. 2020;38(15_suppl) [Google Scholar]
- 118.Hein K.Z., Yao S., Fu S. Wilms’ tumour 1 (WT1): the vaccine for cancer. J Immunother Precis Oncol. 2020;3(4):165–171. doi: 10.36401/JIPO-20-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Feng H., Qin Z., Zhang X. Opportunities and methods for studying alternative splicing in cancer with RNA-Seq. Cancer Lett. 2013;340(2):179–191. doi: 10.1016/j.canlet.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 120.Vorlová S., Rocco G., Lefave C.V., et al. Induction of antagonistic soluble decoy receptor tyrosine kinases by intronic polyA activation. Mol Cell. 2011;43(6):927–939. doi: 10.1016/j.molcel.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yanagisawa M., Huveldt D., Kreinest P., et al. A p120 catenin isoform switch affects Rho activity, induces tumour cell invasion, and predicts metastatic disease. J Biol Chem. 2008;283(26):18344–18354. doi: 10.1074/jbc.M801192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karni R., de Stanchina E., Lowe S.W., Sinha R., Mu D., Krainer A.R. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14(3):185–193. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Climente-González H., Porta-Pardo E., Godzik A., Eyras E. The functional impact of alternative splicing in cancer. Cell Rep. 2017;20(9):2215–2226. doi: 10.1016/j.celrep.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 124.Sebestyén E., Singh B., Miñana B., et al. Large-scale analysis of genome and transcriptome alterations in multiple tumors unveils novel cancer-relevant splicing networks. Genome Res. 2016;26(6):732–744. doi: 10.1101/gr.199935.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grundy E.E., Diab N., Chiappinelli K.B. Transposable element regulation and expression in cancer. FEBS J. 2022;289(5):1160–1179. doi: 10.1111/febs.15722. [DOI] [PubMed] [Google Scholar]
- 126.Bonté P.E., Arribas Y.A., Merlotti A., et al. Single-cell RNA-seq-based proteogenomics identifies glioblastoma-specific transposable elements encoding HLA-I-presented peptides. Cell Rep. 2022;39(10) doi: 10.1016/j.celrep.2022.110916. [DOI] [PubMed] [Google Scholar]
- 127.Kong Y., Rose C.M., Cass A.A., et al. Transposable element expression in tumors is associated with immune infiltration and increased antigenicity. Nat Commun. 2019;10(1):5228. doi: 10.1038/s41467-019-13035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cobbs C.S., Harkins L., Samanta M., et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62(12):3347–3350. [PubMed] [Google Scholar]
- 129.Mitchell D.A., Xie W., Schmittling R., et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008;10(1):10–18. doi: 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yamashita Y., Ito Y., Isomura H., et al. Lack of presence of the human cytomegalovirus in human glioblastoma. Mod Pathol. 2014;27(7):922–929. doi: 10.1038/modpathol.2013.219. [DOI] [PubMed] [Google Scholar]
- 131.Garcia-Martinez A., Alenda C., Irles E., et al. Lack of cytomegalovirus detection in human glioma. Virol J. 2017;14(1):216. doi: 10.1186/s12985-017-0885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zapatka M., Borozan I., Brewer D.S., et al. The landscape of viral associations in human cancers. Nat Genet. 2020;52(3):320–330. doi: 10.1038/s41588-019-0558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dziurzynski K., Chang S.M., Heimberger A.B., et al. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro Oncol. 2012;14(3):246–255. doi: 10.1093/neuonc/nor227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peredo-Harvey I., Rahbar A., Söderberg-Nauclér C. Presence of the human cytomegalovirus in glioblastomas-A systematic review. Cancers. 2021;13(20):5051. doi: 10.3390/cancers13205051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scheurer M.E., Bondy M.L., Aldape K.D., Albrecht T., El-Zein R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol. 2008;116(1):79–86. doi: 10.1007/s00401-008-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brait N., Külekçi B., Goerzer I. Long range PCR-based deep sequencing for haplotype determination in mixed HCMV infections. BMC Genom. 2022;23(1):31. doi: 10.1186/s12864-021-08272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith C., Lineburg K.E., Martins J.P., et al. Autologous CMV-specific T cells are a safe adjuvant immunotherapy for primary glioblastoma multiforme. J Clin Invest. 2020;130(11):6041–6053. doi: 10.1172/JCI138649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schuessler A., Smith C., Beagley L., et al. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res. 2014;74(13):3466–3476. doi: 10.1158/0008-5472.CAN-14-0296. [DOI] [PubMed] [Google Scholar]
- 139.Weathers S.P., Penas-Prado M., Pei B.L., et al. Glioblastoma-mediated immune dysfunction limits CMV-specific T cells and therapeutic responses: results from a phase I/II trial. Clin Cancer Res. 2020;26(14):3565–3577. doi: 10.1158/1078-0432.CCR-20-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goto Y., Iwata S., Miyahara M., Miyako E. Discovery of intratumoral oncolytic bacteria toward targeted anticancer theranostics. Adv Sci. 2023;10(20) doi: 10.1002/advs.202301679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen Y.E., Bousbaine D., Veinbachs A., et al. Engineered skin bacteria induce antitumor T cell responses against melanoma. Science. 2023;380(6641):203–210. doi: 10.1126/science.abp9563. [DOI] [PubMed] [Google Scholar]
- 142.Naghavian R., Faigle W., Oldrati P., et al. Microbial peptides activate tumour-infiltrating lymphocytes in glioblastoma. Nature. 2023;617(7962):807–817. doi: 10.1038/s41586-023-06081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fluckiger A., Daillère R., Sassi M., et al. Cross-reactivity between tumour MHC class I-restricted antigens and an enterococcal bacteriophage. Science. 2020;369(6506):936–942. doi: 10.1126/science.aax0701. [DOI] [PubMed] [Google Scholar]
- 144.Zitvogel L., Kroemer G. Cross-reactivity between cancer and microbial antigens. OncoImmunology. 2021;10(1) doi: 10.1080/2162402X.2021.1877416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nejman D., Livyatan I., Fuks G., et al. The human tumour microbiome is composed of tumour type-specific intracellular bacteria. Science. 2020;368(6494):973–980. doi: 10.1126/science.aay9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang M.M., Coupland S.E., Aittokallio T., Figueiredo C.R. Resistance to immune checkpoint therapies by tumour-induced T-cell desertification and exclusion: key mechanisms, prognostication and new therapeutic opportunities. Br J Cancer. 2023;129(8):1212–1224. doi: 10.1038/s41416-023-02361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li W., Sun T., Li M., et al. GNIFdb: a neoantigen intrinsic feature database for glioma. Database. 2022;2022 doi: 10.1093/database/baac004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen B., Khodadoust M.S., Olsson N., et al. Predicting HLA class II antigen presentation through integrated deep learning. Nat Biotechnol. 2019;37(11):1332–1343. doi: 10.1038/s41587-019-0280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang L., Shamardani K., Babikir H., et al. The evolution of alternative splicing in glioblastoma under therapy. Genome Biol. 2021;22(1):48. doi: 10.1186/s13059-021-02259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shraibman B., Barnea E., Kadosh D.M., et al. Identification of tumour antigens among the HLA peptidomes of glioblastoma tumors and plasma. Mol Cell Proteomics. 2019;18(6):1255–1268. doi: 10.1074/mcp.RA119.001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 152.Yadav M., Jhunjhunwala S., Phung Q.T., et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 153.Freudenmann L.K., Marcu A., Stevanović S. Mapping the tumour human leukocyte antigen (HLA) ligandome by mass spectrometry. Immunology. 2018;154(3):331–345. doi: 10.1111/imm.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Matsushita H., Vesely M.D., Koboldt D.C., et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482(7385):400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chongsathidkiet P., Jackson C., Koyama S., et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–1468. doi: 10.1038/s41591-018-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dimberg A. The glioblastoma vasculature as a target for cancer therapy. Biochem Soc Trans. 2014;42(6):1647–1652. doi: 10.1042/BST20140278. [DOI] [PubMed] [Google Scholar]
- 157.Ramachandran M., Vaccaro A., van de Walle T., et al. Tailoring vascular phenotype through AAV therapy promotes anti-tumour immunity in glioma. Cancer Cell. 2023;41(6):1134–1151.e10. doi: 10.1016/j.ccell.2023.04.010. [DOI] [PubMed] [Google Scholar]
- 158.Sautès-Fridman C., Petitprez F., Calderaro J., Fridman W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 159.Sattiraju A., Kang S., Giotti B., et al. Hypoxic niches attract and sequester tumour-associated macrophages and cytotoxic T cells and reprogram them for immunosuppression. Immunity. 2023;56(8):1825–1843.e6. doi: 10.1016/j.immuni.2023.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gilbert M.R., Dignam J.J., Armstrong T.S., et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Widodo S.S., Dinevska M., Furst L.M., Stylli S.S., Mantamadiotis T. IL-10 in glioma. Br J Cancer. 2021;125(11):1466–1476. doi: 10.1038/s41416-021-01515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Groux H., Bigler M., de Vries J.E., Roncarolo M.G. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160(7):3188–3193. [PubMed] [Google Scholar]
- 163.Fecci P.E., Mitchell D.A., Whitesides J.F., et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 164.Hussain S.F., Yang D., Suki D., Aldape K., Grimm E., Heimberger A.B. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8(3):261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ohue Y., Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110(7):2080–2089. doi: 10.1111/cas.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fecci P.E., Sweeney A.E., Grossi P.M., et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006;12(14 Pt 1):4294–4305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- 167.Ahmadzadeh M., Pasetto A., Jia L., et al. Tumour-infiltrating human CD4(+) regulatory T cells display a distinct TCR repertoire and exhibit tumour and neoantigen reactivity. Sci Immunol. 2019;4(31) doi: 10.1126/sciimmunol.aao4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bonertz A., Weitz J., Pietsch D.H., et al. Antigen-specific Tregs control T cell responses against a limited repertoire of tumour antigens in patients with colorectal carcinoma. J Clin Invest. 2009;119(11):3311–3321. doi: 10.1172/JCI39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Charles N.A., Holland E.C., Gilbertson R., Glass R., Kettenmann H. The brain tumour microenvironment. Glia. 2012;60(3):502–514. doi: 10.1002/glia.21264. [DOI] [PubMed] [Google Scholar]
- 170.Hambardzumyan D., Gutmann D.H., Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–27. doi: 10.1038/nn.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Antonios J.P., Soto H., Everson R.G., et al. Immunosuppressive tumour-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma. Neuro Oncol. 2017;19(6):796–807. doi: 10.1093/neuonc/now287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang P., Miska J., Lee-Chang C., et al. Therapeutic targeting of tumour-associated myeloid cells synergizes with radiation therapy for glioblastoma. Proc Natl Acad Sci USA. 2019;116(47):23714–23723. doi: 10.1073/pnas.1906346116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen Z., Feng X., Herting C.J., et al. Cellular and molecular identity of tumour-associated macrophages in glioblastoma. Cancer Res. 2017;77(9):2266–2278. doi: 10.1158/0008-5472.CAN-16-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gabrusiewicz K., Rodriguez B., Wei J., et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI insight. 2016;1(2) doi: 10.1172/jci.insight.85841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Khan F., Pang L., Dunterman M., Lesniak M.S., Heimberger A.B., Chen P. Macrophages and microglia in glioblastoma: heterogeneity, plasticity, and therapy. J Clin Invest. 2023;133(1) doi: 10.1172/JCI163446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rodrigues J.C., Gonzalez G.C., Zhang L., et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12(4):351–365. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Raychaudhuri B., Rayman P., Ireland J., et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(6):591–599. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kohanbash G., McKaveney K., Sakaki M., et al. GM-CSF promotes the immunosuppressive activity of glioma-infiltrating myeloid cells through interleukin-4 receptor-α. Cancer Res. 2013;73(21):6413–6423. doi: 10.1158/0008-5472.CAN-12-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hailemichael Y., Dai Z., Jaffarzad N., et al. Persistent antigen at vaccination sites induces tumour-specific CD8⁺ T cell sequestration, dysfunction and deletion. Nat Med. 2013;19(4):465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hashimoto M., Kamphorst A.O., Im S.J., et al. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu Rev Med. 2018;69:301–318. doi: 10.1146/annurev-med-012017-043208. [DOI] [PubMed] [Google Scholar]
- 181.Jhunjhunwala S., Hammer C., Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21(5):298–312. doi: 10.1038/s41568-021-00339-z. [DOI] [PubMed] [Google Scholar]
- 182.Pombo Antunes A.R., Scheyltjens I., Duerinck J., Neyns B., Movahedi K., Van Ginderachter J.A. Understanding the glioblastoma immune microenvironment as basis for the development of new immunotherapeutic strategies. Elife. 2020;9 doi: 10.7554/eLife.52176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Eisenbarth D., Wang Y.A. Glioblastoma heterogeneity at single cell resolution. Oncogene. 2023;42(27):2155–2165. doi: 10.1038/s41388-023-02738-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang Q., Hu B., Hu X., et al. Tumour evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32(1):42–56.e6. doi: 10.1016/j.ccell.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Verhaak R.G., Hoadley K.A., Purdom E., et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Patel A.P., Tirosh I., Trombetta J.J., et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Körber V., Yang J., Barah P., et al. Evolutionary trajectories of IDH(WT) glioblastomas reveal a common path of early tumorigenesis instigated years ahead of initial diagnosis. Cancer Cell. 2019;35(4):692–704.e12. doi: 10.1016/j.ccell.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 188.Spiteri I., Caravagna G., Cresswell G.D., et al. Evolutionary dynamics of residual disease in human glioblastoma. Ann Oncol. 2019;30(3):456–463. doi: 10.1093/annonc/mdy506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kuga D., Mizoguchi M., Guan Y., et al. Prevalence of copy-number neutral LOH in glioblastomas revealed by genomewide analysis of laser-microdissected tissues. Neuro Oncol. 2008;10(6):995–1003. doi: 10.1215/15228517-2008-064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang Q., Jiang N., Zou H., et al. Alterations in 3D chromatin organization contribute to tumorigenesis of EGFR-amplified glioblastoma. Comput Struct Biotechnol J. 2022;20:1967–1978. doi: 10.1016/j.csbj.2022.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sesé B., Ensenyat-Mendez M., Iñiguez S., Llinàs-Arias P., Marzese D.M. Chromatin insulation dynamics in glioblastoma: challenges and future perspectives of precision oncology. Clin Epigenet. 2021;13(1):150. doi: 10.1186/s13148-021-01139-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.DuPage M., Mazumdar C., Schmidt L.M., Cheung A.F., Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482(7385):405–409. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Campoli M., Ferrone S. Tumour escape mechanisms: potential role of soluble HLA antigens and NK cells activating ligands. Tissue Antigens. 2008;72(4):321–334. doi: 10.1111/j.1399-0039.2008.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zagzag D., Salnikow K., Chiriboga L., et al. Downregulation of major histocompatibility complex antigens in invading glioma cells: stealth invasion of the brain. Lab Investig. 2005;85(3):328–341. doi: 10.1038/labinvest.3700233. [DOI] [PubMed] [Google Scholar]
- 195.Varn F.S., Johnson K.C., Martinek J., et al. Glioma progression is shaped by genetic evolution and microenvironment interactions. Cell. 2022;185(12):2184–2199.e16. doi: 10.1016/j.cell.2022.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hazini A., Fisher K., Seymour L. Deregulation of HLA-I in cancer and its central importance for immunotherapy. J Immunother Cancer. 2021;9(8) doi: 10.1136/jitc-2021-002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sun T., Li Y., Wu J., et al. Downregulation of exosomal MHC-I promotes glioma cells escaping from systemic immunosurveillance. Nanomed Nanotechnol Biol Med. 2022;46 doi: 10.1016/j.nano.2022.102605. [DOI] [PubMed] [Google Scholar]
- 198.Minata M., Audia A., Shi J., et al. Phenotypic plasticity of invasive edge glioma stem-like cells in response to ionizing radiation. Cell Rep. 2019;26(7):1893–1905.e7. doi: 10.1016/j.celrep.2019.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yeung J.T., Hamilton R.L., Ohnishi K., et al. LOH in the HLA class I region at 6p21 is associated with shorter survival in newly diagnosed adult glioblastoma. Clin Cancer Res. 2013;19(7):1816–1826. doi: 10.1158/1078-0432.CCR-12-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Preusser M., de Ribaupierre S., Wöhrer A., et al. Current concepts and management of glioblastoma. Ann Neurol. 2011;70(1):9–21. doi: 10.1002/ana.22425. [DOI] [PubMed] [Google Scholar]
- 201.Miller K.D., Ostrom Q.T., Kruchko C., et al. Brain and other central nervous system tumour statistics, 2021. CA A Cancer J Clin. 2021;71(5):381–406. doi: 10.3322/caac.21693. [DOI] [PubMed] [Google Scholar]
- 202.White J.T., Cross E.W., Burchill M.A., et al. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat Commun. 2016;7 doi: 10.1038/ncomms11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mittelbrunn M., Kroemer G. Hallmarks of T cell aging. Nat Immunol. 2021;22(6):687–698. doi: 10.1038/s41590-021-00927-z. [DOI] [PubMed] [Google Scholar]
- 204.Yan S.X., Wei W. Castration reverses immunosenescence in aged mice. Acta Pharmacol Sin. 2011;32(9):1085–1086. doi: 10.1038/aps.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Albayram M.S., Smith G., Tufan F., et al. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat Commun. 2022;13(1):203. doi: 10.1038/s41467-021-27887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ahn J.H., Cho H., Kim J.H., et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572(7767):62–66. doi: 10.1038/s41586-019-1419-5. [DOI] [PubMed] [Google Scholar]
- 207.Da Mesquita S., Louveau A., Vaccari A., et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560(7717):185–191. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Itano A.A., Jenkins M.K. Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol. 2003;4(8):733–739. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 209.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chambers E.S., Akbar A.N. Can blocking inflammation enhance immunity during aging? J Allergy Clin Immunol. 2020;145(5):1323–1331. doi: 10.1016/j.jaci.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 211.Fahy G.M., Brooke R.T., Watson J.P., et al. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell. 2019;18(6) doi: 10.1111/acel.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Louveau A., Smirnov I., Keyes T.J., et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nedergaard M. Neuroscience. Garbage truck of the brain. Science. 2013;340(6140):1529–1530. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Louveau A., Herz J., Alme M.N., et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21(10):1380–1391. doi: 10.1038/s41593-018-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Møllgård K., Beinlich F.R.M., Kusk P., et al. A mesothelium divides the subarachnoid space into functional compartments. Science. 2023;379(6627):84–88. doi: 10.1126/science.adc8810. [DOI] [PubMed] [Google Scholar]
- 216.Sano S., Takahama Y., Sugawara T., et al. Stat3 in thymic epithelial cells is essential for postnatal maintenance of thymic architecture and thymocyte survival. Immunity. 2001;15(2):261–273. doi: 10.1016/s1074-7613(01)00180-7. [DOI] [PubMed] [Google Scholar]
- 217.Rezzani R., Nardo L., Favero G., Peroni M., Rodella L.F. Thymus and aging: morphological, radiological, and functional overview. Age. 2014;36(1):313–351. doi: 10.1007/s11357-013-9564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Velardi E., Tsai J.J., van den Brink M.R.M. T cell regeneration after immunological injury. Nat Rev Immunol. 2021;21(5):277–291. doi: 10.1038/s41577-020-00457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lin S.J., Chen A.T., Welsh R.M. Immune system derived from homeostatic proliferation generates normal CD8 T-cell memory but altered repertoires and diminished heterologous immune responses. Blood. 2008;112(3):680–689. doi: 10.1182/blood-2008-01-132464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Huang B., Yu Z., Liang R. Effect of long-term adjuvant temozolomide chemotherapy on primary glioblastoma patient survival. BMC Neurol. 2021;21(1):424. doi: 10.1186/s12883-021-02461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Felsberg J., Thon N., Eigenbrod S., et al. Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int J Cancer. 2011;129(3):659–670. doi: 10.1002/ijc.26083. [DOI] [PubMed] [Google Scholar]
- 222.Touat M., Li Y.Y., Boynton A.N., et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580(7804):517–523. doi: 10.1038/s41586-020-2209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cahill D.P., Levine K.K., Betensky R.A., et al. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumour progression during temozolomide treatment. Clin Cancer Res. 2007;13(7):2038–2045. doi: 10.1158/1078-0432.CCR-06-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hotchkiss K.M., Sampson J.H. Temozolomide treatment outcomes and immunotherapy efficacy in brain tumour. J Neuro Oncol. 2021;151(1):55–62. doi: 10.1007/s11060-020-03598-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ashwell J.D., Lu F.W., Vacchio M.S. Glucocorticoids in T cell development and function∗. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 226.Meng X., Zhao R., Shen G., Dong D., Ding L., Wu S. Efficacy and safety of bevacizumab treatment for refractory brain edema: case report. Medicine. 2017;96(44) doi: 10.1097/MD.0000000000008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Reardon D.A., Desjardins A., Vredenburgh J.J., et al. Rindopepimut with bevacizumab for patients with relapsed EGFRvIII-expressing glioblastoma (ReACT): results of a double-blind randomized phase II trial. Clin Cancer Res. 2020;26(7):1586–1594. doi: 10.1158/1078-0432.CCR-18-1140. [DOI] [PubMed] [Google Scholar]
- 228.Sloan A.E., Dansey R., Zamorano L., et al. Adoptive immunotherapy in patients with recurrent malignant glioma: preliminary results of using autologous whole-tumour vaccine plus granulocyte-macrophage colony-stimulating factor and adoptive transfer of anti-CD3-activated lymphocytes. Neurosurg Focus. 2000;9(6):e9. doi: 10.3171/foc.2000.9.6.10. [DOI] [PubMed] [Google Scholar]
- 229.Vik-Mo E.O., Nyakas M., Mikkelsen B.V., et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother. 2013;62(9):1499–1509. doi: 10.1007/s00262-013-1453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Alspach E., Lussier D.M., Miceli A.P., et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574(7780):696–701. doi: 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Borst J., Ahrends T., Bąbała N., Melief C.J.M., Kastenmüller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18(10):635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 232.Rudloff M.W., Zumbo P., Favret N.R., et al. Hallmarks of CD8(+) T cell dysfunction are established within hours of tumour antigen encounter before cell division. Nat Immunol. 2023;24(9):1527–1539. doi: 10.1038/s41590-023-01578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Khong H., Overwijk W.W. Adjuvants for peptide-based cancer vaccines. J Immunother Cancer. 2016;4:56. doi: 10.1186/s40425-016-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhu X., Nishimura F., Sasaki K., et al. Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumour antigen-derived peptide epitopes in murine CNS tumour models. J Transl Med. 2007;5:10. doi: 10.1186/1479-5876-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pulendran B., Arunachalam P.S., O’Hagan D.T. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20(6):454–475. doi: 10.1038/s41573-021-00163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Edelman R., Deming M.E., Toapanta F.R., et al. The SENIEUR protocol and the efficacy of hepatitis B vaccination in healthy elderly persons by age, gender, and vaccine route. Immun Ageing. 2020;17:9. doi: 10.1186/s12979-020-00179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nardin E.H., Oliveira G.A., Calvo-Calle J.M., et al. Phase I testing of a malaria vaccine composed of hepatitis B virus core particles expressing Plasmodium falciparum circumsporozoite epitopes. Infect Immun. 2004;72(11):6519–6527. doi: 10.1128/IAI.72.11.6519-6527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chiang C.L., Kandalaft L.E., Coukos G. Adjuvants for enhancing the immunogenicity of whole tumour cell vaccines. Int Rev Immunol. 2011;30(2–3):150–182. doi: 10.3109/08830185.2011.572210. [DOI] [PubMed] [Google Scholar]
- 239.Reinhardt R.L., Bullard D.C., Weaver C.T., Jenkins M.K. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J Exp Med. 2003;197(6):751–762. doi: 10.1084/jem.20021690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Redmond W.L., Sherman L.A. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22(3):275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 241.Didierlaurent A.M., Morel S., Lockman L., et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183(10):6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 242.Oh J.Z., Kurche J.S., Burchill M.A., Kedl R.M. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood. 2011;118(11):3028–3038. doi: 10.1182/blood-2011-04-348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jelinek I., Leonard J.N., Price G.E., et al. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J Immunol. 2011;186(4):2422–2429. doi: 10.4049/jimmunol.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Haining W.N., Davies J., Kanzler H., et al. CpG oligodeoxynucleotides alter lymphocyte and dendritic cell trafficking in humans. Clin Cancer Res. 2008;14(17):5626–5634. doi: 10.1158/1078-0432.CCR-08-0526. [DOI] [PubMed] [Google Scholar]
- 245.Cho H.I., Celis E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res. 2009;69(23):9012–9019. doi: 10.1158/0008-5472.CAN-09-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Caskey M., Lefebvre F., Filali-Mouhim A., et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med. 2011;208(12):2357–2366. doi: 10.1084/jem.20111171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Decker W.K., Safdar A. Cytokine adjuvants for vaccine therapy of neoplastic and infectious disease. Cytokine Growth Factor Rev. 2011;22(4):177–187. doi: 10.1016/j.cytogfr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 248.Yu T.W., Chueh H.Y., Tsai C.C., Lin C.T., Qiu J.T. Novel GM-CSF-based vaccines: one small step in GM-CSF gene optimization, one giant leap for human vaccines. Hum Vaccines Immunother. 2016;12(12):3020–3028. doi: 10.1080/21645515.2016.1221551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Petrina M., Martin J., Basta S. Granulocyte macrophage colony-stimulating factor has come of age: from a vaccine adjuvant to antiviral immunotherapy. Cytokine Growth Factor Rev. 2021;59:101–110. doi: 10.1016/j.cytogfr.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Jiang M., Chen P., Wang L., et al. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol. 2020;13(1):81. doi: 10.1186/s13045-020-00916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Fu J., Kanne D.B., Leong M., et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7(283) doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Laidlaw B.J., Craft J.E., Kaech S.M. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat Rev Immunol. 2016;16(2):102–111. doi: 10.1038/nri.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zander R., Schauder D., Xin G., et al. CD4(+) T cell help is required for the formation of a cytolytic CD8(+) T cell subset that protects against chronic infection and cancer. Immunity. 2019;51(6):1028–1042.e4. doi: 10.1016/j.immuni.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cui C., Wang J., Fagerberg E., et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumour CD8 T cell responses. Cell. 2021;184(25):6101–6118.e13. doi: 10.1016/j.cell.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Magen A., Hamon P., Fiaschi N., et al. Intratumoral dendritic cell-CD4(+) T helper cell niches enable CD8(+) T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat Med. 2023;29(6):1389–1399. doi: 10.1038/s41591-023-02345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ferris S.T., Durai V., Wu R., et al. cDC1 prime and are licensed by CD4(+) T cells to induce anti-tumour immunity. Nature. 2020;584(7822):624–629. doi: 10.1038/s41586-020-2611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Han S., Zhang C., Li Q., et al. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110(10):2560–2568. doi: 10.1038/bjc.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Schumacher T., Bunse L., Pusch S., et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–327. doi: 10.1038/nature13387. [DOI] [PubMed] [Google Scholar]
- 259.Corthay A., Skovseth D.K., Lundin K.U., et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22(3):371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 260.Boulch M., Cazaux M., Cuffel A., et al. Tumour-intrinsic sensitivity to the pro-apoptotic effects of IFN-γ is a major determinant of CD4(+) CAR T-cell antitumor activity. Nat Cancer. 2023;4(7):968–983. doi: 10.1038/s43018-023-00570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kruse B., Buzzai A.C., Shridhar N., et al. CD4(+) T cell-induced inflammatory cell death controls immune-evasive tumours. Nature. 2023;618(7967):1033–1040. doi: 10.1038/s41586-023-06199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Brightman S.E., Becker A., Thota R.R., et al. Neoantigen-specific stem cell memory-like CD4(+) T cells mediate CD8(+) T cell-dependent immunotherapy of MHC class II-negative solid tumors. Nat Immunol. 2023;24:1345–1357. doi: 10.1038/s41590-023-01543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Oh D.Y., Kwek S.S., Raju S.S., et al. Intratumoral CD4(+) T cells mediate anti-tumour cytotoxicity in human bladder cancer. Cell. 2020;181(7):1612–1625.e13. doi: 10.1016/j.cell.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Speiser D.E., Chijioke O., Schaeuble K., Münz C. CD4(+) T cells in cancer. Nat Cancer. 2023;4(3):317–329. doi: 10.1038/s43018-023-00521-2. [DOI] [PubMed] [Google Scholar]
- 265.Quezada S.A., Simpson T.R., Peggs K.S., et al. Tumour-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Salvatori E., Lione L., Compagnone M., et al. Neoantigen cancer vaccine augments anti-CTLA-4 efficacy. NPJ Vaccines. 2022;7(1):15. doi: 10.1038/s41541-022-00433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Massarelli E., William W., Johnson F., et al. Combining immune checkpoint blockade and tumour-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(1):67–73. doi: 10.1001/jamaoncol.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Aggarwal C., Cohen R.B., Morrow M.P., et al. Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin Cancer Res. 2019;25(1):110–124. doi: 10.1158/1078-0432.CCR-18-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Liu C.J., Schaettler M., Blaha D.T., et al. Treatment of an aggressive orthotopic murine glioblastoma model with combination checkpoint blockade and a multivalent neoantigen vaccine. Neuro Oncol. 2020;22(9):1276–1288. doi: 10.1093/neuonc/noaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhu P., Li S.Y., Ding J., et al. Combination immunotherapy of glioblastoma with dendritic cell cancer vaccines, anti-PD-1 and poly I:C. J Pharm Anal. 2023;13(6):616–624. doi: 10.1016/j.jpha.2023.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sharma P., Aaroe A., Liang J., Puduvalli V.K. Tumour microenvironment in glioblastoma: current and emerging concepts. Neuro Oncol Adv. 2023;5(1) doi: 10.1093/noajnl/vdad009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lang F.F., Conrad C., Gomez-Manzano C., et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419–1427. doi: 10.1200/JCO.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Geletneky K., Hajda J., Angelova A.L., et al. Oncolytic H-1 parvovirus shows safety and signs of immunogenic activity in a first phase I/IIa glioblastoma trial. Mol Ther. 2017;25(12):2620–2634. doi: 10.1016/j.ymthe.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Xu B., Tian L., Chen J., et al. An oncolytic virus expressing a full-length antibody enhances antitumor innate immune response to glioblastoma. Nat Commun. 2021;12(1):5908. doi: 10.1038/s41467-021-26003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Carpenter A.B., Carpenter A.M., Aiken R., Hanft S. Oncolytic virus in gliomas: a review of human clinical investigations. Ann Oncol. 2021;32(8):968–982. doi: 10.1016/j.annonc.2021.03.197. [DOI] [PubMed] [Google Scholar]
- 276.Antonopoulos M., Vang S.W., Dionysiou D., Graf N., Stamatakos G. Immune phenotype correlates with survival in patients with GBM treated with standard temozolomide-based therapy and immunotherapy. Anticancer Res. 2019;39(4):2043–2051. doi: 10.21873/anticanres.13315. [DOI] [PubMed] [Google Scholar]