Abstract
Background
Physical function and its decline in older age may be connected to treatable vascular risk factors in mid-life. This study aimed to evaluate whether these factors affect the underlying rate of decline.
Methods
This prospective cohort included 5 481 older adults aged 67–91 in the Atherosclerosis Risk in Communities Study (mean [standard deviation {SD}] age = 75.8 [5.0], 58% women, 21% Black race) without a history of stroke. The main outcome was the rate of Short Physical Performance Battery (SPPB) decline over a median late-life follow-up of 4.8 years. Primary mid-life (aged 45–64) exposures were Visit 1 hypertension (>140/90 mm Hg or treatment), diabetes (>126 mg/dL or treatment), high cholesterol (>240 mg/dL or treatment), and smoking, and number of decades of vascular risk exposure across Visits 1–4.
Results
The average adjusted rate of SPPB decline (points per 5 years) for older adults was −0.79 (confidence interval [CI]: −0.87, 0.71) and was accelerated by mid-life hypertension (+57% decline vs normotension: additional decline of −0.47, 95% CI: −0.64, −0.30), diabetes (+73% decline vs no diabetes: additional decline of −0.67, 95% CI: −1.09, −0.24), elevated systolic blood pressure (+17% decline per SD: −0.16, 95% CI: −0.23, −0.10), and elevated fasting blood glucose (+16% decline per SD: −0.015, 95% CI: −0.24, −0.06). Each decade greater mid-life exposure to hypertension (+32% decline: −0.93, 95% CI: −1.25, −0.61) and diabetes (+35% decline: −1.03, 95% CI: −1.68, −0.38) was associated with faster SPPB decline.
Conclusions
Mid-life control of blood pressure and diabetes may offset aging-related functional decline.
Keywords: Cardiovascular, Diabetes, Epidemiology, Hypertension, Physical performance
Greater physical function decline across late life is associated with lower quality of life, loss of independence, functional impairment, disability, and dementia (1–7). Among older adults, poor late-life vascular health is associated with poor physical function outcomes, including slow gait speed, poor balance, and weaker lower-limb strength (8). Yet, accumulation of vascular risk factors begins in mid-life, suggesting the etiology of aging-associated physical function declines may also begin decades before manifesting as late-life functional losses. Recent work has linked mid-life vascular health with poor physical function in late life (8–12), but evidence is lacking regarding associations of mid-life vascular health with physical performance declines in late life.
Understanding the association between mid-life vascular risk factors and disease—which may be responsive to established treatment options—and late-life physical function declines are critical to inform public health policies and projections concerning the health of an increasingly aging population; however, few studies have an adequate characterization of risk factors and outcomes across mid- and late life to fill this knowledge gap.
Using a large, geographically diverse cohort of community-based older Black and White adults in the United States, this study aimed to utilize more than 32 years of follow-up to investigate the relationship between mid-life vascular risk factors and late-life physical function declines. We hypothesized that greater vascular risk, both in higher levels of risk and in greater duration of elevated risk throughout mid- and early–late life, is associated with faster physical function decline in late life.
Method
Study Design
The ongoing Atherosclerosis Risk in Communities (ARIC) study recruited a population-based cohort of mid-life adults aged 45–64 years from 4 U.S. communities during 1987–1989. We included the 4 481 participants aged 67–90 years (mean 75.8 years) at Visit 5 (Black participants from Forsyth County, NC, and Jackson, MS, and White participants from Forsyth County, NC; Minneapolis, MN; and Washington County, MD) who did not have Parkinson disease or stroke and had complete physical function testing.
Vascular risk factors were assessed at Visit 1 in mid-life and again at 3 subsequent visits at approximately 3-year intervals (Visit 1, 1987–1989; Visit 2, 1990–1992; Visit 3, 1993–1995; Visit 4, 1996–1998; Supplementary Figure 1). After a 13-year gap, participants were assessed for physical function in late life over 3 study visits (Visit 5, 2011–2013; Visit 6, 2016–2017; Visit 7, 2018–2019).
All participants gave written informed consent at each study visit. Institutional review boards at all participating institutions approved this study.
Mid-life vascular risk factors
We considered mid-life measures of 4 vascular risk factors—hypertension, diabetes, high cholesterol, and smoking—as well as continuous measures of systolic blood pressure, fasting blood glucose, total cholesterol, and pack-years smoking. Systolic and diastolic blood pressure at each study visit were calculated from the average of the final 2 of 3 measurements after a 5-minute rest except at Visit 4 when 2 measurements were obtained and averaged. Systolic blood pressure was considered both as a continuous variable and categorized as having hypertension (systolic ≥140 or diastolic ≥90 mm Hg, or use of antihypertensive medication), prehypertension (139 ≥ systolic > 130 or 89 ≥ diastolic > 80 mm Hg), or normotension at each visit using the American Heart Association 2020 cardiovascular health goals for adults (13). Fasting blood glucose was modeled continuously by mg/dL and categorized as diabetes (≥126 mg/dL or use of diabetes medication), prediabetes (100–125 mg/dL), or no diabetes. Total plasma cholesterol was categorized as high (≥240 mg/dL), elevated (200–239 mg/dL), or low (14). Participants self-reported smoking status, modeled continuously as calculated pack-years, and categorized as never, former (≥100 cigarettes and quit ≥1 year ago), or current smoking. Continuous measures of vascular risk factors were centered by mean and scaled by standard deviation (SD) of the study population at the first visit.
Late-life physical function
Physical function assessments were offered to all participants at Visits 5, 6, and 7 using the validated Short Physical Performance Battery (SPPB) (12). Scores on the SPPB comprised 3 component scores: standing balance (highest achievement of 3 challenge stances for >10 seconds), unassisted chair stands (time to complete 5 seconds), and self-selected usual walking pace over 4 m (fastest of 2 trials, m/s) (15). Each component was scored 0 (lowest) to 4 (highest) according to the established thresholds (16). Inability to complete a component and those not tested due to inability or safety concerns were scored 0 points in that component. A difference of 0.5 SPPB points constitutes the minimal meaningful change and a 1.0 point difference represents a substantial change in physical function among older adults (17).
Covariates
Participants self-reported age, sex, race, and education at Visit 1. Education was categorized as less than high school, high school or equivalent or vocational degree, and greater than high school education. Body mass index (BMI) was calculated as measured weight (kg) divided by height squared (m2). Coronary heart disease events were adjudicated by an expert committee from the review of medical records between Visits 1 and 5 and by self-report prior to Visit 1. Heart failure events were defined by ICD-9/10 codes in medical records (18).
Statistical Analyses
Vascular risk at single point in mid-life
Linear mixed models tested the interaction between mid-life (Visit 1) vascular risk factors and rate of late-life SPPB score decline over Visits 5–7, scaled per 5 years. All models were random-slope, random-intercept with an unstructured covariance structure, used Huber-White robust variance estimates, and were adjusted for Visit 5 age, sex, education, and race site (Forsyth-Black, Forsyth-White, Jackson-Black, Minneapolis-White, Washington-White). Additional adjustment included Visit 5 BMI, heart disease, and heart failure and mutual adjustment for mid-life vascular risk factors. Results were presented for the full covariate adjustment set, including mutual adjustment for mid-life vascular risk factors, unless otherwise stated.
Cumulative mid-life hypertension and diabetes
Cumulative hypertension and diabetes over Visits 1–4 were assessed using average systolic blood pressure and average fasting blood glucose over a participant’s attended visits (minimum of 2 visits, missing visits were not included in the calculation). Years of hypertension or diabetes over Visits 1–4 assumed disease development at the midpoint (1.5 years) between the last measured visit without the condition (eg, normotensive) and the first visit with the condition. We conservatively estimated individuals with vascular disease at Visit 1 developed the disease 1.5 years prior. Disease diagnosis was considered nonreversible. For example, for hypertension, possible values were 0 years (no hypertension in Visits 1–4, each 3 years apart), 1.5 years (hypertension first at Visit 4), 4.5 years (Visit 3), 7.5 years (Visit 2), and 10.5 years (Visit 1).
Data analyses were conducted using Stata version 15 (StataCorp LLC, College Station, TX). Statistical significance was defined by a 2-sided p value of <.05.
Results
Characteristics of the Study Participants
The characteristics of the 5 481 participants included in this study are presented in Table 1. All participants were over 65 years (mean [SD], 75.8 [5.0]), and on average were overweight (28.7 [5.6]), predominately female (3191 [58%]), White race (4323 [79%]), and had no history of coronary heart disease (4678 [85%]) or heart failure (4851 [89%]). Median follow-up of late-life physical function follow-up was 4.8 years with a maximum of 8 years. The median time between the assessment of mid-life vascular risk factors and the first late-life physical function assessment (Visit 5) was 23.7 years. The average SPPB score at the Visit 5 start of late-life follow-up was 9.4 (SD 2.3) points and 26.4% of participants had an SPPB score below 9, indicating physical function limitation. Participants with higher Visit 5 SPPB scores were younger, had lower BMIs, were less likely to be female, had higher educational attainment, and had lower mid-life vascular risk. Participant characteristics of those lost-to-follow-up are presented in Supplementary Table 1.
Table 1.
Participant Characteristics by Late-Life Physical Function Scores
| n = | Visit 5 SPPB Score | |||
|---|---|---|---|---|
| Overall | ≤5 | 6–9 | ≥10 | |
| 5 481 | 424 | 1 864 | 3 193 | |
| Characteristics at Visit 5 | ||||
| Age, mean (SD) | 75.8 (5.0) | 78.9 (5.5) | 76.9 (5.2) | 74.8 (4.6) |
| Female (%) | 3 191 (58) | 300 (71) | 1 187 (64) | 1 704 (53) |
| BMI, mean (SD) | 28.7 (5.6) | 31.3 (7.8) | 29.2 (5.8) | 28.0 (4.9) |
| Race site (%) | ||||
| Forsyth-Black | 83 (2) | 9 (2) | 48 (3) | 26 (1) |
| Forsyth-White | 1 093 (20) | 62 (15) | 420 (23) | 611 (19) |
| Washington-White | 1 543 (28) | 97 (23) | 502 (27) | 944 (30) |
| Minneapolis-White | 1 687 (31) | 84 (20) | 468 (25) | 1 135 (36) |
| Jackson-Black | 1 075 (20) | 172 (41) | 426 (23) | 477 (15) |
| Education (%) | ||||
| <High school | 713 (13) | 125 (30) | 297 (16) | 291 (9) |
| High school, equivalent, or trade school | 2 308 (42) | 164 (39) | 840 (45) | 1 304 (41) |
| >High school | 2 451 (45) | 133 (32) | 725 (39) | 1 593 (50) |
| Coronary heart disease (%) | 803 (15) | 73 (18) | 297 (16) | 433 (14) |
| History of heart failure (%) | 630 (11) | 106 (25) | 286 (15) | 238 (7) |
| Mid-life vascular risk factors at Visit 1 | ||||
| Hypertension, n (%) | 1 240 (23) | 188 (44) | 484 (26) | 568 (18) |
| Diabetes, n (%) | 266 (5) | 55 (13) | 111 (6) | 100 (3) |
| High cholesterol, n (%) | 1 175 (21) | 118 (28) | 458 (25) | 599 (19) |
| Current smokers, n (%) | 944 (17) | 71 (17) | 373 (20) | 500 (16) |
| Systolic blood pressure (mm Hg), mean (SD) | 116.0 (15.5) | 122.8 (15.7) | 117.1 (16.0) | 114.4 (14.9) |
| Fasting blood glucose (mg/dL), mean (SD) | 100.3 (19.84) | 106.9 (34.31) | 101.2 (22.74) | 98.9 (14.59) |
| Total cholesterol (mg/dL), mean (SD) | 210.0 (39.73) | 216.5 (44.43) | 213.2 (40.03) | 207.4 (38.68) |
| Pack-years among ever smokers, mean (SD) | 10.7 (16.7) | 9.5 (16.0) | 11.4 (17.3) | 10.5 (16.4) |
Notes: BMI = body mass index; SD = standard deviation; SPPB = Short Physical Performance Battery.
Main Analysis
Vascular risk at single point in mid-life
The average rate of late-life SPPB decline was −0.94 points per 5 years (95% confidence interval [CI]: −1.01 to −0.88) for never-smoking participants with average visit 1 systolic blood pressure, fasting blood glucose, and total cholesterol (Table 2). Participants declined an additional −0.16 SPPB points per 5 years (95% CI: −0.23 to −0.10) for each SD higher Visit 1 systolic blood pressure, corresponding to 17% and 34% faster rates of late-life decline at 128.3 mm Hg (1 SD higher than mean systolic blood pressure) and 140.6 mm Hg (2 SDs higher), respectively. Participants declined an additional −0.15 SPPB points more than the average per 5 years (95% CI: −0.24 to −0.06) for each SD higher Visit 1 fasting blood glucose, indicating an average participant with 137.9 mg/dL fasting blood glucose at Visit 1 declined at a 32% faster rate than the average participant with a glucose of 100.3 mg/dL.
Table 2.
Differences in Adjusted Rates of Late-Life SPPB Decline by Higher Mid-Life Vascular Risk Factors at Visit 1
| Difference in 5-Year Rate of Late-Life SPPB Decline Per SD of Mid-Life Vascular Risk Factor | ||||
|---|---|---|---|---|
| Mid-Life Vascular Risk Factor | Adjusted Difference* | Decline Rate Ratio† | ||
| SD | Difference (95% CI) p Value | 1 SD higher | 2 SD higher | |
| Systolic blood pressure | 12.3 mm Hg | −0.16 (−0.23, −0.10) p < .001 | 1.17 | 1.34 |
| Fasting blood glucose | 18.84 mg/dL | −0.15 (−0.24, −0.06) p = .002 | 1.16 | 1.32 |
| Total cholesterol | 39.58 mg/dL | −0.04 (−0.11, 0.02) p = .23 | 1.04 | 1.09 |
| Pack-years smoking | 16.1 pack-years | −0.06 (−0.13, 0.01) p = .11 | 1.06 | 1.13 |
Notes: BMI = body mass index; CI = confidence interval; SD = standard deviation; SPPB = Short Physical Performance Battery.
*Estimates of differences in rates of late-life SPPB decline are derived from random-slope, random-intercept linear mixed models with unstructured covariance, adjusted for age, sex, race site, education, BMI, prevalent coronary heart disease, and prevalent heart failure at Visit 5 and mutually adjusted for systolic blood pressure, fasting blood glucose, total cholesterol, and pack-years smoking. Vascular risk factors were centered to Visit 1 population averages (systolic blood pressure 116.0 mm Hg, fasting blood glucose, 100.3 mg/dL, total cholesterol 210.0 mg/dL, and smoking 0 pack-years).
†Decline rate ratio is equal to the average rate of decline (−0.94 SPPB points per 5 years, 95% CI: −1.01 to −0.88, p < .001) of participants with mean vascular risk factors at Visit 1 plus the rate difference per SD of vascular risk divided by the average rate of decline.
We did not observe an association between total cholesterol (additional decline of −0.04 SPPB points per 5 years, 95% CI: −0.11 to 0.02) or pack-years of smoking (additional decline of −0.06 SPPB points per 5 years, 95% CI: −0.13 to 0.01) and rate of SPPB decline in this study population. Removing cholesterol and smoking from subsequent analyses did not alter the magnitude of the association between higher systolic blood pressure or higher blood glucose and faster rates of SPPB decline (Supplementary Table 2).
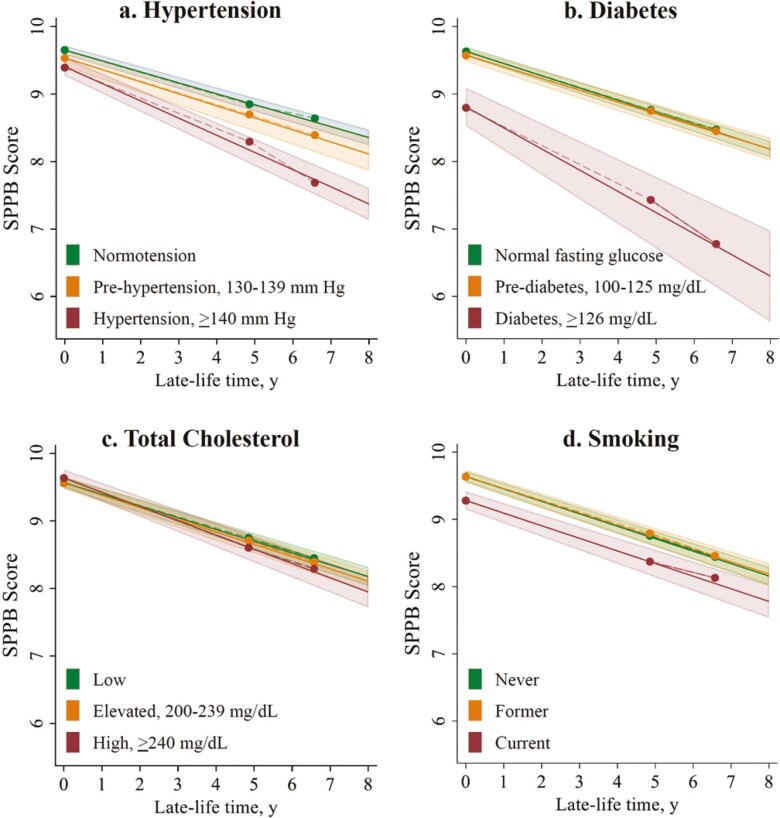
The results of the association between Visit 1 vascular risk factors categorized into high-, intermediate-, and low-risk levels and SPPB point decline over late-life follow-up are presented in Figure 1, displaying both average SPPB at Visit 5 and average decline rates over Visits 5–7 late-life follow-up. On average, participants with hypertension at Visit 1 declined at a rate of −1.27 SPPB points per 5 years (95% CI: −1.42 to −1.11), 57% faster than their peers with normotension (additional decline of −0.47 SPPB points per 5 years, 95% CI: −0.64 to −0.30; Table 3). Participants with no diabetes at Visit 1 had an average rate of decline of −0.90 SPPB points per 5 years (95% CI: −0.98 to −0.83). The average participant with diabetes at Visit 1 declined in late life at a rate of −1.56 SPPB points per 5 years (95% CI: −1.98 to −1.15), 73% faster than a peer without diabetes (rate difference −0.67 SPPB points per 5 years, 95% CI: −1.09 to −0.24). Rates of decline did not differ significantly among peers with prehypertension or with prediabetes.
Figure 1.
Adjusted late-life SPPB decline by mid-life vascular risk factors at Visit 1. Clockwise from top left: the association between Visit 1 mid-life (A) hypertension risk, (B) diabetes risk, (C) total cholesterol level, and (D) smoking status and late-life rate of SPPB decline (Visits 5–7). Time (x-axes) indicates years of late-life follow-up from Visit 5. Estimated linear rates of SPPB decline (solid lines) and their 95% CI (shaded areas) are derived from linear mixed models with random-slope, random-intercept, and unstructured covariance structure. Piece-wise linear splines (dashed lines) show goodness of fit of the average SPPB scores (solid circle markers) at Visit 5, Visit 6 (mean late-life follow-up time = 4.8 years), and Visit 7 (mean late-life follow-up time = 6.6 years) to the linear trend. All models adjust for age, sex, race site, education, BMI, prevalent coronary heart disease, prevalent heart failure at the start of late-life follow-up (Visit 5), and mutually adjust for mid-life vascular risk factors at Visit 1 (hypertension, diabetes, total cholesterol, and smoking status). BMI = body mass index; CI = confidence interval; SPPB = Short Physical Performance Battery.
Table 3.
Adjusted Rate of Late-Life SPPB Decline by Mid-Life Hypertension and Diabetes Categories at Visit 1
| Adjusted Rate of Decline* | |||
|---|---|---|---|
| Vascular Risk | Rate† (95% CI) p Value | Difference (95% CI) p Value | Decline Rate Ratio |
| Hypertension | |||
| Normotension | −0.81 (−0.88, −0.74) p < .001 | 0 (reference) | 1 (reference) |
| Prehypertension | −0.89 (−1.04, −0.73) p < .001 | −0.09 (−0.26, 0.08) p = .315 | 1.10 |
| Hypertension | −1.27 (−1.42, −1.11) p < .001 | −0.47 (−0.64, −0.30) p < .001 | 1.57 |
| Diabetes | |||
| No diabetes | −0.90 (−0.98, −0.83) p < .001 | 0 (reference) | 1 (reference) |
| Prediabetes | −0.87 (−0.97, −0.76) p < .001 | 0.04 (−0.09, 0.16) p = .593 | 0.97 |
| Diabetes | −1.56 (−1.98, −1.15) p < .001 | −0.67 (−1.09, −0.24) p = .002 | 1.73 |
Notes: BMI = body mass index; CI = confidence interval; SPPB = Short Physical Performance Battery.
*Adjusted for age, sex, race site, education, BMI, prevalent coronary heart disease and heart failure at Visit 5, and mutually adjusted for hypertension and diabetes categories.
†Rates of SPPB decline and differences in rates of SPPB decline are derived from random-slope, random-intercept linear mixed models with unstructured covariance and are given as decline in SPPB points per 5 years.
Cumulative hypertension and diabetes exposure
A decade of hypertension during the Visits 1–4 period was associated with 64% faster late-life rates of SPPB decline (additional decline −0.93, 95% CI: −1.25 to −0.61), and a decade of mid-life diabetes was associated with 71% faster rates of decline (additional decline −1.03, 95% CI −1.68 to −0.38) compared to participants who never had reports of either diabetes or hypertension (Table 4). Higher mean systolic blood pressure and higher mean fasting blood glucose during the Visits 1–4 interval were associated with 24% and 23% faster late-life rates of SPPB decline per SD higher than mean at Visit 1 (12.3 mm Hg and 18.84 mg/dL, respectively). One SD higher mean Visits 1–4 systolic blood pressure was associated with a −0.21 faster rate of SPPB decline per 5 years (95% CI: −0.29 to −0.13) and 1 SD higher mean fasting blood glucose was associated with a −0.20 faster rate of SPPB decline per 5 years (95% CI: −0.31 to −0.10) compared to peers with average systolic blood pressure and fasting blood glucose.
Table 4.
Differences in Adjusted Rates of Late-Life SPPB Decline by Cumulative Mid-Life Vascular Exposure
| Difference in 5-Year Rate of Late-Life SPPB Decline Per Mid-Life Hypertension and Diabetes Exposure | |||
|---|---|---|---|
| Adjusted Difference* | Decline Rate Ratio† | ||
| Mid-Life Vascular Disease (per decade) | Difference‡ (95% CI) p Value | 0.5 Decade | 1 Decade |
| Hypertension | −0.93 (−1.25, −0.61) p < .001 | 1.32 | 1.64 |
| Diabetes | −1.03 (−1.68, −0.38) p = .002 | 1.35 | 1.71 |
| Mean of Visits 1–4 (per SD) | Difference§ (95% CI) p Value | 1 SD higher | 2 SD higher |
|---|---|---|---|
| Systolic blood pressure | −0.21 (−0.29, -0.13) p < .001 | 1.24 | 1.48 |
| Fasting blood glucose | −0.20 (−0.31, −0.10) p < .001 | 1.23 | 1.46 |
Notes: BMI = body mass index; CI = confidence interval; SD =standard deviation; SPPB = Short Physical Performance Battery.
*Estimates of differences in rates of late-life SPPB decline per decade of measured mid-life vascular disease are derived from random-slope, random-intercept linear mixed models with unstructured covariance, adjusted for age, sex, race site, education, BMI, prevalent coronary heart disease and prevalent heart failure at Visit 5, and mutually adjusted for hypertension and diabetes exposures.
†Decline rate ratio is equal to the average rate of decline of participants without the hypertension or diabetes exposure plus the rate difference per unit exposure, divided by the average rate of decline.
‡Difference in decline rate per decade of hypertension or diabetes in Visits 1–4 compared to the average rate of decline (−1.46 SPPB points per 5 years, 95% CI: −1.61 to −1.32, p < .001) of participants without any measurement of hypertension or diabetes in Visits 1–4, mutually adjusted for hypertension and diabetes.
§Difference in decline rate per higher mean Visits 1–4 risk factor compared to the average decline (−0.87 SPPB points per 5 years, 95% CI: −0.93 to −0.81, p < .001) of participants with mean systolic blood pressure and fasting blood glucose, mutually adjusted for mean Visits 1–4 vascular risk factors.
Sensitivity Analyses
When evaluating the associations of cumulative hypertension and diabetes, we also classified the presence of hypertension and diabetes by study visit of the first recorded mid-life instance (Supplementary Table 3). There was no significant evidence of nonlinearity in the dose–response of considering hypertension–decades or diabetes–decades as continuous variables, as reported in Table 4, though the estimate for hypertension at Visit 2 presents an anomaly. Associations were broadly consistent both when adjusting for only demographic covariates and additionally for other health status covariates.
Discussion
In a community population of older adults from 4 geographically diverse U.S. locations followed over 32 years, we observed that higher mid-life hypertension and diabetes vascular risk were robustly associated with faster rates of objectively measured physical function decline in late life. These findings persist regardless of whether risk factors were defined categorically or continuously, and whether data from 1 mid-life time point or an average across 12 years of mid- to late-life assessments were included. Collectively, these results indicate that both higher vascular risk of hypertension and diabetes as well as greater cumulative disease starting in mid-life are associated with faster loss of physical functioning decades later in late life, supporting the idea that even modest public health gains in delaying the onset of hypertension or diabetes in mid-life could possibly have profound effects on ameliorating later poor physical function outcomes.
The National Institute on Aging’s Strategic Directions 2020 highlights the need for a better understanding of interventions that can prevent or mitigate loss of independence and function in late life as a research priority in the field (19). As physical function declines measured by the SPPB have been well established on the path to future disability (3,15,16) and loss of independence (1,2), our results contribute to the identification of preventable factors that may have the potential for elongating healthy physical function in late life. In this study, mid-life hypertension, diabetes, higher systolic blood pressure, and fasting blood glucose were associated with faster rates of SPPB decline across eight years of late-life follow-up. Over a 10-year period, these results constitute meaningful differences in late-life physical function (more than 0.5 additional SPPB points) in late life for the effect estimates of having previously had systolic blood pressure or fasting blood glucose levels 2 SDs above the mean, and they indicate substantial differences (more than 1.0 additional SPPB points) over a 10-year period in late-life physical function for older adults with previous diabetes in mid-life compared to their previously low-risk peers (17). A systolic blood pressure of 116 mm Hg compared to 140 mm Hg at Visit 1 was associated with additional SPPB declines over 5 years that were similar to having the late-life physical function declines of a peer 2 years younger. Similarly, our results estimated that although an older person without previous diabetes at Visit 1 had clinically meaningful SPPB loss over 5 years, they averaged 8.6 years to decline the same amount as a peer with diabetes at Visit 1 declined in 5 years, for approximately 3.6 years more of better functioning. Though the ordinal nature of the SPPB means minimally meaningful SPPB change may not become clinically measurable for twice as long as a substantially meaningful SPPB change, both changes, nevertheless, reflect a loss of physical function that can affect an older adult’s daily life and future clinical outcomes.
Although results from a clinical trial would provide the strongest evidence, the practical difficulty of enacting a trial intervening on blood pressure and fasting blood glucose control in mid-life and following up into late-life elevates the importance of well-controlled observational cohort studies. Over 30 years of prospective assessment of vascular risk factors and physical function in the ARIC Study represent a rare resource of well-characterized exposure and outcome assessment in a community cohort of Black and White older adults across 4 U.S. regions.
Our findings complement previous work indicating that healthy blood pressure and blood glucose metrics in mid-life are associated with better physical performance in late life. Participants with higher Life Simple 7 scores—a multicomponent score of ideal vascular health across blood pressure, blood glucose, total cholesterol, smoking status, BMI, diet, and physical activity—in mid-life were reported to have higher SPPB scores during late life in the ARIC cohort (12). We similarly observed average SPPB scores were higher at the start of late-life follow-up among individuals with previously better mid-life vascular risk profiles. Here we add to the literature by demonstrating that greater mid-life vascular disease burden affects the rate of decline across late-life, even if mid-life burden is only measured at a single time point decades before late-life physical function assessment. The magnitude of these associations strengthens when accounting for multiple measurements during mid-life, highlighting the importance of cumulative exposure measurements of modifiable vascular risk factors earlier in the life course. This suggests that delaying onset of hypertension and diabetes could also have potential benefits in terms of late-life physical function.
Previous studies have shown a link between lower mid-life smoking (20–22) or cholesterol risk (22) and better late-life physical outcomes. Our results support the cross-temporal association with smoking—current smoking at Visit 1 was associated with lower physical function level at Visit 5—but we did not observe an association with smoking or cholesterol risk and the rate of late-life physical function decline across 8 years of follow-up in late life. The effects of mid-life smoking and high cholesterol could have been obscured due to higher mortality among smokers and the greater likelihood that healthier adults survived to Visit 5 and were more likely to attend late-life study visits.
The findings in this analysis have several implications for the connection between vascular disease risk and physical functioning. The use of prior measurements of hypertension and diabetes in mid-life may identify older adults more likely to experience faster physical function decline in a health ecosystem where decades of electronic medical records are increasingly available for older adults, but complete records are rare. Previous research has posited that the connection between poor vascular health and poor physical function lies in repeated accumulations of small vessel insults in the brain that affect both cognitive and mobility functioning (23,24), which could present a pathway for how mid-life hypertension and diabetes risk burden are associated with progression of physical function declines into late life. Future research on the role of vascular disease, physical functioning, and brain health may illuminate more of this connection.
Limitations
Our participant population does not include participants in the United States who are neither Black nor White race, and the effects of race are inextricably linked with geographic location in the ARIC Study. We restricted our study to participants without Parkinson’s disease or previous stroke, leaving us unable to generalize findings to those groups. Stroke is a major contributor to mobility disability (25), which likely results in our findings being conservative. The contributions of arthritis and injury could not be examined in this analysis, but our results, nevertheless, show a substantial contribution of risk associated with vascular risk factor mechanisms. Linear mixed models assume a missing-at-random structure for attrition from Visit 5 through Visit 7 and there may be more informative missingness. However, because those with the highest disease burden and lowest physical function would likely be differentially lost-to-follow-up, our results may underestimate relationships between mid-life vascular risk and late-life physical function decline.
We are not able to generalize our findings to all ARIC participants who attended Visit 1 in mid-life. Our analysis includes only participants who attended the first assessment of objective physical function, conducted in late life at study Visit 5. Therefore, the results of this study should be interpreted within the context of how an older adult’s history of mid-life vascular risk may inform their future rate of physical function decline rather than a projection of late-life function for all middle-aged persons.
Conclusion
In this large, prospectively assessed population of community-based older adults in the United States, previous mid-life experience of higher vascular risk of hypertension and diabetes preceded substantially faster rates of decline in physical functioning. These results provide evidence that uncontrolled modifiable vascular risk factors in mid-life may have a clinically meaningful impact on aging-related decline in objectively measured physical function. The finding that longer and more intense exposures to diabetes and hypertension risk amplified associations with functional declines heightens the importance of better understanding mid-life as a potentially crucial period of intervention for physical function outcomes in late life.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions to this and countless other scientific works, which would not be possible without their time, effort, and support.
Contributor Information
Laura F Skow, Department of Epidemiology, Johns Hopkins School of Public Health, Baltimore, Maryland, USA.
A Richey Sharrett, Department of Epidemiology, Johns Hopkins School of Public Health, Baltimore, Maryland, USA.
Rebecca F Gottesman, National Institute of Neurological Disorders and Stroke Intramural Research Program, National Institutes of Health, Bethesda, Maryland, USA.
Josef Coresh, Department of Epidemiology, Johns Hopkins School of Public Health, Baltimore, Maryland, USA.
Jennifer A Deal, Department of Epidemiology, Johns Hopkins School of Public Health, Baltimore, Maryland, USA.
Priya Palta, Department of Neurology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Kevin J Sullivan, Memory Impairment and Neurodegenerative Dementia (MIND) Center, Department of Medicine, Division of Geriatric Medicine, University of Mississippi Medical Center, Jackson, Mississippi, USA.
Michael E Griswold, Memory Impairment and Neurodegenerative Dementia (MIND) Center, Department of Medicine, Division of Geriatric Medicine, University of Mississippi Medical Center, Jackson, Mississippi, USA.
Jennifer A Schrack, Department of Epidemiology, Johns Hopkins School of Public Health, Baltimore, Maryland, USA.
B Gwen Windham, Memory Impairment and Neurodegenerative Dementia (MIND) Center, Department of Medicine, Division of Geriatric Medicine, University of Mississippi Medical Center, Jackson, Mississippi, USA.
Lewis A Lipsitz, (Medical Sciences Section).
Funding
This work was supported by the National Heart, Lung, and Blood Institute (grant numbers 75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, 75N92022D00005), which collaboratively fund the Atherosclerosis Risk in Communities Study and L.F.S. (T32HL007024-46 to L.F.S.). The ARIC Neurocognitive Study is supported by the National Heart, Lung, and Blood Institute; the National Institute of Neurological Disorders and Stroke; the National Institute on Aging (grant numbers U01HL096812, U01HL096814, U01HL096899, U01HL096902, and U01HL096917).
Conflict of Interest
The authors have no Conflicts of Interest to report.
Author Contributions
L.F.S., A.R.S., R.F.G., J.C., M.G., P.P., K.J.S., J.D., J.A.S., and B.G.W. were involved in the planning of this project and provided substantive editing. L.F.S. lead analysis and writing of manuscript drafts. A.R.S., M.G., and J.A.S. contributed to the methodology. A.R.S., J.C., R.F.G., and B.G.W. additionally contributed to obtaining funding and resources necessary to collect the data used in these analyses. Funders did not have a role in the conception, analysis, preparation, publication, or any other part of this work.
References
- 1. Gill TM, Han L, Gahbauer EA, Leo-Summers L, Allore HG.. Prognostic effect of changes in physical function over prior year on subsequent mortality and long-term nursing home admission. J Am Geriatr Soc. 2018;66(8):1587–1591. 10.1111/jgs.15399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. von Bonsdorff M, Rantanen T, Laukkanen P, Suutama T, Heikkinen E.. Mobility limitations and cognitive deficits as predictors of institutionalization among community-dwelling older people. Gerontology. 2006;52(6):359–365. 10.1159/000094985 [DOI] [PubMed] [Google Scholar]
- 3. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. 10.1093/gerona/55.4.m221 [DOI] [PubMed] [Google Scholar]
- 4. Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J.. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67(8):980–986. 10.1001/archneurol.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68(8):929–937. 10.1093/gerona/gls256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verghese J, Robbins M, Holtzer R, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc. 2008;56(7):1244–1251. 10.1111/j.1532-5415.2008.01758.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beauchet O, Annweiler C, Callisaya ML, et al. Poor gait performance and prediction of dementia: results from a meta-analysis. J Am Med Dir Assoc. 2016;17(6):482–490. 10.1016/j.jamda.2015.12.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Welmer A-K, Liang Y, Angleman S, et al. Vascular risk factor burden, atherosclerosis, and functional dependence in old age: a population-based study. Int J Behav Med. 2014;21(4):597–604. 10.1007/s12529-013-9352-8 [DOI] [PubMed] [Google Scholar]
- 9. Sabia S, Elbaz A, Rouveau N, Brunner EJ, Kivimaki M, Singh-Manoux A.. Cumulative associations between midlife health behaviors and physical functioning in early old age: a 17-year prospective cohort study. J Am Geriatr Soc. 2014;62(10):1860–1868. 10.1111/jgs.13071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stenholm S, Sainio P, Rantanen T, et al. High body mass index and physical impairments as predictors of walking limitation 22 years later in adult Finns. J Gerontol A Biol Sci Med Sci. 2007;62(8):859–865. 10.1093/gerona/62.8.859 [DOI] [PubMed] [Google Scholar]
- 11. von Bonsdorff MB, Haapanen MJ, Törmäkangas T, Pitkälä KH, Stenholm S, Strandberg TE.. Midlife cardiovascular status and old age physical functioning trajectories in older businessmen. J Am Geriatr Soc. 2019;67(12):2490–2496. 10.1111/jgs.16150 [DOI] [PubMed] [Google Scholar]
- 12. Windham BG, Harrison KL, Lirette ST, et al. Relationship between midlife cardiovascular health and late-life physical performance: the ARIC study. J Am Geriatr Soc. 2017;65(5):1012–1018. 10.1111/jgs.14732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Virani SS, Alonso A, Benjamin EJ, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 14. Grundy M, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143. 10.1161/cir.0000000000000699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 16. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB.. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perera S, Mody SH, Woodman RC, Studenski SA.. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 18. Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997;146(6):483–494. 10.1093/oxfordjournals.aje.a009302 [DOI] [PubMed] [Google Scholar]
- 19. National Institute of Aging. The National Institute on Aging: strategic directions for research, 2020-2025. 1997.
- 20. Agahi N, Fors S, Fritzell J, Shaw BA.. Smoking and physical inactivity as predictors of mobility impairment during late life: exploring differential vulnerability across education level in Sweden. J Gerontol B Psychol Sci Soc Sci. 2018;73(4):675–683. 10.1093/geronb/gbw090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lafortune L, Martin S, Kelly S, et al. Behavioural risk factors in mid-life associated with successful ageing, disability, dementia and frailty in later life: a rapid systematic review. PLoS One. 2016;11(2):e0144405. 10.1371/journal.pone.0144405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palta P, Griswold M, Ranadive R, et al. Midlife cardiovascular health and robust versus frail late-life status: the Atherosclerosis Risk in Communities Study. J Gerontol A Biol Sci Med Sci. 2022;77(6):1222–1229. 10.1093/gerona/glab310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Power MC, Tingle JV, Reid RI, et al. Midlife and late-life vascular risk factors and white matter microstructural integrity: the Atherosclerosis Risk in Communities Neurocognitive Study. J Am Heart Assoc. 2017;6(5):e005608. 10.1161/JAHA.117.005608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosano C, Kuller LH, Chung H, Arnold AM, LongstrethWT, Jr, Newman AB.. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53(4):649–654. 10.1111/j.1532-5415.2005.53214.x [DOI] [PubMed] [Google Scholar]
- 25. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–e639. 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.