Abstract
Background and Aims
Olive (Olea europaea subsp. europaea var. europaea) is the most extensively cultivated fruit crop worldwide. It is considered a wind-pollinated and strictly outcrossing crop. Thus, elevated pollen production is crucial to guarantee optimum fruit set and yield. Despite these facts, the variability of pollen production within the cultivated olive has been scarcely studied. This study aimed to characterize this feature by analysing a representative set of worldwide olive cultivars.
Methods
We evaluated the average number of pollen grains per anther in 57 principal cultivars over three consecutive years. We applied a standard generalized linear model (GLM) approach to study the influence of cultivar, year and the previous year’s fruit load on the amount of pollen per anther. Additionally, the K-means method was used for cluster analysis to group cultivars based on their pollen production capacity.
Key Results
Pollen production per anther was highly variable among olive cultivars. The cultivar significantly accounted for 51.3 % of the variance in pollen production and the year for 0.3 %. The interaction between the two factors explained 8.4 % of the variance, indicating that not all cultivars were equally stable in producing pollen across the years. The previous year’s fruit load and its interaction with the year were significant, but barely accounted for 1.5 % of the variance. Olive cultivars were classified into four clusters according to their capacity to produce pollen. Interestingly, the fourth cluster was composed of male-sterile cultivars, which presumably share this character by inheritance.
Conclusions
Pollen production per anther varied extensively within the cultivated olive. This variation was mainly driven by the cultivar and its interaction with the year. The differential capacity of olive cultivars to produce pollen should be considered not only for designing new orchards but also gardens where this species is used as an ornamental.
Keywords: Olea europaea, pollen per anther, wind pollination, fruit load, androsterility, clustering, variability, biennial bearing
INTRODUCTION
Cultivated olive (Olea europaea subsp. europaea var. europaea) is a species of essential historical, social and economic importance in the Mediterranean Basin. Cultivated olive is present in more than 40 countries and occupies a total area of 12.5 million hectares, being the most extensively planted fruit crop. Spain has the largest olive cultivated area and production, with ~2.5 million hectares and 35 % of olive oil production worldwide (FAOSTAT, 2020).
Olive cultivation is still primarily based on growing traditional olive cultivars under extensive rainfed conditions. However, the continued increase in demand for productivity has caused the olive sector to adopt new management strategies: harvest mechanization, super high-density systems, irrigation, and the use of new and more productive cultivars (Díez et al., 2015; Rallo et al., 2018; Lo Bianco et al., 2021). The olive tree is also expanding beyond the Mediterranean boundaries in places such as China and the Americas, where its adaptation is occasionally uncertain (Ruiz et al., 2019; Sánchez-Estrada and Cuevas, 2019).
It is thought that the selection of cultivars with high flower production has caused the olive tree pollination process to change over time, from entomophilous to anemophilous pollination (Rallo and Cuevas, 2007). Olive is currently considered a wind-pollinated crop and a strict outcrosser (Lavee et al., 2002; Díaz et al., 2006; Selak et al., 2011), with few reported cases of auto-pollination, always giving rise to low fruit set (Farinelli et al., 2008; Serrano et al., 2008). Wind pollination requires the production of a very large amount of pollen during the flowering period to achieve successful fertilization (Cruden, 2000; Rallo and Cuevas, 2007). In fact, there is a positive correlation between annual pollen production and the subsequent olive yield (Galán et al., 2004; Ribeiro et al., 2007; Orlandi et al., 2017), with the potential for fruit production forecasting.
Olive is an andromonoecious species with hermaphrodite flowers and a minor percentage of male flowers (Cuevas and Polito, 2004; Famiani et al., 2019; Maniriho, 2022). Olive pollen grains are trizonocolporate with a reticulate exine (Trigo et al., 2008), and their ultrastructure was the subject of extensive research during the 1980s and 1990s (Fernández and Rodríguez-García, 1988, 1989; Pacini and Juniper, 1979a, b; Rodríguez-García and Fernández, 1990). This pollen is highly allergenic and causes severe health problems in areas where the olive tree is present (Bousquet et al., 1984; D’Amato, 1998). Twelve allergenic proteins in olive pollen have been identified (from Ole e 1 to Ole e 12) (Rodríguez et al., 2007; Villalba et al., 2014), whose expression and allergenic effects differ depending on the cultivar and the environment (Alché et al., 2007; Quiralte et al., 2007). In aerobiological studies, airborne olive pollen is considered as a whole because possible differences between cultivars (size, shape, apertures, thickness of the exine, and maximum length of the lumens) are not distinguishable under an optical microscope (Koubouris et al., 2012; Ribeiro et al., 2012; Messora et al., 2017; Khaleghi et al., 2019). Therefore, classifying olive cultivars according to their capacity to produce pollen could help to interpret allergy data and generate information for treatment and prevention (Galan et al., 2007, 2013). Likewise, this information could lead to the selection of the most appropriate olive cultivars to be used as ornamental trees in public gardens due to their low or null pollen production.
An elevated pollen production capacity of olive cultivars is crucial to guarantee an optimum fruit set, especially in new olive-growing areas with no airborne olive pollen from surrounding orchards (Reale et al., 2006; El-Soda et al., 2017). In fact, even in traditional olive-growing areas, the use of several cultivars as pollen donors has been related to a fruit set increase (El-Soda et al., 2017; Rallo et al., 2018; Gencer et al., 2023). Despite these facts, there have been few studies characterizing the pollen production capacity of olive cultivars and its possible variability over years (Tous et al., 2004; Mazzeo et al., 2014; Rojo et al., 2015). Some studies showed high pollen production variability among olive cultivars but screened a restricted number of genotypes during a limited time (Tormo-Molina et al., 1996; Cuevas and Polito, 2004; Ferrara et al., 2007; Mazzeo et al., 2014). Remarkably, the presence of several androsterile cultivars was also detected, but the pervasiveness, stability and cause of this character were not studied (Besnard et al., 2000; Tous et al., 2004).
Several factors, such as genotype and climatic and meteorological conditions, determine olive pollen production (Tous et al., 2004; Rojo et al., 2015). Specifically, temperature, water availability and solar radiation are some environmental variables causing alterations in olive vegetative and floral development (Rapoport, 2014; Benlloch-González et al., 2018; Navas-Lopez et al., 2019). In addition, flowering intensity and consequently pollen production per tree are strongly and negatively affected by the previous year’s fruit load, especially in species with a marked alternate bearing behaviour, such as olive (Famiani et al., 2019). However, the possible effect of biennial bearing on the amount of pollen grains per anther has been scarcely tested in olive and other fruit species (Mazzeo et al., 2014; Delgado et al., 2021). Also, the applicability of pollen per anther production in yield forecasting is controversial in other species with studies of this aspect, such as grapevine (Fernández-González et al., 2011; González-Fernández et al., 2020).
Pollen per anther is one of the most robust metrics to estimate the pollen production of a species (Severova et al., 2022). Pollen production per anther has been studied in fruit species, such as the apple tree (Javid and Rather, 2019; Delgado et al., 2021) and mango (Pérez et al., 2019), and in numerous forest species (Hidalgo et al., 1999; Gómez-Casero et al., 2004; Fernández-González et al., 2020; Katz et al., 2020) and grasses (Severova et al., 2022; Prieto-Baena et al., 2003). In olive, some studies have estimated pollen production per anther using different methodologies but always in a limited panel of genotypes (Orlandi et al., 2003; Tous et al., 2004; Cuevas and Polito, 2004; Ferrara et al., 2007; Palasciano et al., 2008; Aguilera and Valenzuela, 2012; Mazzeo et al., 2014; Rojo et al., 2015).
This study sought to examine the pollen production per anther of 57 of the most representative and economically significant olive cultivars worldwide over three years to (1) characterize the agronomical aptitude of the cultivars as pollen donors; (2) estimate the interannual variability in anther pollen production per cultivar as well as the influence of the genotype, the fruit load and the year; and (3) classify the olive cultivars according to their pollen production capacity.
MATERIALS AND METHODS
Location and plant material
This study was carried out in the World Olive Germplasm Bank (WOGB) – University of Cordoba (UCO) collection, located on the Campus of Rabanales in the UCO (Cordoba, Spain, 37°55ʹ56.5″N, 4°43ʹ13.3″W and 173 m a.s.l.). The WOGB includes more than 450 cultivars, and each cultivar is represented by two replicated trees. Olives were planted in 2011 and grown under drip irrigation (2000 m3 per hectare and year) at a spacing of 7 × 6 m. The entire collection was identified molecularly and morphologically (Trujillo et al., 2013). The sanitary status of the olive plants was tested before planting (Morello et al., 2016).
Pollen production was evaluated in 57 olive cultivars, with two trees per cultivar, during three consecutive years, from 2019 to 2021. The olive cultivars were selected according to their economic importance, genetic diversity (nuclear and chloroplastic) and distribution in the Mediterranean Basin (Besnard et al., 2013; Díez et al., 2015), with particular emphasis on cultivars of Spanish origin (Table 1). We also selected only those cultivars with both trees with the same bearing status, ‘on’ or ‘off’. Seven additional varieties from different areas of the Mediterranean Basin (‘Cerasuola’, ‘Chemlal de Kabilye’, ‘Escarabajuelo de Posadas’, ‘Frantoio’, ‘Koroneiki’, ‘Racimal de Jaén’ and ‘Sikitita’) were evaluated in 2020 and 2021 with the aim of expanding the genetic diversity of the studied cultivars.
Table 1.
List of the 57 olive cultivars under evaluation and their countries of origin, genetic cluster and chlorotypes according to previous studies.
| Cultivar name | Country | Genetic cluster† (Díez et al., 2015) |
Chlorotype‡ (Besnard et al. 2013) |
|---|---|---|---|
| ‘Adkam’ | SYR | Q3 | NA¢ |
| ‘Alameño Blanco’ | SP | Q1 | E1.1 |
| ‘Arbequina’ | SP | Q2 | E1.1 |
| ‘Arbosana’ | SP | Mosaic | E1.1 |
| ‘Belluti’ | TUR | Q3 | E1.1 |
| ‘Blanqueta’ | SP | Q2 | E1.3 |
| ‘Bolvino’ | SP | Q2 | E1.1 |
| ‘Borriolenca’ | SP | Q2 | E3.1 |
| ‘Buidiego’ | SP | Q1 | NA |
| ‘Canetera’ | SP | Q2 | NA |
| ‘Carrasqueño de Porcuna’ | SP | Q1 | NA |
| ‘Cerasuola’ | ITA | NA | E3.2 |
| ‘Chemlal de Kabilye’ | DZA | Mosaic | E3.2 |
| ‘Arracada de Aldover’ | SP | Q2 | E3.1 |
| ‘Curivell’ | SP | Mosaic | NA |
| ‘Empeltre’ | SP | Q2 | E1.1 |
| ‘Escarabajuelo de Posadas’ | SP | Q1 | E1.1 |
| ‘Farga’ | SP | Q2 | E3.1 |
| ‘Fishomi’ | IRA | Q3 | NA |
| ‘Frantoio’ | ITA | Q2 | E1.1 |
| ‘Fulla de Salce’ | SP | Mosaic | E3.1 |
| ‘Genotype 92’* | SP | NA | NA |
| ‘Grosal Vimbodí’ | SP | Q2 | NA |
| ‘Hemblasi’ | SYR | Q3 | E1.1 |
| ‘Hojiblanca’ | SP | Q1 | E1.1 |
| ‘Imperial de Jaén’ | SP | Q1 | NA |
| ‘Joanenca’ | SP | Mosaic | NA |
| ‘Kato Drys’ | CYP | Q3 | E1.1 |
| ‘Koroneiki’ | GRC | Q2 | E1.1 |
| ‘Llumeta’ | SP | Q2 | E3.1 |
| ‘Loaime’ | SP | Q1 | NA |
| ‘Manzanilla de Sevilla’ | SP | Q1 | E1.1 |
| ‘Marfil’ | SP | NA | NA |
| ‘Mari’ | IRA | Q3 | NA |
| ‘Mawi’ | SYR | Q3 | NA |
| ‘Menya’ | SP | Q2 | NA |
| ‘Morona’ | SP | Q1 | NA |
| ‘Morrut’ | SP | Mosaic | E1.1 |
| ‘Palomar’ | SP | Mosaic | NA |
| ‘Perafort’ | SP | Mosaic | NA |
| ‘Picual’ | SP | Q1 | E1.1 |
| ‘Picual Blanco de Estepa’ | SP | Q1 | NA |
| ‘Racimal de Jaén’ | SP | Q1 | E1.1 |
| ‘Royal de Sabiñán’ | SP | Q2 | NA |
| ‘Safrawi’ | SYR | Q3 | NA |
| ‘Sevillenca’ | SP | Mosaic | E1.1 |
| ‘Shami’ | SYR | Q3 | E1.1 |
| ‘Shengue’ | IRA | Q3 | NA |
| ‘Sikitita’ | SP | NA | NA |
| ‘Toffahi’ | EGY | Mosaic | E1.2 |
| ‘Vallesa’ | SP | Mosaic | NA |
| ‘Vera’ | SP | Q2 | NA |
| ‘Verdal de Manresa’ | SP | Mosaic | NA |
| ‘Verdala’ | SP | Q1 | E1.1 |
| ‘Verdale’ | FRA | Mosaic | E1.1 |
| ‘Verdiell’ | SP | Q2 | E1.1 |
| ‘Villalonga’ | SP | Mosaic | E1.3 |
NA, not available.
*Genotype 92: cultivar from UCO breeding programme.
†Genetic clusters: Q1, Western Mediterranean Basin; Q2, Central Mediterranean Basin; Q3, Eastern Mediterranean Basin and Mosaics = admixture between clusters.
‡Maternal genetic lineage.
The area of the collection is typically Mediterranean with dry summers and mild winters. Daily meteorological data were obtained from the European Climate Assessment and Dataset (ECA&D) project, which provides gridded data at a resolution of 0.1° latitude and 0.1° longitude. The data are available on the ECA&D website at http://www.ecad.eu/. The average annual temperature from 2019 to 2021 was 18.1 °C, with average high and low temperatures of 25.2 and 11.1 °C, respectively. The average annual precipitation was 323, 407 and 364 mm in 2019, 2020 and 2021, respectively (Supplementary Data Table S1).
Fruit load and pollen production per anther
Fruit load intensity was assessed every year by estimating the percentage of the canopy covered by fruits using a visual scale from 0 to 3, where 0 indicated the absence of fruits; 1 indicated fruits covering ≤33 % of the canopy; 2 indicated fruits occupying 33–66 % of the canopy; and 3 indicated fruits covering >66 % of the canopy (Díez et al., 2016).
The number of pollen grains per anther was estimated according to Cruden (1977). Briefly, for each tree we selected three different inflorescences immediately before anthesis and collected three mature hermaphrodite flowers per inflorescence in the same phenological stage; hence, we sampled 9 flowers per tree and 18 flowers per cultivar (two trees per cultivar were analysed). One undehisced anther from each mature flower was extracted and crushed in 100 µL of an aqueous fuchsin solution. Of this, 10 µL was deposited on a slide, and, once water had been evaporated with a heater, it was stained with fuchsin-stained glycerine gelatine to facilitate pollen grain counting according to Galan et al. (2007). Finally, the slides were sealed with coverslips and transparent nail polish. Pollen grain counts were performed under an optical microscope (Eclipse 80i, Nikon, Tokyo, Japan). Pollen grains were counted on each slide by scanning until reaching a minimum of 300 pollen grains. The total number of pollen grains per anther was calculated as the product of pollen grain counts (Pc) and the total surface area of the coverslip (Sc) divided by eyepiece diameter (Ed), length of a scan (Ls) and number of scans (Ns).
Statistical analysis
The pollen count data per anther were analysed using Statistix software (version 10; Statistix, Tallahassee, FL, USA) and the R statistical software package. Given that our data did not satisfy the requirements of parametric tests (Shapiro‒Wilk test; P < 0.05), we used a standard generalized linear model (GLM) approach to study the influence of genotype and sampling year on the amount of pollen produced by different cultivars. The contribution of each factor (cultivar, year, previous-year fruit load and the interactions between factors) to the overall explained variance was calculated as the partial ω2 value (ωp2) (Yigit and Mendes, 2018). However, the partial η2 values (ηp2) are also presented due to their wide use to estimate the proportion of population variance explained. Subsequently, we applied Bonferroni-corrected comparisons to examine the main effects and possible interactions between cultivars and the Kolmogorov–Smirnov test to determine the year’s impact on pollen production. Additionally, the K-means clustering method was used for cluster analysis to group cultivars based on their pollen production capacity. It should be noted that the two trees examined per cultivar were considered as a single individual since they were clones, and no statistically significant difference in pollen production was found between them.
RESULTS
Pollen production variability
Pollen production was highly variable among olive cultivars. Among the 57 evaluated cultivars, we found excellent pollen donors, while other cultivars did not produce pollen at all. For instance, ‘Alameño Blanco’ and ‘Mawi’, from Spain and Syria, respectively, were the largest pollen donors (>65 000 pollen grains per anther), along with the widely grown cultivar ‘Arbequina’. Other major cultivars, such as ‘Arbosana’ and ‘Manzanilla de Sevilla’ from Spain and ‘Frantoio’ from Italy, showed high pollen production (from 44 000 to 60 000 pollen grains per anther). In addition, important cultivars such as ‘Hojiblanca’ from Spain and ‘Koroneiki’ from Greece showed a medium pollen production capacity (44 402 and 43 150 pollen grains per anther, respectively). In contrast, some local cultivars, such as the Spanish ‘Royal de Sabiñán’ and ‘Racimal de Jaén’ cultivars, did not exceed the average value of 23 000 pollen grains per anther and had a coefficient of variation >90 % between years. Notably, 12 cultivars from Spain, one from Italy and another from Algeria behaved as androsterile and produced no pollen during the three years (Table 2). Therefore, we could consider the androsterility of these cultivars as a stable character.
Table 2.
Summary of average pollen grain production per anther of the 57 olive cultivars screened during three consecutive crop seasons and their classification into significant groups (Bonferroni test; P < 0.05).
| Cultivar | Average | Maximum | Minimum | Standard deviation | Standard error | CV (%) | Significant groups* |
|---|---|---|---|---|---|---|---|
| Androsterile cultivars† | 0 | 0 | 0 | 0 | 0 | 0 | A |
| ‘Royal de Sabiñán’ | 15 946 | 69 240 | 0 | 18 861 | 2 567 | 118 | AB |
| ‘Racimal de Jaén’ | 22 752 | 85 320 | 0 | 21 054 | 3 509 | 93 | ABC |
| ‘Kato Drys’ | 33 632 | 99 120 | 0 | 23 443 | 3 190 | 70 | ABCD |
| ‘Shami’ | 36 786 | 78 360 | 3 860 | 16 279 | 2 215 | 44 | ABCDE |
| ‘Carrasqueño de Porcuna’ | 43 599 | 66 360 | 7 630 | 16 168 | 2 695 | 37 | ABCDEF |
| ‘Koroneiki’ | 43 150 | 81 000 | 0 | 22 679 | 3 780 | 53 | ABCDEF |
| ‘Sikitita’ | 43 121 | 80 280 | 90 | 20 811 | 3 468 | 48 | ABCDEF |
| ‘Joanenca’ | 40 323 | 136 680 | 0 | 33 254 | 4 525 | 82 | BCDE |
| ‘Adkam’ | 48 191 | 93 480 | 13 800 | 20 020 | 2 724 | 42 | BCDEF |
| ‘Belluti’ | 55 197 | 125 640 | 12 800 | 26 635 | 3 625 | 48 | BCDEF |
| Bolvino’ | 50 136 | 100 920 | 9 990 | 21 471 | 2 922 | 43 | BCDEF |
| ‘Empeltre’ | 52 118 | 124 680 | 7 300 | 28 961 | 3 941 | 56 | BCDEF |
| ‘Escarabajuelo de Posadas’ | 58 634 | 131 400 | 12 210 | 34 388 | 8 105 | 59 | BCDEF |
| ‘Frantoio’ | 59 267 | 128 640 | 20 520 | 27 946 | 4 658 | 47 | BCDEF |
| ‘Hojiblanca’ | 44 402 | 197 400 | 11 970 | 29 125 | 3 963 | 66 | BCDEF |
| ‘Imperial de Jaén’ | 43 479 | 101 760 | 610 | 20 031 | 2 726 | 46 | BCDEF |
| ‘Loaime’ | 47 171 | 163 320 | 5 370 | 31 239 | 4 251 | 66 | BCDEF |
| ‘Picual Blanco de Estepa’ | 44 565 | 103 440 | 13 920 | 21 762 | 2 961 | 49 | BCDEF |
| ‘Mari’ | 47 490 | 109 560 | 13 230 | 20 636 | 2 808 | 43 | BCDEF |
| ‘Morona’ | 40 969 | 107 520 | 0 | 23 476 | 3 195 | 57 | BCDEF |
| ‘Picual’ | 44 818 | 82 680 | 11 430 | 20 968 | 2 853 | 47 | BCDEF |
| ‘Shengue’ | 44 387 | 102 000 | 5 970 | 19 770 | 2 690 | 45 | BCDEF |
| ‘Toffahi’ | 48 133 | 131 760 | 9 600 | 25 347 | 3 449 | 53 | BCDEF |
| ‘Verdala’ | 48 974 | 136 440 | 4 640 | 30 122 | 4 099 | 62 | BCDEF |
| ‘Verdale’ | 45 914 | 97 800 | 630 | 20 755 | 3 459 | 45 | BCDEF |
| ‘Verdiell’ | 48 129 | 141 840 | 9 630 | 24 918 | 3 391 | 52 | BCDEF |
| ‘Villalonga’ | 52 117 | 254 400 | 0 | 43 073 | 5 862 | 83 | BCDEF |
| ‘Arbosana’ | 59 012 | 226 080 | 4 600 | 49 973 | 6 800 | 85 | CDEF |
| ‘Blanqueta’ | 60 574 | 146 880 | 11 910 | 30 503 | 4 151 | 50 | CDEF |
| ‘Buidiego’ | 58 910 | 112 440 | 13 320 | 25 477 | 3 467 | 43 | CDEF |
| ‘Fishomi’ | 60 746 | 139 080 | 18 120 | 30 710 | 4 179 | 51 | CDEF |
| ‘Hemblasi’ | 57 249 | 116 640 | 11 490 | 25 777 | 3 508 | 45 | CDEF |
| ‘Manzanilla de Sevilla’ | 58 072 | 121 920 | 9 390 | 30 214 | 4 112 | 52 | CDEF |
| ‘Marfil’ | 61 167 | 185 760 | 14 760 | 29 499 | 4 014 | 48 | CDEF |
| ‘Menya’ | 62 121 | 141 000 | 7 370 | 30 222 | 4 113 | 49 | CDEF |
| ‘Morrut’ | 56 956 | 119 760 | 12 570 | 27 765 | 3 778 | 49 | CDEF |
| ‘Perafort’ | 57 957 | 116 760 | 2 610 | 25 417 | 3 459 | 44 | CDEF |
| ‘Safrawi’ | 61 018 | 183 840 | 13 200 | 34 268 | 4 663 | 56 | CDEF |
| ‘Sevillenca’ | 56 345 | 176 640 | 10 710 | 32 868 | 4 473 | 58 | CDEF |
| ‘Grossal Vimbodí’ | 67 912 | 184 800 | 6 660 | 35 636 | 4 849 | 52 | DEF |
| ‘Arbequina’ | 76 561 | 155 760 | 9 900 | 33 544 | 4 565 | 44 | EF |
| ‘Mawi’ | 76 744 | 221 760 | 18 060 | 39 358 | 5 356 | 51 | EF |
| ‘Alameño Blanco’ | 80 701 | 267 840 | 18 120 | 48 715 | 6 629 | 60 | F |
*The letters indicate the homogeneous groups according to Bonferroni correction (P < 0.05). Different letters indicate significant differences according to Bonferroni correction.
†Non-pollen-producing cultivars: ‘Borriolenca’, ‘Canetera’, ‘Cerausola’, ‘Chemlal de Kabylye’, ‘Arracada de Aldover’, ‘Curivell’, ‘Farga’, ‘Fulla de Salce’, ‘Genotype 92’, ‘Llumeta’, ‘Palomar’, ‘Vallesa’, ‘Vera’, ‘Verdal de Manresa’.
Generally, the amount of pollen per anther and cultivar followed a normal distribution (Fig. 1). However, that of ‘Joanenca’, ‘Kato Drys’, ‘Royal de Sabiñán’, ‘Racimal de Jaén’ and ‘Villalonga’ showed a bimodal distribution due to the production of contrasting amounts of pollen per anther, i.e. anthers with large amounts of pollen (>100 000 pollen grains) and others with low or null pollen (<1000 pollen grains). Other cultivars, such as ‘Koroneiki’ and ‘Morona’, occasionally showed some anthers with no pollen, but this phenomenon was not pervasive. Remarkably, ‘Verdale’ and ‘Carrasqueño de Porcuna’ did not produce flowers in 2019 and 2020, respectively, due to a marked alternate bearing behaviour.
Fig. 1.
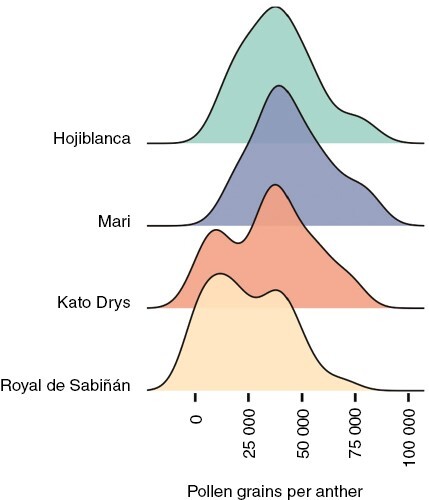
Distribution of olive pollen production during three consecutive years represented as density charts. Cultivars ‘Hojiblanca’ and ‘Mari’ presented a normal distribution, while ‘Kato Drys’ and ‘Royal de Sabiñán’ showed a bimodal distribution.
Factors contributing to pollen production variability
In our study, we evaluated and quantified the possible effect of cultivar (genotype), year, previous-year fruit load (fruit load) and the interaction between these factors on pollen production. To do so, we applied a GLM analysis considering cultivar, year, fruit load and the interactions between them as categorical variables (Table 3).
Table 3.
Generalized linear model analysis of pollen production per anther showing the percentages of variance explained by the evaluated factors [cultivar, interannual variability (year), previous year’s load (fruit load) and their interactions] and their significance level.
| Factor | ω p 2 ( %) | η p 2 ( %) | F ratio | P-value |
|---|---|---|---|---|
| Cultivar | 51.3 | 53.2 | 53.91 | <0.001 |
| Year | 0.3 | 0.1 | 9.53 | <0.01 |
| Fruit load | 0.1 | 0 | 6.48 | <0.05 |
| Cultivar × year | 8.4 | 8.5 | 5.56 | <0.001 |
| Cultivar × fruit load | 1.3 | 3.1 | 1.8 | <0.01 |
| Year × fruit load | 0 | 0 | 0.31 | >0.05 |
| Cultivar × year × fruit load | 1.8 | 2.6 | 4.59 | <0.001 |
| Residual | 36.8 | 32.5 |
ω p 2 and ηp2 are the percentages of pollen production variability explained by each factor and their interaction.
F ratio is the variation between samples/variation within the samples.
Predictably, cultivar was the most determinant factor (GLM test; P < 0.001), explaining >51.3 % of the total variation (ωp2). The effects of year and fruit load on pollen production were also statistically significant (GLM test; P < 0.01 and P < 0.05, respectively) but accounted for <~0.5 % of the variation (ωp2). The cultivar × year interaction (GLM test; P < 0.001) accounted for ~8 % of the variation (ωp2), indicating that the cultivars were not homogeneously affected by year in terms of their capacity to produce pollen. For instance, ‘Carrasqueño de Porcuna’ showed stable pollen production across the three years, while others, such as ‘Arbosana’, produced contrasting amounts of pollen depending on the year. Similarly, the interactions cultivar × fruit load and the three-way interaction cultivar × year × fruit load were significant (GLM test; P < 0.01 and P < 0.001, respectively), but barely contributed ~3 % to the total variation (Supplementary Data Fig. 1).
Global pollen production differed significantly between 2020 and 2021 (Kolmogorov–Smirnov test; P value < 0.01) but neither between 2019 and 2020, nor 2019 and 2021 (Kolmogorov–Smirnov test; P value > 0.05). The highest frequency of pollen production per anther was recorded in 2021, the lowest frequency was recorded in 2020, and the frequency in 2019 was intermediate, with average values of 42 040 ± 36 800, 34 770 ± 31 480 and 38 560 ± 36 910 pollen grains per anther, respectively. The significant effect of year on pollen production was evident when we plotted the frequency distribution of the average pollen production per anther for the whole dataset of cultivars (Fig. 2).
Fig. 2.
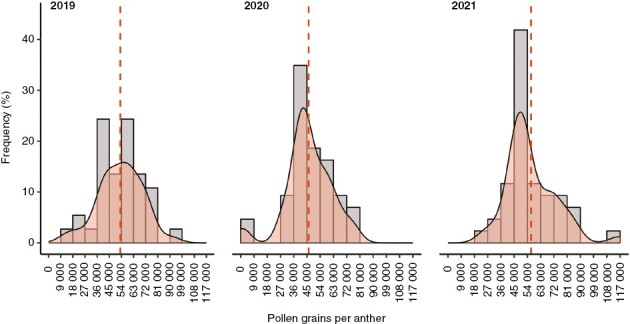
Frequency distribution of pollen production shown by olive cultivars during the three evaluated years. The relative frequency was calculated for the average of all the repetitions for the same cultivar. Each graph shows the mean number of pollen grains per anther (red dashed line; 2019 = 53 228 ± 16 339; 2020, 46 118 ± 15 757; 2021, 56 469 ± 17 052) and density (light grey area).
Classification of cultivars according to their pollen production
Given that cultivar was the most crucial factor determining pollen production, we classified the cultivars according to this feature. To this end, we applied a hierarchical cluster analysis using mean values of pollen production. According to the results, we categorized the olive genotypes into four main clusters: excellent (Cluster I), medium (Cluster II), poor (Cluster III) and androsterile pollen donors (Cluster IV) (Table 4; Fig. 3).
Table 4.
Olive cultivar classification according to pollen grain production per anther, name and group size, and summary statistics.
| Cluster | Cultivar | n | Mean | s.d. | Min | Max |
|---|---|---|---|---|---|---|
| I | ‘Alameño Blanco’, ‘Arbequina’, ‘Arbosana’, ‘Belluti’, ‘Blanqueta’, ‘Buidiego’, ‘Escarabajuelo de Posadas’, ‘Fishomi’, ‘Frantoio’, ‘Grossal Vimbodí’, ‘Hemblasi’, ‘Manzanilla de Sevilla’, ‘Marfil’, ‘Mawi’, ‘Menya’, ‘Morrut’, ‘Perafort’, ‘Safrawi’, ‘Sevillenca’ | 19 | 63 270 | 7490 | 55 190 | 80 700 |
| II | ‘Adkam’, ‘Bolvino’, ‘Carrasqueño de Porcuna’, ‘Empeltre’, ‘Hojiblanca’, ‘Imperial de Jaén’, ‘Joanenca’, ‘Koroneiki’, ‘Loaime’, ‘Mari’, ‘Morona’, ‘Picual’, ‘Picual Blanco de Estepa’, ‘Shami’, ‘Shengue’, ‘Sikitita’, ‘Toffahi’, ‘Verdala’, ‘Verdale’, ‘Verdiell’, ‘Villalonga’ | 21 | 45 610 | 3830 | 36 780 | 52 120 |
| III | ‘Kato Drys’, ‘Racimal de Jaén’, ‘Royal de Sabiñán’ | 3 | 24 110 | 8910 | 15 950 | 33 630 |
| IV | ‘Borriolenca’, ‘Canetera’, ‘Cerasuola’, ‘Chemlal de Kabylye’, ‘Arracada de Aldover’, ‘Curivell’, ‘Farga’, ‘Fulla de Salce’, ‘Genotype 92’, ‘Llumeta’, ‘Palomar’, ‘Vallesa’, ‘Vera’, ‘Verdal de Manresa’ | 14 | 0 | 0 | 0 | 0 |
Fig. 3.
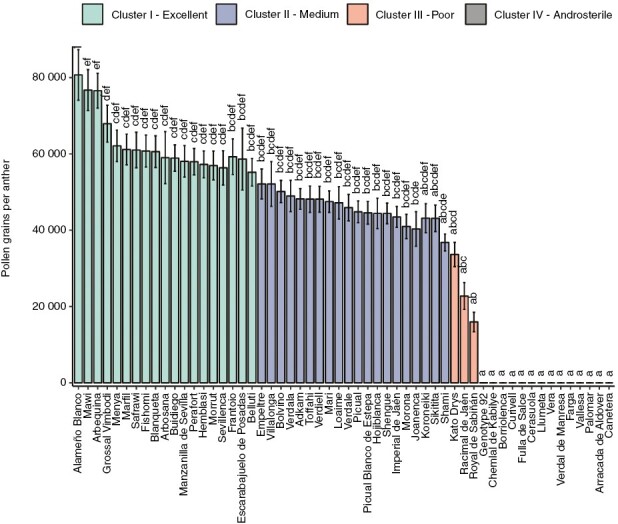
Frequency distribution of average pollen production of the cultivars during the three evaluated years (2019–21). Relative frequency was calculated for the average of all repetitions for the same cultivar. Different colours show the three homogeneous clusters according to their pollen production capacity: Cluster I in blue, Cluster II in green and Cluster III in red. Letters indicate significant differences after Bonferroni correction (P < 0.05).
Cluster I comprised 19 cultivars (~33 %) and showed the highest average count of pollen grains per anther (from 55 190 to 80 700); ‘Arbequina’ and ‘Arbosana’, from Spain, ‘Belluti’ from Turkey and ‘Fishomi’ from Iran were included in this cluster. Cluster II was the most abundant group, with 21 cultivars (~37 %), such as ‘Villalonga’ and ‘Empeltre’, which produced the most pollen (52 117 ± 43 073 and 52 118 ± 28 961 pollen grains per anther, respectively), and important cultivars, such as ‘Mari’ from Iran and ‘Toffahi’ from Egypt. Cluster III was composed of ‘Kato Drys’, ‘Racimal de Jaén’ and ‘Royal de Sabiñán’, which were considered poor pollen donors because they had the lowest pollen counts, with an average value of 24 110 ± 8910 pollen grains/anther. The 14 androsterile cultivars (‘Borriolenca’, ‘Canetera’, ‘Cerasuola’, ‘Chemlal de Kabylye’, ‘Arracada de Aldover’, ‘Curivell’, ‘Farga’, ‘Fulla de Salce’, ‘Genotype 92’, ‘Llumeta’, ‘Palomar’, ‘Vallesa’, ‘Vera’ and ‘Verdal de Manresa’) (~25 %) produced no pollen in any of the evaluated years and were grouped into Cluster IV. This group had normal flowers, but in many cases their anthers showed a brownish colour instead of being bright yellow (Fig. 4), a characteristic previously mentioned by Riera (1950).
Fig. 4.

Inflorescences and undeveloped pollen grains stained with fuchsin (scale bar = 50 μm) in the androsterile cultivar ‘Vera’ (A and C) and in the excellent pollen donor ‘Arbequina’ (B and D).
We also tested the consistency of these groups over the years. To this end, the K-means algorithm was applied to generate groups considering the average values of pollen production per year. We found that ~37 % of the varieties remained in the same group among the three years, and ~44 % did so in two years. It is worth mentioning that these latter cultivars changed between contiguous categories; for instance, ‘Alameño Blanco’ was classified in Cluster II in 2019 and in Cluster I in 2020 and 2021. On the other hand, the classification of ~19 % of the cultivars was quite variable, with them being eventually included in the three different production categories over the study period. These cultivars strongly contributed to the significant effect of the cultivar × year interaction.
DISCUSSION
Pollen production variability
This study presents the most extensive and exhaustive characterization of pollen production of different olive cultivars. Our results showed that olive has a large degree of variability in its capacity to produce pollen, with genotype being the most determinant factor for this feature, but it is also affected by other factors, such as biennial bearing. This result is in agreement with those of previous studies, which revealed marked differences in pollen production between olive cultivars but considered a restricted number of cultivars and evaluated years (Rallo and Cuevas, 2007; Mazzeo et al., 2014). We found cultivars producing on average >60 000 pollen grains per anther, such as ‘Blanqueta’, ‘Safrawi’ and ‘Grossal Vimbodí’, while others were unable to produce half this amount, such as ‘Royal de Sabiñán’, ‘Racimal de Jaén’ and ‘Kato Drys’. Notably, the pollen production of most cultivars was closer to the upper classification extreme and could be considered medium-high (54 440 ± 5660 pollen grains per anther).
Our average pollen counts per anther were lower than those previously reported when applying the same methodology (Tormo-Molina et al., 1996), but the study used only three olive trees of uncertain status, cultivated or wild. On the other hand, our counts were in line with those of other previous studies (Orlandi et al., 2003; Cuevas and Polito, 2004; Aguilera and Valenzuela, 2012; Rojo et al., 2015), which applied a different methodology based on measuring the pollen of a set of anthers from different flowers as samples (Dafni, 1992). Other studies showed higher counts than ours, but these combined the latter methodology with the use of a Bürker haemocytometer for pollen counting instead of an optical microscope (Ferrara et al., 2007; Palasciano et al., 2008; Mazzeo et al., 2014; Rojo et al., 2015).
Three cultivars, ‘Kato Drys’, ‘Royal de Sabiñán’ and ‘Racimal de Jaén’, presented a significantly low average pollen count. Moreover, in these cultivars we observed a heterogeneous pollen distribution per anther, which gave rise to a bimodal distribution (Fig. 1). This feature was caused by some anthers having an average pollen count of ~24 000 pollen grains per anther, while others showed a reduced or null amount. This phenomenon is called partial male sterility, and it was previously reported as a genetic feature in other olive cultivars, such as ‘Tanche’ (Besnard et al., 2000) and ‘Swan Hill’ (Fernandez-Escobar and Martin, 1986). This phenomenon was also observed in other plant species, such as sunflower, maize, and citrus (Putt and Heiser, 1966; Weider et al., 2009; Raveh et al., 2020).
The most extreme deviation regarding pollen production was observed in 14 cultivars with no pollen counts over the three years of evaluation. Twelve of them were from northeastern Spain and, presumably, shared genetic ancestry according to their phylogenetic relationships (Besnard et al., 2013; Díez et al., 2015). Given these facts, the absence of pollen production could be a character shared by descendants, although further genetic and physiological studies are necessary to corroborate this hypothesis. Most of these cultivars (except ‘Borriolenca’ and ‘Genotype 92’) were previously reported as androsterile elsewhere (Amane et al., 1999; Besnard et al., 2000; Tous et al., 2004). Other cases of olive cultivars with male sterility were also cited in France for the ‘Lucques’ and ‘Tanche’ cultivars, in Algeria for ‘Aaroun’ and ‘Hamra’, and in Tunisia for ‘Zarazi’ (Besnard et al., 2000; Cavallotti et al., 2003). It is worth mentioning that the complete reference genome for the olive tree is from one of the androsterile cultivars, ‘Farga’ (Cruz et al., 2016). Therefore, this feature should be seriously taken into account before considering this cultivar as the standard for the species.
Factors contributing to pollen production variability
Cultivar (genotype) accounted for the largest part of the variability observed in pollen production between olive cultivars, contributing 51.3 % of the variance. A major influence of genotype on pollen production per anther was reported but not quantified in other species, such as haskap berries, sweet cherry and grapes (Bieniasz et al., 2019; Dziedzic et al., 2019; Kowalczyk et al., 2022).
In this study, year had a significant but much weaker influence on the variance, contributing barely 0.3 %. Annual fluctuations in environmental conditions affect pollen production (Raja et al., 2019; Yu et al., 2019); indeed, changes in temperature and precipitation might cause irregularities in microsporogenesis and ultrastructural changes in pollen grains (Chaturvedi et al., 2021; Lamin-Samu et al., 2021; Ullah et al., 2022). For instance, olive pollen develops during the 6–4 weeks prior to flowering (approximately the first week of May); therefore, the temperatures during this time (March and April) are determinants of pollen production (Pacini et al., 1985; Fernández and Rodríguez-García, 1988; Fodale et al., 1994; Ateyyeh et al., 2000). Given the limited number of years evaluated in this study, we were unable to establish any robust relationships between pollen production and meteorological variables. The year with the highest average pollen production (2021) also had the highest accumulated precipitation from January to April, but we did not detect any other remarkable meteorological features able to explain the observed pollen variation between years.
Interestingly, environmental factors do not have the same impact on all cultivars (Lavee, 2007; Medina-Alonso et al., 2020; Fernández-González et al., 2021). In our study, the interaction cultivar × year accounted for 8.4 % of the total variance, considered a medium effect in multifactorial experiments (Cohen, 1988). In line with this result, not all the cultivars were equally stable in pollen production over the years; some of them, such as ‘Adkam’, showed a coefficient of variation (CV) of 42 %, while others, such as ‘Arbosana’ and ‘Villalonga’, showed double this number (CV ≈ 80 %). Longer time series are required to assess the specific cause of this unequal variation. We hypothesize that cultivars with larger CVs have a more unstable behaviour, and thus they could be more susceptible to climate change. However, there are other variables affecting the performance of the tree, such as its nutritional status, which were considered in this study. Remarkably, ‘Joanenca’ and ‘Villalonga’ were reported as androsterile by Tous et al. (2004); however, we classified them as partially male-sterile because they showed pollen production, although it was reduced and heterogeneous. This phenomenon, reported in other species, might be triggered by specific climatic conditions, such as heat stress (Begcy et al., 2019; Li et al., 2022).
Biennial bearing affects the number of fruits, although previously it was found to affect the number of flowers (Rallo and Cuevas, 2007; Seifi et al., 2015; Maniriho, 2022) and of pollen grains per anther (Mazzeo et al., 2014). We confirmed this latter observation; the previous year’s fruit load (GLM test; P < 0.05), as well as its interactions with cultivar and cultivar × year, were significant (GLM test; P < 0.01 and P < 0.001, respectively). However, the contribution of these categorical variables to the overall variance barely exceeded 3 %. Resource limitation before inflorescence formation caused by a previous heavy fruit load affects the number of inflorescences, the number of flowers per inflorescence, the floral quality and fruit set (Lavee et al., 1996; Cuevas and Polito, 2004; Rallo and Cuevas 2007). According to Mazzeo et al. (2014), olive compensates for the lower pollen production in ‘off’ years by increasing pollen viability. However, studies in other fruit species, such as apple (Delgado et al., 2021) and apricot (Gallotta et al., 2014), did not find significant differences in pollen production per anther between ‘on’ and ‘off’ years. These discrepancies highlight the need for further research to fully understand the effect of biennial bearing on pollen production in perennial fruit crops.
Classification of cultivars according to their pollen production
The major and more significant effect of cultivar on pollen production allowed us to apply a K-means clustering analysis to classify the cultivars according to this feature. As a result, we classified the evaluated cultivars into four groups: excellent (Cluster I), medium (Cluster II), poor (Cluster III) and androsterile (Cluster IV) pollen donors. We did not detect any previously documented genetic relationship between the cultivars included in every cluster, other than the one mentioned above for androsterile cultivars.
Cluster I included 19 cultivars characterized by the highest pollen production, such as ‘Arbequina’ and ‘Arbosana’, in agreement with the findings of previous studies that already highlighted their outstanding capacity as pollen donors (Ferrara et al., 2007). These cultivars are the most propagated by the nursery industry because of their excellent performance in super-intensive orchards, which constitute the majority of new plantations worldwide (Díez et al., 2016; Rallo et al., 2018). Cluster II included 21 cultivars with medium pollen production, comprising widely spread cultivars, such as ‘Picual’, with >1 000 000 ha in Spain, and ‘Koroneiki’, the main cultivar in Greece (Rallo et al., 2018). Cluster III was composed of ‘Kato Drys’, the main crop in Cyprus, and the Spanish cultivars ‘Racimal de Jaén’ and ‘Royal de Sabiñán’, with little diffusion outside their presumed areas of origin.
Finally, Cluster IV included androsterile cultivars such as ‘Farga’ and ‘Chemlal de Kabylye’, cultivars found in Spain and Algeria, despite its inability to produce pollen. On the other hand, ‘Genotype 92’ was a chance seedling from the UCO breeding programme, being androsterile but also presenting staminate flowers and therefore being unable to produce fruits. These two characteristics make ‘Genotype 92’ a highly valuable variety in an ornamental context (Ashok and Velmurugan, 2020; Culley et al., 2022).
Our classification of olive cultivars according to their aptitude as pollen donors is essential for designing new plantations, especially in non-traditional production countries. In this context, the compatibility between cultivars must also be considered to guarantee the plantation’s success. Olive shows pollen–pistil self-incompatibility, with cultivars apparently grouped into two proposed compatibility groups (i.e. cross-pollination between individuals of the same group causes a response of incompatibility) (Saumitou-Laprade et al., 2010, 2017; Alagna et al., 2019); however, this hypothesis is still controversial (Farinelli et al., 2018; Breton et al., 2021).
Our results contribute to increasing our knowledge about olive cultivar performance, giving rise to new questions regarding olive pollen development, fruit set and floral physiology. Indeed, further studies are needed to complete information regarding possible differences in pollen quality parameters (such as viability) between cultivars, as it was outlined by other authors (Rejón et al., 2010; Ribeiro et al., 2012). Additionally, we aimed to determine the physiological cause of the androsterility observed in some cultivars and to reveal the effect of elevated temperatures on pollen production, pollen quality and possible differences between cultivars.
Conclusions
We found important and significant variability in pollen production per anther within cultivated olive. In fact, genotype accounted for the largest proportion of the variance, followed by year and the previous year’s fruit load. Remarkably, the interaction between the three factors was significant, particularly between cultivar and year, indicating that the stability of pollen production was not a pervasive character. Our results allowed us to classify the olive cultivars into four clusters according to their capacity to produce pollen. Interestingly, the fourth group was composed of androsterile cultivars, which could share a common origin. This study provides novel and useful information for the establishment of new olive plantations and creates new lines of inquiry for the characterization of olive pollen development, quality and floral physiology.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following. Table S1: pollen production and summary of the air temperatures and precipitation during the months prior to flowering in the three evaluated years. Figure S1: distribution of cultivar pollen production per anther according to their previous-year yield.
ACKNOWLEDGEMENTS
We thank Pablo Morello, curator of the World Olive Germplasm Bank of Cordoba – University of Cordoba Collection, for his assistance and help during the sampling of the trees.
Contributor Information
M Rojas-Gómez, Department of Agronomy, Agrifood Campus of International Excellence CeiA3, University of Cordoba, Rabanales Campus, Celestino Mutis Building, E-14071, Córdoba, Spain; Department of Botany, Ecology and Plant Physiology, Agrifood Campus of International Excellence CeiA3, University of Cordoba, Rabanales Campus, Celestino Mutis Building, E-14071, Córdoba, Spain.
J Moral, Department of Agronomy, Agrifood Campus of International Excellence CeiA3, University of Cordoba, Rabanales Campus, Celestino Mutis Building, E-14071, Córdoba, Spain.
R López-Orozco, Department of Botany, Ecology and Plant Physiology, Agrifood Campus of International Excellence CeiA3, University of Cordoba, Rabanales Campus, Celestino Mutis Building, E-14071, Córdoba, Spain; Andalusian Inter-University Institute for Earth System IISTA, University of Cordoba, E-14071, Córdoba, Spain.
D Cabello, Department of Agronomy, Agrifood Campus of International Excellence CeiA3, University of Cordoba, Rabanales Campus, Celestino Mutis Building, E-14071, Córdoba, Spain.
J Oteros, Department of Botany, Ecology and Plant Physiology, Agrifood Campus of International Excellence CeiA3, University of Cordoba, Rabanales Campus, Celestino Mutis Building, E-14071, Córdoba, Spain; Andalusian Inter-University Institute for Earth System IISTA, University of Cordoba, E-14071, Córdoba, Spain.
D Barranco, Department of Agronomy, Agrifood Campus of International Excellence CeiA3, University of Cordoba, Rabanales Campus, Celestino Mutis Building, E-14071, Córdoba, Spain.
C Galán, Department of Botany, Ecology and Plant Physiology, Agrifood Campus of International Excellence CeiA3, University of Cordoba, Rabanales Campus, Celestino Mutis Building, E-14071, Córdoba, Spain; Andalusian Inter-University Institute for Earth System IISTA, University of Cordoba, E-14071, Córdoba, Spain.
C M Díez, Department of Agronomy, Agrifood Campus of International Excellence CeiA3, University of Cordoba, Rabanales Campus, Celestino Mutis Building, E-14071, Córdoba, Spain.
FUNDING
This work was supported by the grant Gen4Olive (Ref. H2020-IA-SFS-2020-101000427).
LITERATURE CITED
- Aguilera F, Valenzuela L.. 2012. Microclimatic-induced fluctuations in the flower and pollen production rate of olive trees (Olea europaea L). Grana 51: 228–239. [Google Scholar]
- Alagna F, Caceres ME, Pandolfi S, et al. 2019. The paradox of self-fertile varieties in the context of self-incompatible genotypes in olive. Frontiers in Plant Science 10: 725. https://www.frontiersin.org/articles/10.3389/fpls.2019.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alché J, Castro A, Jiménez-López J, et al. 2007. Differential characteristics of olive pollen from different cultivars: biological and clinical implications. Journal of Investigational Allergology and Clinical Immunology 17: 7. [PubMed] [Google Scholar]
- Amane M, Lumaret R, Hany V, et al. 1999. Chloroplast-DNA variation in cultivated and wild olive (Olea europaea L). Theoretical and Applied Genetics 99: 133–139. [Google Scholar]
- Ashok A, Velmurugan S.. 2020. Review on seed production techniques in flowering ornamentals. Journal of Pharmacognosy and Phytochemistry 9: 190–198. [Google Scholar]
- Ateyyeh AF, Stösser R, Qrunfleh M.. 2000. Reproductive biology of the olive (Olea europaea L) cultivar ‘Nabali Baladi’. Journal of Applied Botany and Food Quality 74: 255–270. [Google Scholar]
- Begcy K, Nosenko T, Zhou L-Z, Fragner L, Weckwerth W, Dresselhaus T.. 2019. Male sterility in maize after transient heat stress during the tetrad stage of pollen development. Plant Physiology 181: 683–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlloch-González M, Sánchez-Lucas R, Benlloch M, Fernández-Escobar R.. 2018. An approach to global warming effects on flowering and fruit set of olive trees growing under field conditions. Scientia Horticulturae 240: 405–410. [Google Scholar]
- Besnard G, Khadari B, Villemur P, Bervillé A.. 2000. Cytoplasmic male sterility in the olive (Olea europaea L). Theoretical and Applied Genetics 100: 1018–1024. [Google Scholar]
- Besnard G, Khadari B, Navascués M, et al. 2013. The complex history of the olive tree: from Late Quaternary diversification of Mediterranean lineages to primary domestication in the northern Levant. Proceedings Biological Sciences 280: 20122833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieniasz M, Dziedzic E, Słowik G.. 2019. Biological features of flowers influence the fertility of Lonicera spp. cultivars. Horticulture, Environment and Biotechnology 60: 155–166. doi: 10.1007/s13580-018-0110-3. [DOI] [Google Scholar]
- Bousquet J, Cour P, Guerin B, Michel FB.. 1984. Allergy in the Mediterranean area I. Pollen counts and pollinosis of Montpellier. Clinical & Experimental Allergy 14: 249–258. [DOI] [PubMed] [Google Scholar]
- Breton CM, Farinelli D, Koubouris G, Famiani F, Raymond M, Bervillé A.. 2021. A dual-successive-screen model at pollen/stigma and pollen tube/ovary explaining paradoxical self-incompatibility diagnosis in the olive tree—an interpretative update of the literature. Plants 10: 1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallotti A, Regina TMR, Quagliariello C.. 2003. New sources of cytoplasmic diversity in the Italian population of Olea europaea L. as revealed by RFLP analysis of mitochondrial DNA: characterization of the cox3 locus and possible relationship with cytoplasmic male sterility. Plant Science 164: 241–252. [Google Scholar]
- Chaturvedi P, Wiese AJ, Ghatak A, Záveská Drábková L, Weckwerth W, Honys D.. 2021. Heat stress response mechanisms in pollen development. New Phytologist 231: 571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. 1988. Statistical power analysis for the behavioral sciences, 2nd edn. New York: Routledge. [Google Scholar]
- Cruden RW. 1977. Pollen-ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution 31: 32–46. [DOI] [PubMed] [Google Scholar]
- Cruden RW. 2000. Pollen grains: why so many? In: Dafni A., Hesse M., Pacini E, (Eds.), Pollen and pollination Vienna: Springer, 143–165. [Google Scholar]
- Cruz F, Julca I, Gómez-Garrido J, et al. 2016. Genome sequence of the olive tree, Olea europaea. GigaScience 5: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas J, Polito VS.. 2004. The role of staminate flowers in the breeding system of Olea europaea (Oleaceae): an andromonoecious, wind‐pollinated taxon. Annals of Botany 93: 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley TM, Dreisilker K, Clair Ryan M, et al. 2022. The potential role of public gardens as sentinels of plant invasion. Biodiversity and Conservation 31: 1845–1846. [Google Scholar]
- D’Amato G. 1998. Pollen allergy in the Mediterranean area. Revue Française d’Allergologie et d’Immunologie Clinique 38: S160–S162. [Google Scholar]
- Dafni A. 1992. Pollination ecology: a practical approach. Brittonia 46: doi: 10.2307/2807163. [DOI] [Google Scholar]
- Delgado A, Quinet M, Dapena E.. 2021. Analysis of the variability of floral and pollen traits in apple cultivars—selecting suitable pollen donors for cider apple orchards. Agronomy 11: 1717. [Google Scholar]
- Díaz A, Martín A, Rallo P, Barranco D, Rosa R.. 2006. Self-incompatibility of ‘Arbequina’ and ‘Picual’ olive assessed by SSR markers. Journal of the American Society for Horticultural Science 131: 250–255. [Google Scholar]
- Díez CM, Trujillo I, Martinez-Urdiroz N, et al. 2015. Olive domestication and diversification in the Mediterranean Basin. New Phytologist 206: 436–447. [DOI] [PubMed] [Google Scholar]
- Díez CM, Moral J, Cabello D, Morello P, Rallo L, Barranco D.. 2016. Cultivar and tree density as key factors in the long-term performance of super high-density olive orchards. Frontiers in Plant Science 7: 1226. https://www.frontiersin.org/articles/10.3389/fpls.2016.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzic E, Bieniasz M, Kowalczyk B.. 2019. Morphological and physiological features of sweet cherry floral organ affecting the potential fruit crop in relation to the rootstock. Scientia Horticulturae 251: 127–135. [Google Scholar]
- El-Soda A, El-Husseiny A, Hammad A.. 2017. Effect of using some pollinators on productivity of some olive cultivars. Egyptian Journal of Agricultural Sciences 68: 397–409. [Google Scholar]
- Famiani F, Farinelli D, Gardi T, Rosati A.. 2019. The cost of flowering in olive (Olea europaea L). Scientia Horticulturae 252: 268–273. [Google Scholar]
- FAOSTAT Statistical Database. 2020. Food and Agriculture Organization of the United Nations. Rome. https://www.fao.org/faostat/en/#data/QCL/visualize (March 2023, date last accessed).
- Farinelli D, Tombesi A, Hassani D.. 2008. Self-sterility and cross-pollination responses of nine cultivars in central Italy. Acta Horticulturae 791: 127–136. [Google Scholar]
- Fernández MC, Rodríguez-García MI.. 1988. Pollen wall development in Olea europaea L. New Phytologist 108: 91–99. [DOI] [PubMed] [Google Scholar]
- Fernández MC, Rodríguez-García MI.. 1989. Developmental changes in the aperture during pollen grain ontogeny in Olea europaea L. New Phytologist 111: 717–723. [DOI] [PubMed] [Google Scholar]
- Fernandez-Escobar R, Martin G.. 1986. ‘Swan Hill’ as an ornamental olive cultivar. California Agriculture 40: 18–18. [Google Scholar]
- Fernández-González M, Rodríguez-Rajo FJ, Jato V, Escuredo O, Aira MJ.. 2011. Estimation of yield ‘Loureira’ variety with an aerobiological and phenological model. Grana 50: 63–72. [Google Scholar]
- Fernández-González M, González-Fernández E, Ribeiro H, Abreu I, Rodríguez-Rajo FJ.. 2020. Pollen production of Quercus in the North-Western Iberian Peninsula and airborne pollen concentration trends during the last 27 years. Forests 11: 702. [Google Scholar]
- Fernández-González M, Ribeiro H, Pereira SG, Rodríguez-Rajo FJ, Abreu I.. 2021. Pollen Ole e 1 content variations in olive cultivars of different Portugal regions. Aerobiologia 37: 205–216. [Google Scholar]
- Ferrara G, Camposeo S, Palasciano M, Godini A.. 2007. Production of total and stainable pollen grains in Olea europaea L. Grana 46: 85–90. [Google Scholar]
- Fodale AS, Mulè R, Iannotta N, Tucci A.. 1994. Development of the pollen grain in Olea europaea L. Acta Horticulturae 356: 249–251. [Google Scholar]
- Galán C, Vázquez L, García-Mozo H, Domínguez E.. 2004. Forecasting olive (Olea europaea) crop yield based on pollen emission. Field Crops Research 86: 43–51. [Google Scholar]
- Galan C, Cariñanos P, Alcázar P.Domínguez E.. 2007. Spanish Aerobiology Network (REA): Management and quality manual. Cordoba: Servicio de Publicaciones de la Universidad de Córdoba. [Google Scholar]
- Galan C, Antunes C, Brandao R, et al.;HIALINE working group. 2013. Airborne olive pollen counts are not representative of exposure to the major olive allergen Ole e 1. Allergy 68: 809–812. [DOI] [PubMed] [Google Scholar]
- Gallotta A, Palasciano M, Mazzeo A, Ferrara G.. 2014. Pollen production and flower anomalies in apricot (Prunus armeniaca L.) cultivars. Scientia Horticulturae 172: 199–205. [Google Scholar]
- Gencer CD, Özkaya MT, Eti S, Karabıyık Ş. 2023. Evaluation of the effect of open-, self-and cross pollinations on fruit set in Domat, Gemlik and Sarı Ulak olive cultivars. Scientia Horticulturae 311: 111780. [Google Scholar]
- Gómez-Casero TM, Hidalgo PJ, García-Mozo H, Domínguez E, Galán C.. 2004. Pollen biology in four Mediterranean Quercus species. Grana 43: 22–30. [Google Scholar]
- González-Fernández E, Piña-Rey A, Fernández-González M, Aira MJ, Rodríguez-Rajo FJ.. 2020. Prediction of grapevine yield based on reproductive variables and the influence of meteorological conditions. Agronomy 10: 714. [Google Scholar]
- Hidalgo PJ, Galán C, Domínguez E.. 1999. Pollen production of the genus Cupressus. Grana 38: 296–300. [Google Scholar]
- Javid R, Rather G.. 2019. Functional pollen ability of different crab apples used as pollinizers for apple. Journal of Pharmacognosy and Phytochemistry 8: 617–620. [Google Scholar]
- Katz DSW, Morris JR, Batterman SA.. 2020. Pollen production for 13 urban North American tree species: allometric equations for tree trunk diameter and crown area. Aerobiologia 36: 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaleghi E, Karamnezhad F, Moallemi N.. 2019. Study of pollen morphology and salinity effect on the pollen grains of four olive (Olea europaea) cultivars. South African Journal of Botany 127: 51–57. [Google Scholar]
- Koubouris GC, Metzidakis IT, Vasilakakis MD.. 2012. Intraspecific variation in pollen viability, germination and ultrastructure of Olea europaea L. African Journal of Biotechnology 11: 13442–13446. [Google Scholar]
- Kowalczyk BA, Bieniasz M, Kostecka-Gugała A.. 2022. Flowering biology of selected hybrid grape cultivars under temperate climate conditions. Agriculture 12: 655. [Google Scholar]
- Lamin-Samu AT, Farghal M, Ali M, Lu G.. 2021. Morpho-physiological and transcriptome changes in tomato anthers of different developmental stages under drought stress. Cells 10: 1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavee S. 2007. Biennial bearing in olive (Olea europaea). Annales Series Historia Naturalis 1: 101–112. [Google Scholar]
- Lavee S, Rallo L, Rapoport HF, Troncoso A. 1996. The floral biology of the olive: effect of flower number, type and distribution on fruitset. Scientia Horticulturae 66: 149-158. doi: 10.1016/S0304-4238(96)00941-7. [DOI] [Google Scholar]
- Lavee S, Taryan J, Levin J, Haskal A.. 2002. The significance of cross-pollination for various olive cultivars under irrigated intensive growing conditions. Olivae 91: 25–36. [Google Scholar]
- Li Y, Li Y, Su Q, et al. 2022. High temperature induces male sterility via MYB66–MYB4–casein kinase I signaling in cotton. Plant Physiology 189: 2091–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Bianco R, Proietti P, Regni L, Caruso T.. 2021. Planting systems for modern olive growing: strengths and weaknesses. Agriculture 11: 494. [Google Scholar]
- Maniriho F. 2022. Flower differentiation and fruiting dynamics in olive trees (Olea europaea): eco-physiological analysis in the Mediterranean basin. Advances in Horticultural Science 36: 53–62. [Google Scholar]
- Mazzeo A, Palasciano M, Gallotta A, Camposeo S, Pacifico A, Ferrara G.. 2014. Amount and quality of pollen grains in four olive (Olea europaea L.) cultivars as affected by ‘on’ and ‘off’ years. Scientia Horticulturae 170: 89–93. [Google Scholar]
- Medina-Alonso MG, Navas JF, Cabezas JM, et al. 2020. Differences on flowering phenology under Mediterranean and Subtropical environments for two representative olive cultivars. Environmental and Experimental Botany 180: 104239. [Google Scholar]
- Messora R, Florenzano A, Torri P, Mercuri AM, Muzzalupo I, Arru L.. 2017. Morphology and discrimination features of pollen from Italian olive cultivars (Olea europaea L). Grana 56: 204–214. [Google Scholar]
- Morello P, Díez CM, Codes M, et al. 2016. Sanitation of olive plants infected by Verticillium dahliae using heat treatments. Plant Pathology 65: 412–421. [Google Scholar]
- Navas-Lopez JF, León L, Rapoport HF, Moreno-Alías I, Lorite IJ, de la Rosa R.. 2019. Genotype, environment and their interaction effects on olive tree flowering phenology and flower quality. Euphytica 215: 184. [Google Scholar]
- Orlandi F, Ferranti F, Romano B, Fornaciari M.. 2003. Olive pollination: flowers and pollen of two cultivars of Olea europaea. New Zealand Journal of Crop and Horticultural Science 31: 159–168. [Google Scholar]
- Orlandi F, Aguilera F, Galán C, Msallem M, Fornaciari M.. 2017. Olive yields forecasts and oil price trends in Mediterranean areas: a comprehensive analysis of the last two decades. Experimental Agriculture 53: 71–83. [Google Scholar]
- Pacini E, Juniper BE.. 1979a. The ultrastructure of pollen-grain development in the olive (Olea europaea). 1. Proteins in the pore. New Phytologist 83: 157–163. [Google Scholar]
- Pacini E, Juniper BE.. 1979b. The ultrastructure of pollen-grain development in the olive (Olea europaea). 2. Secretion by the tapetal cells. New Phytologist 83: 165–174. [Google Scholar]
- Pacini E, Franchi GG, Bellani LM.. 1985. Pollen grain development in the olive (Olea europaea L.): ultrastructure and anomalies. In: Willemse MTM, Van Went JL (Eds.), Sexual reproduction in seed plants, ferns and mosses (pp. 25-27). Pudoc, Wageningen. [Google Scholar]
- Palasciano M, Camposeo S, Ferrara G, Godini A.. 2008. Pollen production by popular olive cultivars. Acta Horticulturae 791: 489–492. [Google Scholar]
- Pérez V, Herrero M, Hormaza JI.. 2019. Pollen performance in mango (Mangifera indica L., Anacardiaceae): andromonoecy and effect of temperature. Scientia Horticulturae 253: 439–446. [Google Scholar]
- Prieto-Baena JC, Hidalgo PJ, Domínguez E, Galán C.. 2003. Pollen production in the Poaceae family. Grana 42: 153–159. [Google Scholar]
- Putt ED, Heiser CB Jr. 1966. Male sterility and partial male sterility in sunflowers. Crop Science 6: 165–168. [Google Scholar]
- Quiralte J, Palacios L, Rodríguez R, et al. 2007. Modelling diseases: the allergens of Olea europaea pollen. Journal of Investigational Allergology and Clinical Immunology 17: 76–82. [PubMed] [Google Scholar]
- Raja MM, Vijayalakshmi G, Naik ML, et al. 2019. Pollen development and function under heat stress: from effects to responses. Acta Physiologiae Plantarum 41: 47. [Google Scholar]
- Rallo L, Cuevas J. 2007. Fructificación y producción. In: Barranco D, Fernández-Escobar R, Rallo L (Eds.), El cultivo del olivo. Madrid: Ediciones Mundi-Prensa, 147-186. [Google Scholar]
- Rallo L, Barranco D, Díez CM, et al. . 2018. Strategies for olive (Olea europaea L.) breeding: cultivated genetic resources and crossbreeding. In: Al-Khayri JM, Jain SM, Johnson DV (Eds.), Advances in Plant Breeding Strategies: Fruits: Vol. 3. New York City, USA: Springer International Publishing, 535–600.. [Google Scholar]
- Rapoport H. 2014. The reproductive biology of the olive tree and its relationship to extreme environmental conditions. Acta Horticulturae 1057: 41–50. [Google Scholar]
- Raveh E, Goldenberg L, Porat R, Carmi N, Gentile A, La Malfa S.. 2020. Conventional breeding of cultivated citrus varieties. Gentile A., La Malfa S., Deng Z. eds., The citrus genome. Cham: Springer, 33–48. [Google Scholar]
- Reale L, Sgromo C, Bonofiglio T, et al. 2006. Reproductive biology of olive (Olea europaea L) DOP Umbria cultivars. Sexual Plant Reproduction 19: 151–161. [Google Scholar]
- Rejón JD, Suárez CG, Alché JD, Castro AJ, Rodríguez-García MI.. 2010. Evaluación de diferentes métodos para estimar la calidad del polen en distintos cultivares de olivo (Olea europaea L.). Polen 20: 60–72. [Google Scholar]
- Ribeiro H, Cunha M, Abreu I.. 2007. Improving early-season estimates of olive production using airborne pollen multi-sampling sites. Aerobiologia 23: 71–78. [Google Scholar]
- Ribeiro H, Cunha M, Calado L, Abreu I.. 2012. Pollen morphology and quality of twenty olive (Olea europaea L.) cultivars grown in Portugal. Acta Horticulturae 949: 259–264. [Google Scholar]
- Riera FJ. 1950. Morphologically and cyto-logically conditioned forms of sterility in the olive. In: 13th Congres International Oleicult. 3. Actas Oleicult., 1.
- Rodríguez R, Villalba M, Batanero E, Palomares O, Salamanca G.. 2007. Emerging pollen allergens. Biomedicine & Pharmacotherapy 61: 1–7. [DOI] [PubMed] [Google Scholar]
- Rodríguez-García MI, Fernández MC.. 1990. Ultrastructural evidence of endoplasmic reticulum changes during the maturation of the olive pollen grain (Olea europaea L., Oleaceae). Plant Systematics and Evolution 171: 221–231. [Google Scholar]
- Rojo J, Salido P, Pérez-Badia R.. 2015. Flower and pollen production in the ‘Cornicabra’ olive (Olea europaea L.) cultivar and the influence of environmental factors. Trees 29: 1235–1245. [Google Scholar]
- Ruiz-S LM, Carvajal-R DC, García-M JF, et al. 2019. Olives and olive oil production in the Alto Ricaurte climate region in Boyaca, Colombia. Revista Colombiana de Ciencias Hortícolas 13: 108–119. [Google Scholar]
- Sánchez-Estrada A, Cuevas J.. 2019. Pollination strategies to improve fruit set in orchards of ‘Manzanillo’ olive in a nontraditional producing country, Mexico. HortTechnology 29: 258–264. [Google Scholar]
- Saumitou-Laprade P, Vernet P, Vassiliadis C, et al. 2010. A self-incompatibility system explains high male frequencies in an androdioecious plant. Science 327: 1648–1650. [DOI] [PubMed] [Google Scholar]
- Saumitou-Laprade P, Vernet P, Vekemans X, et al. 2017. Elucidation of the genetic architecture of self-incompatibility in olive: evolutionary consequences and perspectives for orchard management. Evolutionary Applications 10: 867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifi E, Guerin J, Kaiser B, Sedgley M.. 2015. Flowering and fruit set in olive: a review. Iranian Journal of Plant Physiology 5: 1263–1272. [Google Scholar]
- Selak GV, Perica S, Ban SG, Radunic M, Poljak M.. 2011. Reproductive success after self-pollination and cross-pollination of olive cultivars in Croatia. HortScience 46: 186–191. [Google Scholar]
- Serrano I, Suárez C, Olmedilla A, Rapoport HF, Rodríguez-García MI.. 2008. Structural organization and cytochemical features of the pistil in olive (Olea europaea L.) cv. Picual at anthesis. Sexual Plant Reproduction 21: 99–111. [Google Scholar]
- Severova E, Kopylov-Guskov Y, Selezneva Y, Karaseva V, Yadav SR, Sokoloff D.. 2022. Pollen production of selected grass species in Russia and India at the levels of anther, flower and inflorescence. Plants 11: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo-Molina R, Rodríguez AM, Palaciso IS, López FG.. 1996. Pollen production in anemophilous trees. Grana 35: 38–46. [Google Scholar]
- Tous J, Del Río C, Caballero J.M, Rallo L. 2004. Libro II. Variabilidad y selección. In: Rallo L, Barranco D, Caballero M, Del Río C, Martín A, Tous J, Trujillo I (Eds.), Variedades de olivo en España. Madrid: Junta de Andalucía, MAPA y ediciones Mundi-Prensa, 295-300. [Google Scholar]
- Trigo MM, Jato V, Fernández D, Galán C.. 2008. Atlas aeropalinológico de España (pp. 110). Secretariado de Publicaciones de la Universidad de Leon, León, España. https://publicaciones.unileon.es/product/atlas-aeropalinologico-de-espana/. [Google Scholar]
- Trujillo I, Ojeda MA, Urdiroz N, et al. 2013. Identification of the Worldwide Olive Germplasm Bank of Córdoba (Spain) using SSR and morphological markers. Tree Genetics & Genomes 10: 141–155. [Google Scholar]
- Ullah A, Nadeem F, Nawaz A, Siddique KHM, Farooq M.. 2022. Heat stress effects on the reproductive physiology and yield of wheat. Journal of Agronomy and Crop Science 208: 1–17. [Google Scholar]
- Villalba M, Rodríguez R, Batanero E.. 2014. The spectrum of olive pollen allergens. From structures to diagnosis and treatment. Methods 66: 44–54. [DOI] [PubMed] [Google Scholar]
- Weider C, Stamp P, Christov N, et al. 2009. Stability of cytoplasmic male sterility in maize under different environmental conditions. Crop Science 49: 77–84. [Google Scholar]
- Yigit S, Mendes M.. 2018. Which effect size measure is appropriate for one-way and two-way ANOVA models?: A Monte Carlo simulation study. REVSTAT – Statistical Journal 16: 295–313. [Google Scholar]
- Yu J, Jiang M, Guo C.. 2019. Crop pollen development under drought: from the phenotype to the mechanism. International Journal of Molecular Sciences 20: 1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


