Highlights
-
•
Growing evidence suggests that persistent microvascular inflammation, clumping/clotting of blood cells and thrombotic complications may be key causes of Long COVID.
-
•
Plasma levels of von Willebrand factor and Factor VIII were uniformly higher in all gynecologic patients with Long COVID vs controls without Long COVID.
-
•
Persistently elevated levels of Von Willebrand and Factor VIII may represent the results of lingering microvascular damage (i.e., spike-induced endotheliosis).
Keywords: Long COVID, PASC, Von Willebrand factor, Factor VIII, Microclotting
Abstract
Up to 30 % of COVID-infected patients may develop post-acute sequelae of COVID-19 (PASC), also known as Long COVID (LC), a syndrome characterized by a variety of debilitating symptoms lasting for more than 3 months after the acute infection. While the pathophysiological mechanisms behind PASC/LC are not completely understood, growing evidence suggests that an important component of this syndrome may be related to persistent microvascular inflammation causing clumping/clotting of red blood cells and platelets and thrombotic complications. We retrospectively evaluated the plasma levels of von Willebrand factor (VWF), Factor VIII and D-dimer in 10 gynecologic patients (60 % with an endometrial or ovarian cancer diagnosis) affected by PASC/LC vs 5 control patients (60 % harboring endometrial or ovarian tumors). We found elevated VWF and Factor VIII levels in all 10 PASC/LC patients (means of 254 % and 229 %, respectively) vs none of the 5 randomly selected cancer control patients (means of 108 % and 95 %, respectively), p = 0.0046 and p < 0.0001, respectively. In contrast, no significant difference was noted in the levels of D-dimer in PASC/LC. Importantly, abnormally elevated VWF and Factor VIII levels were found to persist for at least 2 years in patients with Long COVID symptoms. VWF and Factor VIII but not D-dimer levels are significantly elevated in the plasma of PASC/LC cancer patients. Abnormally and persistently elevated VWF and Factor VIII levels may represent the results of persistent microvascular damage (i.e., spike-induced endotheliosis) and may be biomarkers of persistent inflammation in gynecologic patients with PASC/LC.
1. Introduction
Up to 30 % of previously healthy individuals and up to 60 % of cancer patients recovering from COVID-19 infection and followed up for up to 14 months may develop prolonged debilitating symptoms consistent with Post-Acute Sequelae of COVID-19 (PASC), also known as Long COVID (LC). These symptoms include, but are not limited to decreased exercise tolerance, shortness of breath, brain fog, cognitive dysfunction, fatigue, postural orthostatic tachycardia syndrome (POTS), chest pain, chronic pain, palpitations, and gastrointestinal symptoms (Soriano et al., 2022, Ceban et al., 2022, Dagher et al., 2023, Cortellini et al., 2023). In many of these studies, female cancer patients were more likely than male patients to report persistence of PASC/LC symptoms, suggesting a gender-dependent predisposition for developing this syndrome (Bai et al., 2022).
While the pathophysiological mechanisms behind PASC/LC are not completely understood and are likely multifactorial, growing evidence suggests that an important component of the syndrome may be related to a persistent COVID-19-associated coagulopathy, triggered by the ability of viral Spike-1 protein to cause clumping and clotting of red blood cells and platelets (Scheim et al., 2023, Boschi et al., 2022) as well as disruption of normal endothelial function leading to prolonged thrombotic and microvascular complications (Kruger et al., 2022, Pretorius et al., 2022, Turner et al., 2023, Kell et al., 2022). Consistent with this view, while acute COVID-19 infection typically initiates with penetration of the virus into the respiratory epithelium to cause lung inflammation, multiple studies have identified the vascular endothelium as the primary target of COVID-19 pathology, causing microvascular occlusion in lungs and other organ systems with accompanying morbidities such as intravascular clotting and peripheral ischemia (Ackermann et al., 2020, O'Sullivan et al., 2020). Accordingly, autopsy studies have reported widespread microthrombi disseminated throughout the pulmonary vasculature, demonstrating that vasculopathy is paramount in COVID-19 pathogenesis (Wichmann et al., 2020). Taken all together, these data suggest that endothelial cell damage may play a key role in orchestrating not only the unusual pulmonary intravascular coagulopathy associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection but also PASC/LC.
Several reports have recently provided strong experimental evidence to demonstrate that SARS-CoV-2 may persist for a prolonged amount of time in the body of many PASC/LC patients, including the human olfactory epithelium, lung cells, blood, feces and gastrointestinal tissues after the initial diagnosis of COVID-19 (de Melo et al., 2021, Bussani et al., 2020, Craddock et al., 2023, Zollner et al., 2022, Cheung et al., 2022), as well as in non-classical CD16 + monocytes up to 15 months post-infection (Patterson et al., 2022). All these infected cells may potentially cause prolonged Spike-1 production and trigger chronic vessels and organs’ inflammation (Kruger et al., 2022, Pretorius et al., 2022, Turner et al., 2023, Kell et al., 2022). Taken together, these studies suggest that multiple tissues and organs including vessels and subpopulations of inflammatory cells such as monocyte/macrophages, dendritic cells and mast cells (Schultheiß et al., 2023) may represent not only a potential SARS-CoV-2 viral -antigen reservoir but also a persistent source of inflammation in at least a subset of PASC/LC patients.
In this retrospective study, we evaluated the levels of three biomarkers of endothelial inflammation (VWF, factor VIII, and D-dimer) in 10 gynecologic PASC/LC patients vs 5 control patients with or without a cancer diagnosis followed in our gynecologic oncology clinic. We found a significant and persistent increase in the plasma levels of VWF and factor VIII in PASC/LC patients vs controls while no significant differences were noted in D-dimer levels. Abnormally and persistently elevated VWF and factor VIII levels in gynecologic patients with or without cancer may represent novel biomarkers for PASC/LC.
2. Cases
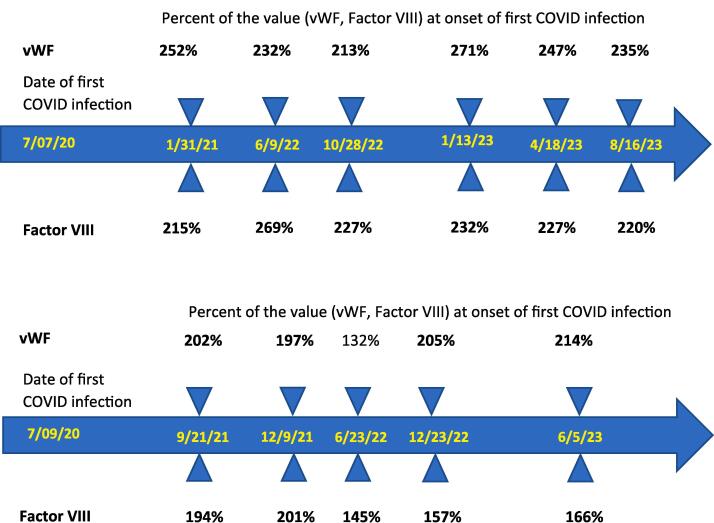
We retrospectively evaluated the plasmatic levels of VWF, Factor VIII and D-dimer, in gynecologic patients affected by PASC/LC symptoms (i.e., six (60 %) with a history of gynecologic tumors and four with non-malignant gynecologic conditions) followed in our clinic. We compared their levels to those detected in five randomly selected gynecologic patients without PASC/LC symptoms (three out of five (60 %) harboring gynecologic tumors) where plasmatic levels of VWF, Factor VIII and/or D-dimer were collected in our clinic during the same time periods of the COVID-19 pandemic as part of their blood work up to evaluate the risk of thrombosis. The characteristics of the study patients are described in Table 1. Biomarkers were pre-tested for normality and equal variances, respectively, with the Shapiro-Wilk and folded-F tests, then compared for group differences with Student’s t-test if normally distributed with equal variances, with Welch’s t-test if normally distributed with unequal variances, or with the Wilcoxon two-sample test if not normally distributed. We considered the biomarker levels to be elevated if they exceeded the Yale Laboratory’s reference range of 62–175 % for the VWF, the range of 66––143 % for Factor VIII and a value ≥ 0.50 mg/L for D-dimer. A p value < 0.05 was considered statistically significant. As depicted in Fig. 1, we found elevated VWF levels in all 10 of the PASC/LC patients (mean ± SD = 254 % ± 71 %) vs none of the 5 randomly selected cancer control patients (mean ± SD = 108 % ± 21 %) (Wilcoxon p = 0.0046). Similarly, Factor VIII levels were elevated in all 10 of the PASC/LC patients (mean ± SD = 229 % ± 63 %) vs none of the 5 randomly selected cancer control patients (mean ± SD = 95 % ± 15 %) (Welch’s t-test p < 0.0001). In contrast, no significant difference was noted in the levels of D-dimer in PASC/LC (mean ± SD = 0.63 ± 0.62 mg/L) vs controls (0.39 ± 0.18 mg/L, Wilcoxon p = 0.4962) (Fig. 1). As representatively demonstrated in Fig. 2 for PASC/LC patients where multiple longitudinal VWF and Factor VIII blood collections were available, prolonged abnormalities in the levels of both endothelial biomarkers were noted. Importantly, both these PASC/LC cancer patients reported long-lasting (i.e., over 2 years) debilitating symptoms (i.e., shortness of breath, fatigue, etc.) since the onset of the acute COVID infection.
Table 1.
Characteristics of the patients.
| Number | Age | Race | Gyn Pathology | Date of first COVID infection | Long COVID Symptoms |
|---|---|---|---|---|---|
| 1 | 64 | White | endometrial cancer | n/a | no |
| 2 | 63 | White | cervical dysplasia | n/a | no |
| 3 | 63 | White | ovarian cancer | 04/17/2020 | no |
| 4 | 46 | White | endometriosis | 05/30/2022 | no |
| 5 | 69 | White | ovarian cancer | 07/07/2020 | yes |
| 6 | 70 | White | endometrial cancer | 07/09/2020 | yes |
| 7 | 41 | White | cervical dysplasia | 11/10/2021 | yes |
| 8 | 71 | White | complex ovarian cyst | 12/09/2022 | yes |
| 9 | 40 | White | cervical dysplasia | 08/14/2023 | yes |
| 10 | 65 | White | endometrial cancer | 09/01/2023 | yes |
| 11 | 64 | White | ovarian cancer | 10/01/2022 | yes |
| 12 | 82 | Black | endometrial cancer | 12/08/2020 | yes |
| 13 | 58 | Black | ovarian cancer | 10/01/2020 | yes |
| 14 | 65 | White | ovarian cancer | n/a | no |
| 15 | 51 | White | None | 11/20/2020 | yes |
Fig. 1.
Levels of VWF (upper panel), Factor VIII (middle panel) and D-dimer (lower panel) in 10 PASC/LC patients vs 5 controls. The dotted vertical lines show the lower limits (blue) and upper limits (green) of the Yale Laboratory’s reference ranges. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Longitudinal von Willebrand and Factor VIII plasma values in two representative PASC/LC cancer patients (upper panel patient #5, lower panel patient #6) with multiple blood collection available over 2 years. Values shown in bold are above the normal reference range for both assays used.
3. Discussion
Several studies have reported that cancer patients may be more susceptible to SARS‐CoV‐2 infection and are at a higher risk of severe COVID‐19 complications than the general population (Wang et al., 2020). Consistent with this higher vulnerability, recent data have suggested that cancer patients may be more susceptible to develop long-lasting debilitating symptoms including but not limited to shortness of breath, decreased exercise tolerance, brain fog, cognitive dysfunction, fatigue, dysautonomia including POTS, chest pain and chronic pain, palpitations, and gastrointestinal symptoms after the acute infection, with up to 60 % reported to develop PASC/LC (Dagher et al., 2023, Cortellini et al., 2023).
The pathophysiologic mechanisms underlying the post-COVID prolonged and disabling symptoms remain poorly understood and are likely multifactorial. However, several recent studies have demonstrated that SARS‐CoV‐2 may remain detectable for months in a subset of PASC/LC patients after the acute infection, hiding in multiple organs and inflammatory cells such as monocytes/macrophages (de Melo et al., 2021, Bussani et al., 2020, Craddock et al., 2023, Zollner et al., 2022, Cheung et al., 2022, Patterson et al., 2022, Schultheiß et al., 2023, Wang et al., 2020). Chronic production of spike protein in the body of PASC/LC patients might therefore trigger a persistent state of inflammation in vessels/organs, in particular in the microcirculation, causing a status of chronic coagulopathy and systemic microthrombosis (Kruger et al., 2022, Pretorius et al., 2022, Turner et al., 2023, Kell et al., 2022). Consistent with this view, SARS-CoV-2 spike protein has been demonstrated to mediate not only direct lung injury but also vascular damage by inducing endothelial barrier dysfunction and inflammation (i.e., endotheliitis), platelet activation and formation of difficult to lyse “fibrinaloid” microclots after the recovery from the acute infection (Kruger et al., 2022, Pretorius et al., 2022, Turner et al., 2023, Kell et al., 2022, Hattori et al., 2022).
Previous studies have reported elevated levels of VWF, Factor VIII and D-dimer in the plasma of critically and noncritically ill COVID-19 patients, with a greater increase in these levels associated with critical illness (Goshua et al., 2020). These results obtained in acutely infected patients provided biochemical evidence that endotheliopathy and platelet activation are ubiquitous in COVID-19 patients and may lead to the clinical prothrombotic manifestations of COVID-19-associated coagulopathy, which include microvascular thrombosis even in the non-critical (i.e., mild/moderate) form of COVID-19 infection (Goshua et al., 2020). Indeed, since the production of VWF is exclusive to endothelial cells and megakaryocytes (Lenting et al., 2015), and since VWF is secreted by endothelial cells in response to high shear stress and other inflammatory mediators (Lenting et al., 2015), we conjecture that resting endothelial cells may continue to release large amounts of long VWF multimers into circulation when activated/damaged by the persistent production/presence of spike protein in PASC/LC patients. In addition, VWF is also known to act as a carrier—and stabilizer—of the procoagulant factor VIII (FVIII) in circulation (Lenting et al., 2015), which is achieved by the formation of a non-covalently bound VWF-FVIII complex that protects FVIII from being degraded by activated protein C (Lenting et al., 2015). Taken all together, these studies suggest that chronic endotheliopathy and platelet activation manifested by chronically elevated VWF and Factor VIII factors might be important in the pathophysiology of acute as well as PASC/LC-associated coagulopathy. However, to our knowledge, no information is currently available about levels of vascular inflammatory biomarkers such as VWF, Factor VIII and D-dimer in cancer patient affected by PASC/LC.
In our retrospective analysis we found plasma levels of VWF and Factor VIII to be significantly elevated in gynecologic patients with and without cancer affected by PASC/LC symptoms vs controls. In contrast, no significant differences were detected in the plasma levels of D-dimer. These findings strongly suggest that persistently elevated VWF and Factor VIII concentrations play a role in the pathogenesis underpinning PASC/LC COVID-19 vasculopathy and microthrombosis in these patients. One, VWF is able to bind to platelet receptors and thus modulate platelet adhesion and aggregation. Two, pathological VWF multimers are responsible for thrombotic microangiopathy. Three, VWF multimers also play a role in the pathogenesis of microvasculature occlusion in other infectious disease causing clumping/clotting of red blood cells and platelets as well as disruption of normal endothelial function leading to thrombotic and microvascular complications (i.e., cerebral malaria) (O'Sullivan et al., 2016). Importantly, in our small series, abnormalities in the levels of VWF and Factor VIII were found to persist for at least 2 years in gynecologic cancer patients with prolonged Long COVID symptoms. These findings are consistent with a recent report on a large number of PASC/LC patients demonstrating that their significant activity limitations did not significantly improve over time (Ford et al., 2023). More importantly, the association between persistent symptoms and a procoagulant state support the hypothesis that an ongoing process of clumping/clotting of red blood cells and platelets thrombi formation in combination with persistent microthrombosis may be responsible for the main physical symptoms experienced by a large subset of PASC/LC patients.
In conclusion, we report that VWF and Factor VIII but not D-dimer levels were significantly elevated in the plasma of gynecologic PASC/LC patients with and without cancer. Abnormally and persistently elevated VWF and Factor VIII levels may represent the results of persistent microvascular damage (i.e., spike-induced endotheliosis), and be novel biomarkers of persistent inflammation in gynecologic patients with PASC/LC.
Consent
The retrospective chart review was approved by the Yale Human Research Protection Program, Institutional Review Boards, IRB Protocol ID: 2000030512.
Author contributions
Stefania Bellone, Eric Siegel, David Scheim, and Alessandro D. Santin participated in drafting and revising this manuscript. All authors read and approved this manuscript to be submitted.
CRediT authorship contribution statement
Stefania Bellone: Data curation, Formal analysis, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. Eric R. Siegel: Data curation, Formal analysis, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. David E. Scheim: Data curation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. Alessandro D. Santin: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F., Tomasoni D., Falcinella C., et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2022;28 doi: 10.1016/j.cmi.2021.11.002. 611.e619-611.e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschi C., Scheim D.E., Bancod A., et al. SARS-CoV-2 spike protein induces hemagglutination: Implications for COVID-19 morbidities and therapeutics and for vaccine adverse effects. Int. J. Mol. Sci. 2022;23:15480. doi: 10.3390/ijms232415480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussani R., Schneider E., Zentilin L., et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020;61 doi: 10.1016/j.ebiom.2020.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceban F., Ling S., Lui L.M.W., et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C.C.L., Goh D., Lim X., et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut. 2022;71:226–229. doi: 10.1136/gutjnl-2021-324280. [DOI] [PubMed] [Google Scholar]
- Cortellini A., Tabernero J., Mukherjee U., et al. SARS-CoV-2 omicron (B.1.1.529)-related COVID-19 sequelae in vaccinated and unvaccinated patients with cancer: results from the OnCovid registry. Lancet Oncol. 2023;24:335–346. doi: 10.1016/S1470-2045(23)00056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock V., Mahajan A., Spikes L., et al. Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19. J. Med. Virol. 2023;95:e28568. doi: 10.1002/jmv.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher H., Chaftari A.-M., Subbiah I.M., et al. Long COVID in cancer patients: preponderance of symptoms in majority of patients over long time period. Elife. 2023;12:e81182. doi: 10.7554/eLife.81182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo G.D., Lazarini F., Levallois S., et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13:eabf8396. doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford N.D., Slaughter D., Edwards D., et al. Long COVID and significant activity limitation among adults, by age - united states, June 1–13, 2022, to June 7–19, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:866–870. doi: 10.15585/mmwr.mm7232a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7 doi: 10.1016/s2352-3026(20)30216-7. e575-e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y., Hattori K., Machida T., Matsuda N. Vascular endotheliitis associated with infections: Its pathogenetic role and therapeutic implication. Biochem Pharmacol. 2022;197 doi: 10.1016/j.bcp.2022.114909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell D.B., Laubscher G.J., Pretorius E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J. 2022;479:537–559. doi: 10.1042/bcj20220016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger A., Vlok M., Turner S., et al. Proteomics of fibrin amyloid microclots in long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc Diabetol. 2022;21:190. doi: 10.1186/s12933-022-01623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenting P.J., Christophe O.D., Denis C.V. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. 2015;125:2019–2028. doi: 10.1182/blood-2014-06-528406. [DOI] [PubMed] [Google Scholar]
- O'Sullivan J.M., Preston R.J., O'Regan N., O'Donnell J.S. Emerging roles for hemostatic dysfunction in malaria pathogenesis. Blood. 2016;127:2281–2288. doi: 10.1182/blood-2015-11-636464. [DOI] [PubMed] [Google Scholar]
- O'Sullivan J.M., Gonagle D.M., Ward S.E., Preston R.J.S., O'Donnell J.S. Endothelial cells orchestrate COVID-19 coagulopathy. Lancet Haematol. 2020;7 doi: 10.1016/s2352-3026(20)30215-5. e553-e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson B.K., Francisco E.B., Yogendra R., et al. Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection. Front. Immunol. 2022;12:5526. doi: 10.3389/fimmu.2021.746021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius E., Venter C., Laubscher G.J., et al. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC) Cardiovasc. Diabetol. 2022;21:148. doi: 10.1186/s12933-022-01579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheim D.E., Vottero P., Santin A.D., Hirsh A.G. Sialylated glycan bindings from SARS-CoV-2 spike protein to blood and endothelial cells govern the severe morbidities of COVID-19. Int. J. Mol. Sci. 2023;24:17039. doi: 10.3390/ijms242317039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiß C., Willscher E., Paschold L., et al. Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19. J Med Virol. 2023;95:e28364. doi: 10.1002/jmv.28364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22 doi: 10.1016/s1473-3099(21)00703-9. e102-e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S., Khan M.A., Putrino D., et al. Long COVID: pathophysiological factors and abnormalities of coagulation. Trends Endocrinol Metab. 2023;34:321–344. doi: 10.1016/j.tem.2023.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Sun Y., Yuan Y., Mei Q., Yuan X. Clinical challenges in cancer patients with COVID-19: Aging, immunosuppression, and comorbidities. Aging (Albany NY) 2020;12:24462–24474. doi: 10.18632/aging.104205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann D., Sperhake J.-P., Lütgehetmann M., et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann. Intern. Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner A., Koch R., Jukic A., et al. Postacute COVID-19 is characterized by gut viral antigen persistence in inflammatory bowel diseases. Gastroenterology. 2022;163:495–506.e498. doi: 10.1053/j.gastro.2022.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]