Abstract
Background:
Bronchiectasis in individuals with chronic obstructive pulmonary disease (COPD) is associated with greater mortality. However, whether suspected bronchiectasis, defined as incidental bronchiectasis on computed tomography (CT) plus clinical manifestation, is associated with increased mortality in smoking individuals with normal spirometry and preserved ratio impaired spirometry (PRISm) is unknown.
Objective:
To determine the association between suspected bronchiectasis and mortality in individuals with normal spirometry, PRISm, and obstructive spirometry.
Design:
Prospective, observational cohort.
Setting:
COPDGene Study.
Participants:
7662 non-Hispanic Black or White individuals, aged 45-80 years, with ≥10 pack-years of smoking history. Participants who were former and current smokers were stratified into normal spirometry (n=3277), PRISm (n=986), and obstructive spirometry (n=3399).
Measurements:
CT-identified bronchiectasis was ascertained using artificial intelligence-based measurements of airway-to-artery ratio >1 (AAR>1), a measure of bronchial dilatation. The primary outcome of “suspected bronchiectasis” was defined as AAR>1 greater than 1% plus two of the following: cough, phlegm, dyspnea, and history of ≥2 exacerbations.
Results:
Among the 7662 participants (mean age, 60 years; 52% women), 1352 (17.6%) had suspected bronchiectasis. During a median follow-up of 11 years, 2095 died (27.3%). 10-yr mortality risk was higher in participants with suspected bronchiectasis, compared to those without suspected bronchiectasis (normal spirometry, difference in mortality probability (Pr) 0.15 [95% CI 0.09 to 0.21]; PRISm, Pr 0.07 [CI, −0.003 to 0.15]; obstructive spirometry, Pr 0.06 [CI, 0.03 to 0.09]). When only CT was used to identify bronchiectasis, the differences were attenuated in the normal spirometry (Pr, 0.04 [CI, −0.001 to 0.08].
Limitations:
Only two racial groups were studied; only one measurement was used to define bronchiectasis on CT; symptoms of suspected bronchiectasis were nonspecific.
Conclusions:
Suspected bronchiectasis was associated with a heightened risk of mortality in individuals with normal and obstructive spirometry.
Funding:
NHLBI
Introduction
Bronchiectasis is a clinical condition characterized by the pathological widening of airways and repeated infection and inflammation cycles resulting in lung structural damage.(1–3) The term bronchiectasis is also used to refer to the characteristic radiological appearance of this clinical condition.(4) In 2009-2013, the prevalence of bronchiectasis in the United States (US) was 139 cases per 100,000 persons.(5) Bronchiectasis is also a frequent manifestation of chronic obstructive pulmonary disease (COPD), affecting 4%-72% of these individuals.(6–9) Individuals with overlapping COPD-bronchiectasis conditions experience more exacerbations and higher mortality risk than either condition alone.(8, 10–12)
In the US, COPD is mainly caused by exposure to cigarette smoking. Airflow obstruction is the defining spirometric pattern of the disease.(13) Smoking individuals who do not meet the spirometric criteria for COPD include those with normal spirometry and those with a “restrictive” pattern known as “preserved ratio impaired spirometry (PRISm)”, which has a prevalence of 5%-20% globally.(14, 15) Although these smoking individuals do not fall into a disease category, they present with substantial structural lung abnormalities, clinical impairments, and reduced quality of life.(14, 16–18) On computed tomography (CT), incidental bronchiectasis (i.e., radiologic bronchiectasis in asymptomatic, mildly symptomatic, or individuals without a physician-diagnosed condition) is found in 12%-30% of smoking individuals participating in lung cancer screening programs and smoking-related studies.(19–22) Lung cancer screening participants with more severe bronchiectasis on CT had increased hospitalizations due to respiratory events. However, whether incidental bronchiectasis along with clinical manifestations (hereafter termed “suspected” bronchiectasis) is associated with increased mortality in smoking individuals without a spirometry-based diagnosis of COPD has not been systematically evaluated.
Prior studies about mortality in the COPD-bronchiectasis overlap were carried out only in participants with moderate-to-severe lung function impairment, which limits generalizability, and, moreover, they relied on subjective CT measures of bronchiectasis.(7, 8) (23) Visual inspection on CT is highly variable, time-consuming, and unable to assess the entire bronchial tree. Advanced artificial intelligence-based, validated imaging tools now enable quantifying airway-to-artery ratios (AARs) throughout the entire bronchial tree to identify bronchiectasis objectively.(11) In this study, we tested the hypothesis that “suspected bronchiectasis” is associated with increased all-cause mortality in smoking individuals with normal spirometry, PRISm, and obstructive spirometry. We based our hypothesis on prior work demonstrating that airway dilation is associated with inflammation, recurrent infections, and pulmonary vascular changes that contribute to worse outcomes, including mortality.(20, 24–27)
Methods
Participants
In this secondary analysis, we used data collected by the COPDGene (Genetic Epidemiology of Chronic Obstructive Pulmonary Disease) Study (NCT00608764)(28), a study of patients with COPD and non-COPD controls. Participants with COPD included outpatient clinic patients, patients in registries, previous study participants, and COPD Foundation volunteers. Participants without COPD included spouses and friends of study participants with COPD and patients at primary care practices.
The study inclusion criteria for the participants with COPD were 45-80 years of age, non-Hispanic White or non-Hispanic Black race/ethnicity, and current or former smoker of ≥10 pack-years. Participants were excluded if they met any of the following criteria: history of pulmonary disease except for asthma, previous surgical excision of at least one lung lobe (or lung volume reduction procedure), active cancer under treatment, suspected lung cancer (large or highly suspicious lung mass), metal in the chest, exacerbation of COPD treated with antibiotics or steroids in the last month, recent eye surgery, myocardial infarction, other cardiac hospitalization, recent chest or abdominal surgery, inability to use albuterol, history of chest radiation therapy, and first or second-degree relative already enrolled in the study. Participants in the control group had the same inclusion and exclusion criteria except they did not have to have a smoking history.
Participants were recruited from local communities across 21 US clinical centers (See Supplement for a list of centers) from November 2007 to April 2011. Participants with COPD were recruited using several strategies, including outpatient clinics, word-of-mouth communication with friends and spouses of patients with COPD, advertisements, and outreach to community groups and If churches. Participants with a history of smoking but without COPD were recruited from community groups.(17) All participants completed questionnaires and underwent spirometry and standardized volumetric chest CT scanning at baseline, 5 years (phase 1), and 10 years (phase 2).
For our analysis, we excluded those who did not have smoking history, resulting in a sample 7662 remaining participants with complete data on spirometry, AAR, and mortality (Figure 1), including those from three spirometry-based groups: Normal spirometry, PRISm, and obstructive spirometry (i.e., COPD. The terms obstructive spirometry and COPD are used interchangeably. See definitions below). All participants provided written informed consent to participate. The Institutional Review Board at each participating clinical site approved the protocol.
Figure 1.
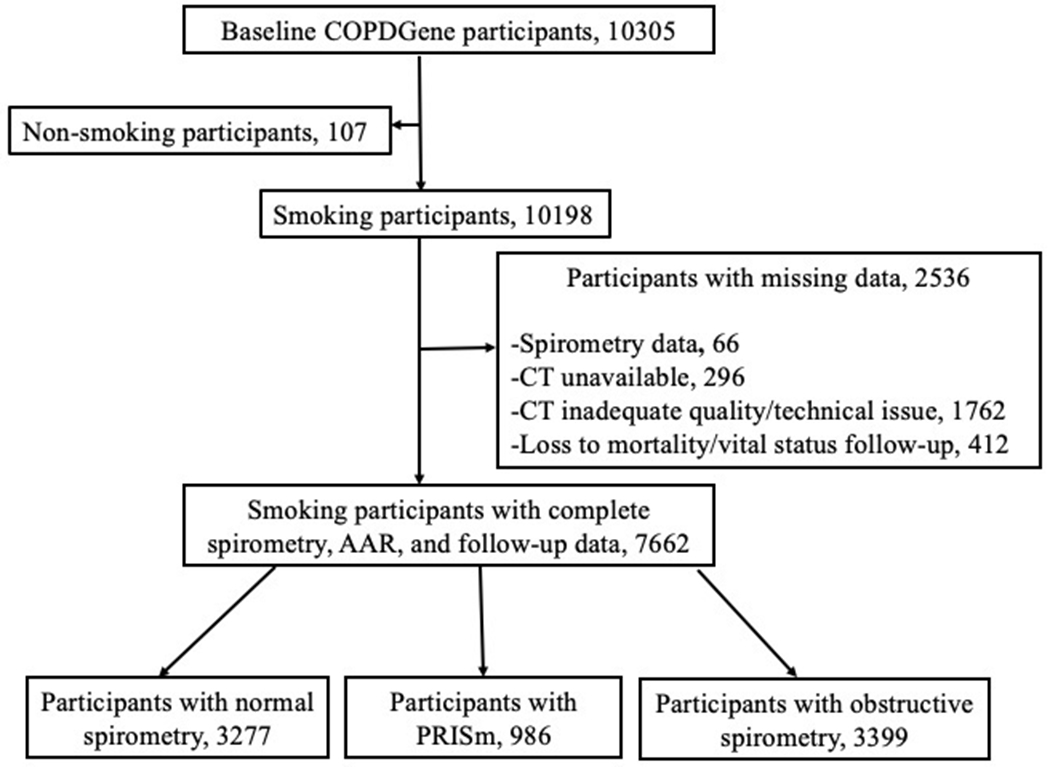
Flowchart of participants selection and their spirometry-based group distribution. Abbreviations, PRISm, preserved ratio impaired spirometry.
Mortality and vitality status
Mortality data were corroborated using searches of the Social Security Death Index (SSDI) and through the Longitudinal Follow-up (LFU) program of COPDGene.(29) The death date was also verified from other sources, including medical records, published obituaries, and next-of-kin reports. Mortality or vitality status obtained via SSDI was back-censored for 3 months from the search date to account for the expected lag between the event and its appearance in the SSDI record. For participants evaluated through the LFU program, the dates of the most recent contact, including COPDGene phase 2 and phase 3 dates and dates of LFU surveys between phase visits, were used to calculate the follow-up time.(30) The survival time was calculated from the date of the baseline visit to the date of death or censored at the most recent contact date before or on August 31, 2022, the close-out date of the all-cause mortality dataset.
Identification of bronchiectasis
We identified suspected bronchiectasis based on a combination of clinical manifestations reported on questionnaires and chest CT scans acquired at baseline.
CT features consistent with bronchiectasis
Bronchiectasis on CT scans was ascertained with AARs. The CT protocols of COPDGene have been described in a previous report.(28) Assessment of AARs comprised the following five steps, with two of them involving deep learning techniques (3 and 4, below),(11): 1) segmentation of the lungs; 2) three-dimensional (3D) reconstruction of the airway and vessel trees; 3) separation of pulmonary arteries from pulmonary veins; 4) sizing of the airways and vessels; and 5) pairing of the airways and pulmonary arteries. Airway-and-artery pairs were selected to compute AARs and determine the percentage of airways with AARs>1 (hereafter, percentage of AAR>1) obtained from all airway-and-artery pairs with an artery cross-sectional area ≥5 mm2, where higher the value greater the extent of airway enlargement on CT. If a scan yielded 16,000 AARs and 160 were greater than 1, the percentage of AAR>1 was 1%. In clinical and research settings, a cutoff point of AAR>1 is typically used to mark a positive bronchiectasis diagnosis.(23) The intra-CT scan (i.e., running the algorithm 3 times) and inter-kernel (i.e., image reconstruction for CT) intraclass correlation coefficient for the percentage of AAR>1 was 1.00 and 0.82, respectively. Based on the distribution of the percentage of AAR>1 in this cohort, the median value was used to define bronchiectasis (i.e., presence, >1% vs absence, ≤1%), an approach previously reported.(11) (See Supplement for additional details). As part of an improvement process, step 2 of the above algorithm was enhanced by excluding the central airways (i.e., the main bronchi and lobar bronchi) to decrease mismatching of the airway and nearby arteries that might result in inaccurate AAR measurements.
Clinical manifestations of bronchiectasis
Information about clinical manifestations was obtained with standardized questionnaires (available at www.copdgene.org). If a participant responded “yes” to the questions “Do you usually have a cough (excluding clearing of throat)?” and “Do you usually bring up phlegm from your chest?”, then the participant was considered to have cough and phlegm, respectively. Dyspnea was evaluated with the modified Medical Research Council (mMRC) Scale (range, 0-4) and dichotomized as 0 vs ≥1. An exacerbation episode was defined as an increase or new onset of respiratory symptoms (cough, phlegm, dyspnea) treated with antibiotics or systemic corticosteroids.(1) The number of episodes the year before enrollment was recorded.
Definition of suspected bronchiectasis
We combined the following clinical and imaging criteria to designate that a participant has suspected bronchiectasis: 1) ≥2 of cough, phlegm, mMRC dyspnea score ≥1, and ≥2 exacerbations in the year before enrollment (31) and 2) percentage of AAR>1 greater than 1% (11). We defined 4 clinical/imaging groups: 1) Non-suspected bronchiectasis (no criteria met); 2) Clinical manifestation only (only met criterium 1); 3) CT manifestation only (only met criterium 2); and 4) Suspected bronchiectasis (met both criteria).(11) Suspected bronchiectasis definition is not a physician-based diagnosis, as the COPDGene study did not include patients with a history of this condition. Additionally, the clinical feature of suppurative disease linked to bronchiectasis was not assessed in this cohort. We applied the definition to participants with a history of smoking without COPD because they usually experience symptoms and exacerbation episodes and have structural lung damage (i.e., emphysema and bronchiectasis on CT) as those with COPD, as previously demonstrated.(16, 17, 20) (See the Supplement for more details).
Spirometry
Spirometric measure of lung function was performed before and after administering bronchodilator albuterol.(32) Forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) after bronchodilator use was expressed as a percentage of predicted values.(33) Spirometric groups were defined as follows: normal spirometry, FEV1:FVC ratio ≥0.7 and FEV1 ≥80%; PRISm, FEV1:FVC ≥0.7 and FEV1<80%; and obstructive spirometry, FEV1:FVC<0.7. In participants with COPD (i.e., obstructive spirometry), severity was classified into four grades as per the Global Initiative for Obstructive Lung Disease (GOLD): 1 (mild), FEV1 % predicted ≥80; 2 (moderate), FEV1 % predicted ≥50 - <80; 3 (severe) FEV1 % predicted ≥30 - <50; and 4 (very severe), FEV1 % predicted <30.(13)
Other covariates
Data on demographics (sex, race, body mass index [BMI]) and clinical information (current smoking status, pack-years smoked, comorbidities, and oxygen saturation) were collected with standardized questionnaires and procedures. Participants self-reported their race. (18) Seven comorbidities were included (hypertension, coronary artery disease, congestive heart failure, stroke, diabetes mellitus, high cholesterol, and cancer [i.e., lung, colon, breast, bladder, and prostate cancers]) and collapsed as a count variable termed the number of comorbidities (see Supplement for more details).(18) Oxygen saturation was taken at rest with a pulse oximeter.
Statistical methods
The association between suspected bronchiectasis and all-cause mortality was assessed with Cox proportional hazard regression analysis. Our a priori multivariable model included 12 groupings, each made of a combination of one of four clinical/imaging and one of three spirometric groups to allow interactions between those groups. The covariates included age, sex, race, BMI, pack-years, smoking status (current vs. former), the number of comorbidities, oxygen saturation, FEV1 after bronchodilator use, CT measures of emphysema and airway wall thickness, and CT scanner make/model. A robust sandwich estimate of the covariance matrix was applied to handle clustered death events within clinical centers. The adjusted probabilities of death were calculated with the direct standardization method.(34) We evaluated proportionality assumptions by visual inspection and multiplying each covariate by the log transform of the follow-up time. The assumed proportionality did not present major violations, and the model fit was adequate. Analyses were performed using the SAS 9.4 (SAS Institute, Cary, NC) software.
Role of the funding source
The funding source had no role in the design, conduct, and analysis of the current study and in the decision to submit the manuscript for publication.
Results
Participants Characteristics
Of the 10305 COPDGene study participants, 107 had no smoking history and 2536 of the remaining participants were excluded for missing data, leaving a sample size of 7662 (Figure 1). Characteristics of excluded participants who had a smoking history due to missing data and the distribution of those lost to follow-up are provided in Supplement Tables 1 and 2.
Among the 7662 study participants, the median number of AARs per CT was 16285 (interquartile range [IQR], 11940-21396]), yielding a median percentage of AAR>1 of 1% [IQR, 0-3]. The distribution of AARs across clinical/imaging and spirometry-based groups is shown in Supplement Figure 1. 1352 (17.6%) and 2893 (37.7%) participants met the criteria for suspected bronchiectasis and non-suspected bronchiectasis, respectively, and their characteristics and distribution across the 3 spirometric groups is shown in Table 1 and Supplement Figure 2. 1889 (24.6%) and 1528 (20%) participants met the criteria for Clinical manifestation only and CT manifestation only and their characteristics are provided in Supplement Table 3. Among those with suspected bronchiectasis, the median percentage of AAR>1 was 4% in both the obstructive spirometry GOLD stage 1-2 (mild to moderate COPD, n = 392) and 3-4 (severe-to-very severe COPD, n = 499).
Table 1.
Characteristics of participants across spirometry-based groups by suspected bronchiectasis status*
| Characteristic | Normal spirometry | Preserved ratio impaired spirometry (PRISm) | Obstructive spirometry | |||
|---|---|---|---|---|---|---|
| Non-suspected bronchiectasis | Suspected bronchiectasis | Non-suspected bronchiectasis | Suspected bronchiectasis | Non-suspected bronchiectasis | Suspected bronchiectasis | |
| (n=1598) | (n=296) | (n=399) | (n=165) | (n=896) | (n=891) | |
| Age, yr. | 57 ± 8 | 56 ± 8 | 59 ± 8 | 56 ± 8 | 64 ± 8 | 63 ± 9 |
| Women, n (%) | 945 (59) | 118 (40) | 256 (64) | 75 (45) | 539 (60) | 303 (34) |
| Men, n (%) | 653 (41) | 178 (60) | 143 (36) | 90 (55) | 357 (40) | 588 (66) |
| Non-Hispanic Black, n (%) | 526 (33) | 172 (58) | 117 (29) | 98 (59) | 155 (17) | 234 (26) |
| Non-Hispanic White, n (%) | 1072 (67) | 124 (42) | 282 (71) | 67 (41) | 741 (83) | 657 (74) |
| BMI | 28.6 ± 5.5 | 30.5 ± 6.7 | 30.5 ± 6.0 | 35.9 ± 8.0 | 27.7 ± 5.6 | 29.1 ± 6.9 |
| Current Smoking Status, n (%) | 792 (50) | 218 (74) | 206 (52) | 122 (74) | 325 (36) | 338 (38) |
| Former Smoking Status, n (%) | 806 (50) | 78 (26) | 193 (48) | 43 (26) | 571 (64) | 553 (62) |
| Pack-Years Smoked | 35.9 ± 20.0 | 39.8 ± 22.1 | 41.62 ± 22.3 | 48.1 ± 32.1 | 47.2 ± 23.5 | 55.9 ± 30.7 |
| Cough, n (%) | 144 (9) | 246 (83) | 16 (4) | 128 (78) | 59 (7) | 638 (72) |
| Phlegm, n (%) | 128 (8) | 232 (78) | 28 (7) | 112 (68) | 72 (8) | 699 (78) |
| mMRC dyspnea score ≥1, n (%) | 334 (21) | 237 (80) | 139 (35) | 153 (93) | 406 (46) | 831 (93) |
| ≥2 Exacerbations the year before enrollment, n (%) | 4 (0.3) | 31 (10) | 1 (0.3) | 31 (19) | 9 (1) | 292 (33) |
| Comorbidities, n (%) | ||||||
| Hypertension, n (%) | 645 (40) | 145 (49) | 211 (53) | 115 (70) | 484 (54) | 563 (63) |
| Coronary Artery Disease, n (%) | 108 (7) | 34 (11) | 48 (12) | 38 (23) | 101 (11) | 183 (21) |
| Congestive Heart Failure, n (%) | 17 (1) | 6 (2) | 9 (2) | 18 (11) | 20 (2) | 64 (7) |
| Stroke, n (%) | 21 (1) | 5 (2) | 9 (2) | 9 (5) | 24 (3) | 37 (4) |
| Diabetes, n (%) | 170 (11) | 57 (19) | 72 (18) | 59 (36) | 87 (10) | 149 (17) |
| High Cholesterol, n (%) | 630 (39) | 106 (36) | 201 (50) | 76 (46) | 443 (49) | 443 (50) |
| Cancer, n (%) | 80 (5) | 15 (5) | 25 (6) | 11 (7) | 85 (9) | 111 (12) |
| Mean number of comorbidities | 1.1 ± 1.1 | 1.2 ± 1.3 | 1.4 ± 1.3 | 2.0 ± 1.5 | 1.4 ± 1.2 | 1.7 ± 1.4 |
| FEV1, L | 2.8 ± 0.6 | 2.9 ± 0.7 | 2.0 ± 0.5 | 2.0 ± 0.6 | 1.8 ± 0.7 | 1.4 ± 0.7 |
| FEV1 % predicted | 96.9 ± 11.2 | 97.4 ± 11.8 | 72.0 ± 7.0 | 66.5 ± 11 | 66.9 ± 21.1 | 47.6 ± 20.2 |
| FVC, L | 3.5 ± 0.8 | 3.7 ± 1.0 | 2.7 ± 0.6 | 2.6 ± 0.8 | 3.1 ± 1.0 | 2.9 ± 1.0 |
| FVC % predicted | 96.1 ± 11.6 | 96.0 ± 12.6 | 73.1 ± 7.9 | 68.4 ± 11.3 | 88.0 ± 19.1 | 74.6 ± 19.0 |
| FEV1/FVC Ratio | 0.79 ± 0.05 | 0.80 ± 0.06 | 0.76 ± 0.05 | 0.76 ± 0.05 | 0.57 ± 0.12 | 0.48 ± 0.13 |
| Oxygen Saturation at rest, % | 97.1 ± 1.8 | 96.9 ± 2.9 | 96.6 ± 2.2 | 96.1 ± 2.6 | 95.8 ± 2.9 | 94.4 ± 3.7 |
| Emphysema on CT, % | 1.8 ± 2.4 | 2.0 ± 2.8 | 1.3 ± 2.5 | 1.9 ± 3.1 | 8.6 ± 10.3 | 15.2 ± 13.4 |
| Airway wall thickness, mm | 2.04 ± 0.41 | 2.02 ± 0.55 | 2.37 ± 0.46 | 2.69 ± 0.74 | 2.41 ± 0.47 | 2.90 ± 0.65 |
| AAR>1, % | 0.0 [0.0 - 0.0] | 4.5 [3.0 - 7.0] | 0.0 [0.0 - 0.0] | 4.0 [3.0 - 6.0] | 0.0 [0.0 - 1.0] | 4.0 [3.0 - 7.0] |
Abbreviations: BMI, body mass index; mMRC, modified Medical Research Council; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; CT, computed tomography; AAR, airway to artery ratio.
Data are presented as mean ± standard deviation and frequency (percentages) for continuous and categorical variables, except for AAR>1, which is presented as median [interquartile range]
Race and ethnic group were reported by the participant. Only non-Hispanic White and non-Hispanic Black participants were included.
Emphysema on CT was measured as the percentage of voxels <−950 Hounsfield Units.
Airway wall thickness was measured on CT as the square root of the wall area of an ideal 10-mm-inner-perimeter airway
The number of participants with missing data for the overall cohort: pack-years of smoking, 2; mMRC scale, 10; hypertension, 1; stroke, 2; oxygen saturation at rest, 3; CT measures of emphysema and airway wall thickness, 309.
Association between suspected bronchiectasis and all-cause mortality across spirometry-based groups
In a median follow-up of 11.0 years (IQR, 6.2–12.4), 2095 (27.3%) participants died. In adjusted models (Table 2), suspected bronchiectasis vs non-suspected bronchiectasis was associated with a higher risk of all-cause mortality in all spirometry-based groups (difference in 10-yr mortality probability (Pr), 0.15, 0.07, and 0.06 for normal, PRISm, and obstructive groups, respectively). In PRISm, the estimate was similar to the obstructive group’s, but the confidence interval crossed zero (Table 2 and Figure 2). The mortality risk estimates were similar when using the seven comorbidities separately in the adjusted model (Supplemental Table 4). Further analyzing the obstructive (i.e., COPD) group, the association between suspected bronchiectasis and all-cause mortality was comparable between mild-to-moderate (GOLD 1–2, Pr 0.29 [95%CI 0.26 to 0.32]) and severe-to-very severe COPD (GOLD 3–4, Pr 0.28 [95%CI 0.25 to 0.31])
Table 2.
Association of suspected bronchiectasis and CT manifestation only with 10-yr all-cause mortality across spirometry-based groups.*
| Spirometry-based group | Non-suspected bronchiectasis | CT manifestation only | Suspected bronchiectasis | Difference in adjusted all-cause mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of participants | 10-yr cumulative adjusted all-cause mortality | Number of participants | 10-yr cumulative adjusted all-cause mortality | Number of participants | 10-yr cumulative adjusted all-cause mortality | CT manifestation only vs Non-suspected bronchiectasis | Suspected bronchiectasis vs Non-suspected bronchiectasis | ||||
| who died | total | probability (95% CI) | who died | total | probability (95% CI) | who died | total | probability (95% CI) | probability (95% CI) | probability (95% CI) | |
| Normal spirometry | 176 | 1598 | 0.20 (0.18 to 0.23) | 107 | 731 | 0.25 (0.21 to 0.28) | 68 | 296 | 0.35 (0.29 to 0.41) | 0.04 (−0.001 to 0.08) | 0.15 (0.09 to 0.21) |
| PRISm | 76 | 399 | 0.23 (0.19 to 0.27) | 41 | 149 | 0.30 (0.23 to 0.36) | 51 | 165 | 0.30 (0.23 to 0.37) | 0.07 (−0.01 to 0.15) | 0.07 (−0.003 to 0.15) |
| Obstructive spirometry | 244 | 896 | 0.20 (0.18 to 0.22) | 291 | 648 | 0.24 (0.22 to 0.26) | 486 | 891 | 0.26 (0.24 to 0.28) | 0.04 (0.01 to 0.07) | 0.06 (0.03 to 0.09) |
Estimates represent 10-yr adjusted cumulative probability of all-cause mortality from a Cox model that included a variable combining 4 clinical/imaging groups and 3 spirometry-based groups as main exposure. Clinical/imaging groups: 1) non-suspected bronchiectasis (ref); 2) Clinical manifestation only; 3) CT manifestation only; and 4) Suspected bronchiectasis. Spirometry-based groups: normal, PRISm, obstructive). Covariates included age (continuous), sex (women (ref) vs men), race (Black (ref) vs White), BMI (continuous), current smoking status (former (ref) vs current), pack-years of smoking (continuous), oxygen saturation (continuous), the number of comorbidities (continuous), FEV1 (continuous), CT measures of emphysema (continuous) and airway wall thickness (continuous), and CT scanner make/model (5 groups). Clinical center was included in the “ID” statement and the option “covs (aggregate)” was specified in the proc PHREG statement to obtain the robust sandwich estimates of the covariance matrix. Adjusted probabilities were calculated using the direct standardization method (baseline variables and “diradj” statements of the PHREG procedure of SAS). See the main text for additional statistical details.
Figure 2.
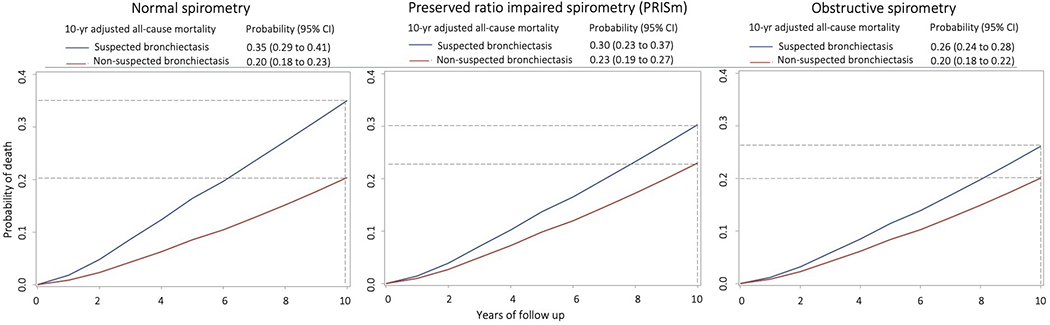
Mortality curve plots by suspected bronchiectasis status across spirometry-based groups. Left, participants with a history of smoking and normal spirometry (defined as FEV1/FVC ratio ≥0.7 and FEV1 % predicted ≥80). Middle, participants with a history of smoking and preserved ratio impaired spirometry (defined as FEV1/FVC ≥0.7 and FEV1 % predicted <80). Right, participants with a history of smoking and obstructive spirometry (defined as FEV1/FVC <0.7). Data are from a Cox survival model adjusted for age, sex, race, body mass index, smoking status, pack-years smoked, the number of comorbidities, FEV1 after bronchodilator use, oxygen saturation, CT measures of emphysema and airway wall thickness, and CT scanner make/model. Suspected bronchiectasis was defined as >1% of bronchi with an airway-to-artery ratio >1 on chest computed tomography scans plus ≥2 of the following: cough, phlegm, dyspnea, and history of ≥2 exacerbations. Participants meeting criteria for suspected bronchiectasis (blue lines) have an increased risk of all-cause mortality compared to those without suspected bronchiectasis (red lines) across the 3 spirometric groups. Horizontal broken lines mark estimated probabilities of 10-yr mortality. Vertical broken lines mark the 10-yr follow-up.
Secondary Analysis
We also conducted an analysis using the objective radiologic metric alone. The percentage of AAR>1 was categorized into quartiles. All-cause mortality increased with increasing quartiles (Q) of AAR>1 among normal and obstructive spirometric groups; among participants with PRISm, the mortality trended to rise from Q1 to Q2, but estimates were similar across Q2-Q4 (Supplement Table 5). Participants meeting the criteria for CT manifestation only vs those with non-suspected bronchiectasis had a higher risk of all-cause mortality across the 3 spirometric groups, but the differences were attenuated among those with normal spirometry and the CIs for the differences were wider and crossed zero in normal and PRISm groups (Table 2, Supplement Figure 3). The association of Clinical manifestation only with all-cause mortality is shown in Supplement Table 6 and Supplement Figure 4. Supplement Figure 5 shows 10-yr adjusted mortality for all 4 clinical/imaging groups across the 3 spirometric groups.
Discussion
In over 7600 COPDGene participants with a history of smoking followed for over 11 years, we found that suspected bronchiectasis is associated with higher all-cause mortality. Participants with normal and obstructive spirometry meeting the criteria for suspected bronchiectasis had an increased risk of all-cause mortality, compared to the non-suspected bronchiectasis group. In participants with PRISm, the increase in mortality risk was inconclusive. Additionally, when only objective CT measures of bronchiectasis were used, compared to the non-bronchiectasis group, the associated risk of mortality was substantially attenuated and not significant in the normal spirometry group.
This study represents one of the largest systematic evaluations of suspected bronchiectasis in distinct spirometry-based groups of smoking individuals. Our study provides new insights into the association of suspected bronchiectasis and mortality by including two understudied groups of individuals with a history of smoking, those with normal spirometry and PRISm, and by using objective CT measurements applied to a large, well-characterized cohort. We found that participants with a history of smoking with normal spirometry meeting the criteria for suspected bronchiectasis have a 15% higher risk of all-cause mortality. The prognostic value of suspected bronchiectasis relies on combining the objective measurements of airway dilation on CT, the AARs>1, with subjective clinical manifestations; thus, the findings should be interpreted cautiously, as the respiratory symptoms are not specific to any airway disease. Further, we found no evidence that in smokers with normal spirometry CT manifestations of bronchiectasis alone (Table 2) portend an adverse prognosis.
The association between suspected bronchiectasis and higher risk of death can be explained by several factors: systemic inflammation that is shared by cigarette smoking and the airway abnormality; vascular changes (such as small pulmonary vessel pruning associated with bronchiectasis); frequent acute exacerbations; and sputum pathogens (e.g., Pseudomonas aeruginosa).(20, 25, 35–37) Further studies are needed to understand the relationship between bronchiectasis, tobacco smoke, and clinical outcomes in smoking populations with preserved lung function.
We also find that participants with a history of smoking and with obstructive spirometry (i.e., COPD) and suspected bronchiectasis have a heightened risk of all-cause mortality. While these findings confirm prior observations, our studies also advance the investigations by including the full spectrum of COPD severity, allowing us to perform subgroup analysis.(8, 10–12, 38) The mortality risk of suspected bronchiectasis is similar in mild-to-moderate and severe-to-very severe COPD, a novel finding involving mild COPD, the most common form of the disease.(15) Further insight is that the presence of the radiographic airway abnormality alone, as objectively measured with AARs>1, is also associated with a 4% increase in mortality risk compared to those with non-suspected bronchiectasis (Table 2). Tobacco smoke induces not only inflammation and remodeling of the airways but also pulmonary vascular changes (e.g., vasoconstriction, vasodilation, pruning, and remodeling of pulmonary arteries),(6, 20) raising the possibility that AARs also represent a “bronchovascular” process. Altogether, prior (8, 10–12, 38) and current findings support lung imaging as a potential tool to evaluate COPD, which can improve patient care because bronchiectasis is typically ruled in or ruled out with CT images.(31) Further work is needed to fully understand how the CT measures of AAR change over time and whether any treatments may halt the structural damage to the airways.
A large study in US adults found that current smoking status and high and low BMI were associated with PRISm. Compared with individuals with normal spirometry, PRISm has poorer clinical outcomes.(14) In this study, we found that in PRISm, the adjusted mortality risks of suspected bronchiectasis and CT manifestation only were not conclusively increased compared to non-suspected bronchiectasis (Table 2); although the sample size of PRISm was smaller than the other two spirometric groups, our analysis shows that other factors, such as the number of comorbidities, current smoking and airway wall thickness (data not shown), were more likely to explain risk of all-cause mortality than suspected bronchiectasis.
Limitations
This study has several limitations. One, this is an observational study; hence, conclusions about the causality between suspected bronchiectasis and death cannot be made. Second, the COPDGene cohort enrolled only non-Hispanic White and non-Hispanic Black participants, so the study findings may not be generalizable to all racial and ethnic groups. Third, the COPDGene study performed volumetric chest CT scans with sub-millimetric slice thickness allowing the deployment of an algorithm to quantify AARs. Such an imaging protocol might not be routinely acquired in clinical settings. Fourth, we used only airway dilation as a radiologic feature in the suspected bronchiectasis definition, which might have underestimated the burden of airway abnormality on CT. Fifth, despite adjustment for multiple potential confounders, including the number of comorbidities, there may have been residual and unmeasured confounding factors (e.g., sputum pathogens) that might have affected the association of interest. Sixth, missing data for some variables, such as CT measures of emphysema and airway wall thickness, could have affected the estimates for the association between suspected bronchiectasis and all-cause mortality.
Conclusions
Our study reveals that individuals with a history of smoking who have normal spirometry and COPD meeting the imaging and clinical criteria for suspected bronchiectasis have an increased risk of all-cause mortality. These findings support including lung imaging as a tool for clinically defining bronchiectasis and for COPD workup to improve patient care.
Supplementary Material
Funding/Support:
The COPDGene project was supported by awards U01 HL089897 and U01 HL089856 from the National Heart, Lung, and Blood Institute. The COPDGene® project was also supported by the COPD Foundation through an Industry Advisory Board comprising AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, and Sunovion. Dr. Diaz is supported by the NIH National Heart, Lung, and Blood Institute grants R01-HL133137, R01-HL149861, and R01-HL164824. Dr. Raul San José Estépar is supported by the NIH grants 1R01HL149877 and 5R21LM013670.
Contributor Information
Alejandro A. Diaz, Division of Pulmonary and Critical Care Medicine, Brigham and Women’s Hospital, Boston, Massachusetts.
Wei Wang, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Boston, Massachusetts.
Jose L. Orejas, Division of Pulmonary and Critical Care Medicine, Brigham and Women’s Hospital, Boston, Massachusetts.
Rim Elalami, Division of Pulmonary and Critical Care Medicine, Brigham and Women’s Hospital, Boston, Massachusetts.
Wojciech R. Dolliver, Division of Pulmonary and Critical Care Medicine, Brigham and Women’s Hospital, Boston, Massachusetts.
Pietro Nardelli, Department of Radiology, Brigham and Women’s Hospital, Boston, Massachusetts.
Ruben San José Estépar, Department of Radiology, Brigham and Women’s Hospital, Boston, Massachusetts.
Bina Choi, Division of Pulmonary and Critical Care Medicine, Brigham and Women’s Hospital, Boston, Massachusetts.
Carrie L. Pistenmaa, Division of Pulmonary and Critical Care Medicine, Brigham and Women’s Hospital, Boston, Massachusetts.
James C. Ross, Department of Radiology, Brigham and Women’s Hospital, Boston, Massachusetts.
Diego J. Maselli, Division of Pulmonary Diseases and Critical Care, the University of Texas San Antonio, San Antonio, Texas.
Andrew Yen, Department of Radiology, University of California San Diego, San Diego, California.
Kendra A. Young, Department of Epidemiology, Colorado School of Public Health, University of Colorado Aurora, Colorado.
Gregory L. Kinney, Department of Epidemiology, Colorado School of Public Health, University of Colorado Aurora, Colorado.
Michael H. Cho, Division of Pulmonary and Critical Care Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, Massachusetts.
Raul San José Estépar, Department of Radiology, Brigham and Women’s Hospital, Boston, Massachusetts.
References
- 1.Maselli DJ, Yen A, Wang W, Okajima Y, Dolliver WR, Mercugliano C, et al. Small Airway Disease and Emphysema Are Associated with Future Exacerbations in Smokers with CT-derived Bronchiectasis and COPD: Results from the COPDGene Cohort. Radiology. 2021;300(3):706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalmers JD, Chang AB, Chotirmall SH, Dhar R, McShane PJ. Bronchiectasis. Nat Rev Dis Primers. 2018;4(1):45. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell AE. Bronchiectasis - A Clinical Review. N Engl J Med. 2022;387(6):533–45. [DOI] [PubMed] [Google Scholar]
- 4.Aliberti S, Goeminne PC, O’Donnell AE, Aksamit TR, Al-Jahdali H, Barker AF, et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med. 2022;10(3):298–306. [DOI] [PubMed] [Google Scholar]
- 5.Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis. 2017;14(4):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polverino E, Dimakou K, Hurst J, Martinez-Garcia MA, Miravitlles M, Paggiaro P, et al. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J. 2018;52(3). [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Garcia MA, de la Rosa Carrillo D, Soler-Cataluna JJ, Donat-Sanz Y, Serra PC, Lerma MA, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(8):823–31. [DOI] [PubMed] [Google Scholar]
- 8.Mao B, Lu HW, Li MH, Fan LC, Yang JW, Miao XY, et al. The existence of bronchiectasis predicts worse prognosis in patients with COPD. Sci Rep. 2015;5:10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jairam PM, van der Graaf Y, Lammers JW, Mali WP, de Jong PA, group PS. Incidental findings on chest CT imaging are associated with increased COPD exacerbations and mortality. Thorax. 2015;70(8):725–31. [DOI] [PubMed] [Google Scholar]
- 10.Dhar R, Singh S, Talwar D, Murali Mohan BV, Tripathi SK, Swarnakar R, et al. Clinical outcomes of bronchiectasis in India: data from the EMBARC/Respiratory Research Network of India registry. Eur Respir J. 2023;61(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz AA, Nardelli P, Wang W, San Jose Estepar R, Yen A, Kligerman S, et al. Artificial Intelligence-based CT Assessment of Bronchiectasis: The COPDGene Study. Radiology. 2022:221109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De la Rosa D, Martinez-Garcia MA, Giron RM, Vendrell M, Olveira C, Borderias L, et al. Clinical impact of chronic obstructive pulmonary disease on non-cystic fibrosis bronchiectasis. A study on 1,790 patients from the Spanish Bronchiectasis Historical Registry. PLoS One. 2017;12(5):e0177931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agusti A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am J Respir Crit Care Med. 2023;207(7):819–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan ES, Balte P, Schwartz JE, Bhatt SP, Cassano PA, Couper D, et al. Association Between Preserved Ratio Impaired Spirometry and Clinical Outcomes in US Adults. JAMA. 2021;326(22):2287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988-1994 to 2007-2010. Chest. 2013;143(5):1395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N Engl J Med. 2016;374(19):1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JH, Grenier PA, et al. Clinical and Radiologic Disease in Smokers With Normal Spirometry. JAMA Intern Med. 2015;175(9):1539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz AA, Martinez CH, Harmouche R, Young TP, McDonald ML, Ross JC, et al. Pectoralis muscle area and mortality in smokers without airflow obstruction. Respir Res. 2018;19(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez-Carpintero Abad M, Sanchez-Salcedo P, de-Torres JP, Alcaide AB, Seijo LM, Pueyo J, et al. Prevalence and burden of bronchiectasis in a lung cancer screening program. PLoS One. 2020;15(4):e0231204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz AA, Maselli DJ, Rahaghi F, Come CE, Yen A, Maclean ES, et al. Pulmonary vascular pruning in smokers with bronchiectasis. ERJ Open Res. 2018;4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan WC, Hague CJ, Leipsic J, Bourbeau J, Zheng L, Li PZ, et al. Findings on Thoracic Computed Tomography Scans and Respiratory Outcomes in Persons with and without Chronic Obstructive Pulmonary Disease: A Population-Based Cohort Study. PLoS One. 2016;11(11):e0166745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai Q, Triphuridet N, Zhu Y, You N, Yip R, Yankelevitz DF, et al. Bronchiectasis in Low-Dose CT Screening for Lung Cancer. Radiology. 2022;304(2):437–47. [DOI] [PubMed] [Google Scholar]
- 23.Meerburg JJ, Veerman GDM, Aliberti S, Tiddens H. Diagnosis and quantification of bronchiectasis using computed tomography or magnetic resonance imaging: A systematic review. Respir Med. 2020;170:105954. [DOI] [PubMed] [Google Scholar]
- 24.Chalmers JD, Moffitt KL, Suarez-Cuartin G, Sibila O, Finch S, Furrie E, et al. Neutrophil Elastase Activity Is Associated with Exacerbations and Lung Function Decline in Bronchiectasis. Am J Respir Crit Care Med. 2017;195(10):1384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189(5):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Garcia MA, de Gracia J, Vendrell Relat M, Giron RM, Maiz Carro L, de la Rosa Carrillo D, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J. 2014;43(5):1357–67. [DOI] [PubMed] [Google Scholar]
- 27.Dolliver WR, Wang W, Nardelli P, Rahaghi FN, Orejas JL, Maselli DJ, et al. Pulmonary arterial pruning is associated with CT-derived bronchiectasis progression in smokers. Respir Med. 2022;202:106971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart JI, Moyle S, Criner GJ, Wilson C, Tanner R, Bowler RP, et al. Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. COPD. 2012;9(5):466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowe KE, Regan EA, Anzueto A, Austin E, Austin JHM, Beaty TH, et al. COPDGene((R)) 2019: Redefining the Diagnosis of Chronic Obstructive Pulmonary Disease. Chronic Obstr Pulm Dis. 2019;6(5):384–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traversi L, Miravitlles M, Martinez-Garcia MA, Shteinberg M, Bossios A, Dimakou K, et al. ROSE: radiology, obstruction, symptoms and exposure - a Delphi consensus definition of the association of COPD and bronchiectasis by the EMBARC Airways Working Group. ERJ Open Res. 2021;7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–36. [DOI] [PubMed] [Google Scholar]
- 33.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. [DOI] [PubMed] [Google Scholar]
- 34.Denz R, Klaassen-Mielke R, Timmesfeld N. A comparison of different methods to adjust survival curves for confounders. Stat Med. 2023;42(10):1461–79. [DOI] [PubMed] [Google Scholar]
- 35.Choi H, Park HY, Han K, Yoo J, Shin SH, Yang B, et al. Non-Cystic Fibrosis Bronchiectasis Increases the Risk of Lung Cancer Independent of Smoking Status. Ann Am Thorac Soc. 2022;19(9):1551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, Chalmers JD. A Comprehensive Analysis of the Impact of Pseudomonas aeruginosa Colonization on Prognosis in Adult Bronchiectasis. Ann Am Thorac Soc. 2015;12(11):1602–11. [DOI] [PubMed] [Google Scholar]
- 37.Chalmers JD, Aliberti S, Filonenko A, Shteinberg M, Goeminne PC, Hill AT, et al. Characterization of the “Frequent Exacerbator Phenotype” in Bronchiectasis. Am J Respir Crit Care Med. 2018;197(11):1410–20. [DOI] [PubMed] [Google Scholar]
- 38.McDonnell MJ, Aliberti S, Goeminne PC, Restrepo MI, Finch S, Pesci A, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med. 2016;4(12):969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


