Abstract
Burkholderia pseudomallei is the causative agent of melioidosis, an infectious disease, which is increasingly recognized as an important public health problem in various tropical regions. This study describes the identification and characterization of a heat-stable extracellular toxin of B. pseudomallei. After cultivation of B. pseudomallei in liquid media, the heated cell-free supernatant was concentrated by ultrafiltration. The concentrate exhibited a cytotoxic and hemolytic activity which showed remarkable resistance against alkaline and acidic treatments. For further purification, reversed-phase chromatography using a fast-performance liquid chromatography system was performed. After elution with an acetonitrile gradient, a single cytotoxic and hemolytic peak was detected. Structural characterization of the toxin was performed by a combination of mass spectrometric and nuclear magnetic resonance spectroscopic techniques. A highly purified glycolipid, 2-O-α-l-rhamnopyranosyl-α-l-rhamnopyranosyl-β-hydroxytetradecanoyl-β-hydroxytetradecanoate (Rha-Rha-C14-C14), with a molecular mass of 762 Da was identified. The purified exolipid showed a time- and dose-dependent cytotoxic effect on phagocytic (HL60) and nonphagocytic (HeLa) cell lines. In addition, a time- and dose-dependent hemolysis of erythrocytes from various species was observed. The toxin structure makes a detergentlike action most probable. Interestingly, the cytotoxic and hemolytic activities of the glycolipid could be neutralized by albumin. Future studies will concentrate on the role of this exolipid as a virulence factor in the pathogenesis of melioidosis.
The gram-negative, saprophytic rod Burkholderia pseudomallei is the causative agent of melioidosis, an infectious disease of humans and animals with a protean clinical spectrum. Southeast Asia and northern Australia are the main areas of endemicity where the bacterium can be found in soil and surface waters (18, 27). In northeastern Thailand, melioidosis is a major public health problem and an important cause of community-acquired septicemia (2). There is evidence that melioidosis might also be endemic in Africa, the Indian subcontinent, and Central and South America (5). However, it is possible that the disease remains greatly underdiagnosed in many areas of the tropics where sophisticated laboratory facilities are not available (5). The clinical manifestation of melioidosis is extremely variable, ranging from acute or chronic localized forms to fulminant septic infections (6, 18). Severe septicemic melioidosis is significantly associated with underlying diseases, such as diabetes and chronic renal failure (2). However, fulminant melioidosis does occur in healthy individuals. Epidemiological studies suggest that mild or undetected infections are common and manifested only by seroconversion (26). The proportion of such seropositive individuals who may harbor viable B. pseudomallei is unknown. Long periods of latency and frequent relapses after antibiotic treatment are characteristic features of melioidosis (6). It is assumed that the majority of infections occur by inoculation of organisms from the environment into minor cuts or abrasions especially in people in regular contact with soil and muddy waters (7), although inhalation might also be an important route of infection under certain circumstances (14); the role of ingestion is unclear.
Laboratory investigations on the pathogenesis of melioidosis and the contribution of single bacterial structures to the virulence of B. pseudomallei have so far been carried out only on a very limited scale. Putative virulence factors of B. pseudomallei include endotoxin (25), a constitutively expressed exopolysaccharide recently identified in this laboratory (28), several relatively uncharacterized extracellular enzymes (1), and heat-labile toxic activities detected in cell-free filtrates of B. pseudomallei liquid cultures (11). In the present study, we have identified a heat-stable, extracellular glycolipid with cytotoxic and hemolytic properties produced by B. pseudomallei. Very pure exolipid was obtained, and the chemical structure has been elucidated.
MATERIALS AND METHODS
Bacteria and culture conditions.
B. pseudomallei NCTC 10274 used in this study was obtained from the National Collection of Type Cultures. Bacteria were stored until use in Luria broth supplemented with 20% (vol/vol) glycerol at −70°C. Working cultures were maintained on Columbia blood agar plates. For the production of the toxic exolipid, bacteria were grown in chemically defined, modified Vogel-Bonner medium (3.3 mM MgSO4, 10 mM citric acid, 28 mM NaNH4HPO4, 37 mM K2HPO4, 214 mM d-gluconic acid; medium pH 7.4). Twenty milliliters of a culture of B. pseudomallei grown overnight served as an inoculum for a 2-liter culture incubated at 37°C on a rotary shaker for 6 days. When bacteria were cultured in a glycerol medium (4% [vol/vol] glycerol, 0.15 M NaCl; medium pH 7), a different inoculum was used. Bacteria were grown overnight on Columbia blood agar plates and suspended in the glycerol medium. Ten milliliters of this bacterial suspension at a concentration of approximately 1011 cells per ml was used as an inoculum for 100 ml of medium. Cultures were incubated at 37°C with shaking under aerobic conditions. The growth of the cultures was monitored by plating properly diluted samples on Columbia blood agar plates and counting the colony-forming units (CFU).
Measurement of hemolytic activity.
Purified-toxin- or toxin-containing supernatants were serially diluted twofold in 10 mM phosphate-buffered saline (PBS) (10 mM sodium phosphate buffer, 0.15 M NaCl, 3 mM KCl; solution pH 7.2). One hundred microliters of diluted toxin was added to 100 μl of 1% (vol/vol) human erythrocyte suspension prepared by washing the cells obtained from EDTA-treated blood with 10 mM PBS. Incubation was carried out in microtiter plates at 37°C for 1 h. After centrifugation for 3 min at 1,000 × g, the highest dilution showing complete hemolysis was defined as 1 hemolytic unit (HU). Comparative testing of mouse and sheep erythrocytes was performed in the same way. For a more precise determination of the hemolytic activity, the microtiter plates were centrifuged and the extent of hemolysis was determined by measuring the absorption of liberated hemoglobin in the supernatant at 540 nm. Human erythrocytes were incubated in 0.15 M NH4Cl, pH 7.3, to determine total lysis, and the background lysis was determined with erythrocytes incubated in 10 mM PBS. A standard curve of various concentrations of lysed erythrocytes was used to determine the percentage of hemolysis. When the time course of hemolysis of different toxin concentrations was determined, 1 ml of diluted toxin and 1 ml of the erythrocyte suspension was incubated in a microcentrifuge tube. Samples of 100 μl were removed at given time intervals, and liberated hemoglobin was determined as described above. The capability of bovine serum albumin to reduce the hemolytic activity of the exolipid was tested by diluting purified toxin with various concentrations of human and bovine serum albumin (Sigma, St. Louis, Mo., and Boehringer, Mannheim, Germany, respectively). The hemolytic activity of these samples was then tested as described above. Human and bovine gamma globulins (both from Sigma) at the appropriate concentrations were used as controls.
Determination of cytotoxity.
The cell lines for the cytotoxicity tests were obtained from the German Collection of Microorganisms and Cell Cultures in Braunschweig, Germany. HeLa cells (DSM ACC 161) were grown in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. For the cytotoxicity test, confluent cells were treated with trypsin-EDTA and diluted in serumfree medium (105 cells per ml) (Hybridomed DIF 1000; Seromed, Berlin, Germany). To prevent adherence of the HeLa cells, culture plates were shaken gently. Three hundred microliters of the cell suspension was incubated with 200 μl of various concentrations of purified toxic exolipid diluted with serumfree medium for 7 h. Samples of 20 μl were removed at given time intervals, stained with trypan blue, and the percentage of viable cells was determined. For a control, 300 μl of the cell suspension was incubated with 200 μl of serumfree medium under the same conditions. The nonadherent phagocytic cell line HL60 (DSM ACC 3) was grown in serumfree medium and tested in the same way.
Purification of the toxic exolipid.
B. pseudomallei supernatants obtained by the two different culture conditions described above served as starting material for the purification process. First, a 2-liter culture of B. pseudomallei grown in modified Vogel-Bonner medium for 6 days was used. The bacteria were pelleted by centrifugation at 6,000 × g for 40 min, and the supernatant was heated for 10 min at 100°C. Two ultrafiltration steps with a ProVario 3 apparatus (Pall-Filtron, Karlstein, Germany) followed. In the first ultrafiltration step, the supernatant was filtered through a membrane with a cutoff of 10 kDa. The filtrate was subjected to a second filtration step with a membrane cutoff of 3 kDa. The hemolytic and cytotoxic retentate was further purified on a 3-ml Resource RP column (Pharmacia) by fast-performance liquid chromatography. The column was equilibrated with 0.1% (vol/vol) trifluoroacetic acid, and the retentate (>3 kDa) was injected onto the column. The material was eluted with a linear gradient of 0 to 90% acetonitrile (vol/vol) in 0.1% trifluoroacetic acid (vol/vol) at a flow rate of 1 ml/min within 70 min. Fractions (1 ml) containing the hemolytic activity were collected and lyophilized. In the second purification procedure, a supernatant of B. pseudomallei that had been cultured in the glycerol medium for 6 days was used. After the bacteria were pelleted and the supernatant was heated, ultrafiltration with a Centricon concentrator with a membrane cutoff of 10 kDa (Amicon) followed. This time the retentate (>10 kDa) exhibited hemolytic and cytotoxic activity and was further purified on the Resource RP column by fast protein liquid chromatography as described above.
Stability after pH treatment.
Solutions of purified toxin were adjusted to pH values ranging from 1 to 10 by adding 5 M HCl or 5 M NaOH. After incubation at 37°C for 1 h, the samples were adjusted to pH 7, and the hemolytic activity was determined.
Structural analysis.
After methanolysis (0.625 M HCl in methanol, 12 h, 70°C), the constituents (monosaccharide methylglycoside and fatty acid methylester) were pertrimethylsilylated and analyzed by gas chromatography-mass spectrometry (GC-MS) on a 30-m DB5 capillary column connected to a Finnigan GCQ ion trap mass spectrometer (Finnigan MAT Corp., San Jose, Calif.) running in the electronic impact mode. The components were identified by their retention time on the GC column and their mass spectra. Exact quantification was achieved by electronic peak integration and the use of correction factors determined by addition of internal standards (1 μg each of fucose and alpha-hydroxytetradecanoic acid). The absolute configuration of the monosaccharide component was determined by separation of its trimethylsilylated S-(+)-but-2-yl glycoside (10) on the same column. A Finnigan MAT TSQ 700 triple-quadrupole mass spectrometer equipped with a Finnigan electrospray ion source (Finnigan MAT Corp.) was used for analysis by ESI-MS. The sample was dissolved in methanol (concentration approximately 10 pmol per μl) and injected at a flow rate of 1 μl per min into the electrospray chamber. In the positive-ion mode, a voltage of +5.5 kV was applied to the electrospray needle. For collision-induced dissociation experiments, parent ions were selectively transmitted by the first mass analyzer and directed into the collision cell with argon as the collision gas at a kinetic energy of ca. −35 eV. For the detection of negative ions, all voltages were reversed.
Prior to all nuclear magnetic resonance (NMR) analyses, the sample (approximately 0.4 mg) was repeatedly lyophilized against D2O (>99.95 atom% D; Fluka) at pD 7 and ambient temperature. All 600-MHz 1H spectra were recorded at 300°K on a Bruker AVANCE DMX 600 NMR spectrometer incorporating a gradient unit and locked to the major deuterium resonance of the CD3OD solvent. 1H chemical shifts are referenced to the residual methanol signal (3.35 ppm). Phase-sensitive two-dimensional (2D) 1H-correlated spectroscopy (COSY), total correlated spectroscopy (TOCSY), and rotating frame nuclear Overhauser enhancement and exchange spectroscopy (ROESY) were performed on the same instrument with mixing times of 80 and 500 ms, respectively, for the two latter techniques.
RESULTS
Identification of a heat-stable exotoxin secreted into the supernatants of B. pseudomallei liquid cultures.
Direct testing of heated culture supernatant of B. pseudomallei grown in modified Vogel-Bonner medium for 6 days showed no hemolytic activity. The supernatant was then subjected to the first ultrafiltration with a membrane cutoff of 10 kDa. Since no hemolytic activity was detected in the retentate, the filtrate was then concentrated 40-fold by using a membrane cutoff of 3 kDa. After this procedure, cytotoxicity to HeLa cells and hemolytic activity to human erythrocytes could be detected in the retentate (data not shown). No loss of activity was observed after acid and alkaline treatment within a pH range from pH 1 to 12. Since significant amounts of either cytotoxic or hemolytic activity were found only in the heated culture supernatant of modified Vogel-Bonner medium after bacteria reached the stationary phase of growth, culture conditions where B. pseudomallei was incubated at high cell densities in a sodium chloride solution containing glycerol were used. Under these conditions, bacteria replicated slowly and toxin formation could be measured in heated supernatant without any concentration step. Exotoxin production was detected after only 24 h of incubation (Fig. 1). Continued incubation led to increasing toxin levels, with a maximum after 5 days. The resistance of the toxic activity in the glycerol medium to acid and alkali treatment was the same as that observed with the modified Vogel-Bonner medium.
FIG. 1.
Time course of production of the exotoxin into the supernatant of a B. pseudomallei culture grown with glycerol. Bacteria were inoculated at high density in 0.15 M NaCl containing 4% (vol/vol) glycerol at pH 7 and incubated for 6 days. Symbols: •, CFU per ml; ▾, HU.
Purification of the exotoxin.
The exotoxin was purified from the supernatants obtained by the two different culture conditions described above. The toxic fraction derived from modified Vogel-Bonner medium was concentrated within the molecular mass range from 3 to 10 kDa by ultrafiltration. Surprisingly, the toxic activity of the culture grown in glycerol was found to be >10 kDa, as it could be concentrated by using a membrane cutoff of 10 kDa. Interestingly, no matter which retentate was further purified by reversed-phase chromatography, in each case, a single sharp toxic peak consisting of four fractions eluted between 73 and 78% acetonitrile. This observation led to the hypothesis that the toxic molecules in the glycerol medium were present in an aggregated form due to its higher concentration. Indeed, when toxin concentrations exceeded 8 HU per ml, toxic activity was found in the retentate after ultrafiltration (10-kDa exclusion size), whereas with concentrations below 8 HU per ml, toxic activity was found in the filtrate. About 15% of the initial toxic activity of the glycerol culture supernatant was recovered after reversed-phase chromatography. The fact that only a small portion of the initial heat-stable toxic activity in the glycerol-grown culture was recovered in purified form could at least partly be explained by a significant loss during the first ultrafiltration step (data not shown). After lyophilization of the purified-toxin-containing fractions, a colorless powder was obtained, which was then subjected to structural analysis.
Structural elucidation of the exotoxin.
Compositional analysis of the exotoxin sample revealed the presence of l-rhamnose and β-hydroxytetradecanoic acid in a ratio of approximately 1:1, suggestive of a glycolipid. The absolute configuration of the lipid moiety was not determined, but published data for bacterial glycolipids (22) and the biosynthesis pathway of β-hydroxy fatty acids in gram-negative bacteria (13) suggest the presence of the d enantiomer. Molecular ions with a m/z of 785 [M+Na]+ in the positive-ion mode and a m/z of 761 [M-H]− in the negative-ion mode were detected by ESI/MS and were compatible with a compound consisting of two residues each of l-rhamnose and β-hydroxytetradecanoic acid. MS/MS of the positively charged molecular ion with a m/z of 785 yielded the daughter ion spectrum depicted in Fig. 2, which clearly indicates a linear arrangement of a disaccharide moiety linked to two β-hydroxytetradecanoic acid residues, as depicted in the fragmentation scheme. Structural details were resolved by 600-MHz 1H 1D and 2D NMR techniques. After assignment of the resonances of the two α-rhamnopyranosyl systems and two β-hydroxytetradecanoic acid residues by 2D COSY and TOCSY techniques (Table 1), the interresidual linkages were determined from 2D ROESY spectra (Table 2). Thus, an 1-2 linkage between the rhamnose residues was established by the detection of an interresidual ROE correlation between H-1 of the terminal and H-2 of the second internal rhamnose moieties. Similarly, this disaccharide unit was linked glycosidically to the β-hydroxyl group of the internal lipid residue, detected by a correlation between H-1 of the internal rhamnose moiety and H-3 of the internal lipid group. The low field shift of H-3 of the terminal lipid moiety was indicative of the ester linkage between the two lipid units. The presence of only single signals for H-1 of the internal rhamnose and for H-3 of both β-hydroxy fatty acid moieties clearly indicated the presence of only one enantiomeric form for the latter, although the NMR data did not allow distinction between d and l enantiomers. Figure 3 shows the proposed structure of the rhamnolipid produced by B. pseudomallei.
FIG. 2.
Positive-ion mode ESI-MS/MS of the molecular ion (sodium adduct) of the exotoxin isolated from B. pseudomallei. The detected fragments and their m/z values are shown in the fragmentation scheme at the top of the figure. Detailed information on the fragmentation of glycoconjugates can be found in reference 8.
TABLE 1.
1H shift NMR data for the exotoxin in CD3OD at 300°Ka
| Moiety of exotoxinb | Atom | Shift (ppm) |
|---|---|---|
| A | H-1 | 4.93 |
| H-2 | 4.01 | |
| H-3 | 3.70 | |
| H-4 | 3.42 | |
| H-5 | 3.72 | |
| H-6 | 1.29 | |
| B | H-1 | 4.95 |
| H-2 | 3.76 | |
| H-3 | 3.83 | |
| H-4 | 3.30 | |
| H-5 | 3.69 | |
| H-6 | 1.28 | |
| C | H-2A | 2.58 |
| H-2B | 2.50 | |
| H-3 | 4.15 | |
| H-4 | 1.59 | |
| H-(5–13)c | 1.36 | |
| H-14 | 0.94 | |
| D | H-2A | 2.51 |
| H-2B | 2.51 | |
| H-3 | 5.37 | |
| H-4 | 1.64 | |
| H-(5–13)c | 1.36 | |
| H-14 | 0.94 |
Sequential ROEs were observed between H-1 of moiety A and H-2 of moiety B and between H-1 of moiety B and H-3 of moiety C.
The moieties of the exotoxin, Rha-Rha-C14-C14, are as follows: A, the first rhamnose; B, the second rhamnose; C, the first C14 sequence; and D, the second C14 sequence.
All methylene group signals form C5 to C13.
TABLE 2.
1H coupling NMR data for the exotoxin in CD3OD at 300°Ka
| Moiety of exotoxinb | Linkage | Coupling (Hz) |
|---|---|---|
| A | 1-2 | 1.8 |
| 2-3 | 3.4 | |
| 3-4 | 9.5 | |
| 4-5 | 9.5 | |
| 5-6 | 6.1 | |
| B | 1-2 | 1.7 |
| 2-3 | 3.1 | |
| 3-4 | 9.7 | |
| 4-5 | 9.7 | |
| 5-6 | 6.1 | |
| C | 2A-2B | 15.0 |
| 2A-3 | 8.0 | |
| 2B-3 | 4.8 | |
| 13-14 | 7.0 | |
| D | 13-14 | 7.0 |
Only couplings to first-order well-resolved signals are given.
The moieties of the exotoxin, Rha-Rha-C14-C14, are as follows: A, the first rhamnose; B, the second rhamnose; C, the first C14 sequence; and D, the second C14 sequence.
FIG. 3.
Proposed structure of the rhamnolipid produced by B. pseudomallei.
Hemolytic characteristics of the exolipid.
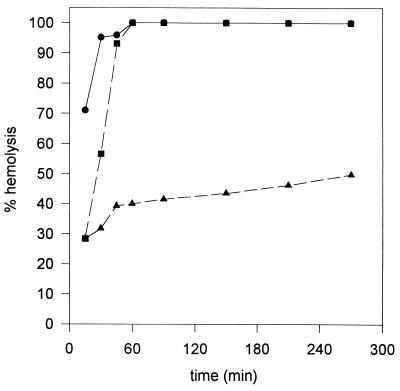
Mouse and sheep erythrocytes were found to be equally susceptible to the lytic action of the rhamnolipid, whereas human erythrocytes were found to be slightly more sensitive (about twofold). The rhamnolipid displayed a dose- and time-dependent hemolytic activity against human erythrocytes. The time course of hemolysis with various levels of purified exolipid is shown in Fig. 4. Approximately 250 μg of rhamnolipid was equivalent to 1 HU. High toxin levels showed complete hemolysis within a short time, whereas low concentrations exhibited a lag period that was inversely proportional to the toxin level. Liu (19) reported the neutralization of a heat-stable hemolytic activity of B. pseudomallei by animal and human sera. Interestingly, the hemolytic activity of the rhamnolipid could be neutralized by either human or bovine serum albumin (Fig. 5), whereas the respective gamma globulins showed no effect.
FIG. 4.
Time- and dose-dependent hemolysis of human erythrocytes by the rhamnolipid at various levels. Symbols: •, 4 HU per ml; ▪, 2 HU per ml; ▴, 1 HU per ml.
FIG. 5.
Neutralization of hemolytic activity (64 HU) of the B. pseudomallei rhamnolipid by bovine serum albumin (•). Bovine gamma globulin (▪) showed no neutralizing effect. The same results were obtained with human albumin and gamma globulin.
Cytotoxic activity of the exolipid.
In addition to the hemolytic activity, the rhamnolipid also displayed a time- and dose-dependent cytotoxicity against nonphagocytic HeLa and phagocytic HL60 cells. As there was also an inhibitory effect of serum albumin on cytotoxicity (data not shown), serumfree medium was used for the cytotoxicity assay. The toxin concentrations showing toxic effects on the cell lines were significantly higher than the levels needed for complete hemolysis. Analogous to the lysis of erythrocytes, the percentages of dead cells for HeLa cells (Fig. 6A) and HL60 cells (Fig. 6B) are proportional to the toxin concentration and the lag period is inversely proportional to the toxin level.
FIG. 6.
Time- and dose-dependent cytotoxicity of the B. pseudomallei rhamnolipid on HL60 cells (A) or HeLa cells (B). Symbols: •, 80 HU per ml; ▪, 40 HU per ml; ▴, 20 HU per ml.
DISCUSSION
It has been known for some time that cell-free filtrates of B. pseudomallei liquid cultures possess toxic activities in mice and hamsters (3, 12). It was suggested that two heat-labile toxic components, which are responsible for either lethal activity or the immediate development of hemorrhagic and necrotic lesions after intradermal inoculation, must be present in these filtrates (12). In crude preparations, necrotoxicity was found to be associated with proteolytic activity, whereas the lethal activity of culture filtrates was correlated with a potent anticoagulant activity (11). In addition, a heat-stable toxic component, which showed a delayed type of skin reaction, was described (23). It was speculated that this heat-stable toxic activity was due to endotoxin present in the filtrates (23). Hemolytic activity was not observed in those filtrates containing the different toxic activities (12). By using a cellophane plate technique, Liu (19) detected a heat-stable hemolytic activity of B. pseudomallei, which was neutralized by animal and human sera, most likely due to albumin. It was suggested that the appearance of only weak hemolysis around confluent growth but not individual colonies could be explained by the inhibition of hemolysis by the serum present in ordinary blood agar medium (19). More recently, Ashdown et al. (1) described the production of small hemolytic zones around heavy growth on brain heart infusion agar containing washed sheep erythrocytes in the majority of clinical strains. These researchers could not detect hemolytic activity in broth culture filtrates of those strains.
It seems likely that the acidic rhamnolipid identified in this study is responsible for the heat-stable hemolytic activity observed by Liu (19). The loss of activity of the purified toxin in the presence of albumin described here is in accordance with this assumption. In this study, hemolytic activity of B. pseudomallei liquid cultures was observed only prior to concentration steps when supernatants of very high bacterial densities and limited growth conditions were tested. This might explain the fact that hemolytic activity was not detected in liquid cultures by others (1, 12). The purification protocol established in this study, which is based on ultrafiltration and reversed-phase chromatography, resulted in a rhamnolipid of very high purity with a molecular mass of 762 Da. The fact that the high toxin levels obtained in the culture grown in glycerol medium could be concentrated by a 10-kDa-cutoff membrane implies that under these conditions the critical micelle concentrations was reached and the rhamnolipid molecules aggregated to form micelles of more than 10 kDa, whereas the toxin production and the degree of aggregation after cultivation in the modified Vogel-Bonner medium were obviously lower. The B. pseudomallei rhamnolipid demonstrated remarkable toxicity on various cell lines and hemolytic activity on erythrocytes from different species. It seems likely that the hemolytic and cytotoxic activities of the amphilic B. pseudomallei rhamnolipid are due to detergentlike properties and cell membranes are most likely perturbed by the introduction of its fatty acid chains into the organized lipid layer of cells.
Pseudomonas aeruginosa strains have been shown to produce a unique glycolipid very similar to the B. pseudomallei rhamnolipid that also contains rhamnose and β-hydroxycarboxylic acids (15). The two principal P. aeruginosa glycolipids produced in liquid cultures consist of either two molecules of rhamnose and two molecules of β-hydroxydecanoic acid (Rha-Rha-C10-C10) or of one molecule of rhamnose and two molecules of β-hydroxydecanoic acid (Rha-C10-C10) (24). The rhamnolipids of P. aeruginosa are responsible for the heat-stable hemolytic activity in culture supernatants (9) and were recently shown to inhibit macrophage functions (20, 21). In contrast, the B. pseudomallei rhamnolipid identified in this study is composed of two molecules of rhamnose and two molecules of β-hydroxytetradecanoic acid (C14). The exolipid preparations in this study had completely homogeneous fatty acid chains. Unlike, e.g., the trehalose lipids of mycobacteria, corynebacteria, and nocardia (4), the fatty acids of the rhamnolipid are not bound to the sugar by acyl bonds. A glycosidic bond with a hydroxyl group of the first fatty acid is formed, and the second acid is bound to the first acid by an ester linkage, resulting in a glycolipid with a free carboxyl group. It is generally assumed that bacteria growing on water-insoluble hydrocarbon substrates benefit from the presence of a surfactant (e.g., glycolipids) with emulsifying capabilities (4). It will be interesting to investigate the influence of the rhamnolipid on the hydrocarbon fermentation of B. pseudomallei. The P. aeruginosa rhamnolipid was detected in sputum samples of cystic fibrosis patients colonized with P. aeruginosa, and the highest concentrations were found in phases of acute exacerbations (17). Further investigations should focus on the role of the B. pseudomallei rhamnolipid on intracellular (16) and extracellular growth in acute and chronic melioidosis. The generation of B. pseudomallei rhamnolipid knockout strains in the future should help to elucidate the role of this toxic exolipid in experimental infections with this organism.
ACKNOWLEDGMENT
We gratefully acknowledge the continuous encouragement of D. Bitter-Suermann.
REFERENCES
- 1.Ashdown L, Koehler J M. Production of hemolysin and other extracellular enzymes by clinical isolates of Pseudomonas pseudomallei. J Clin Microbiol. 1990;28:2331–2334. doi: 10.1128/jcm.28.10.2331-2334.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaowagul W, White N J, Dance D A B, Wattanagoon Y, Naigowit P, Davis T M E, Looareesuwan S, Pitakwatchara N. Melioidosis: a major cause of community acquired septicemia in northeastern Thailand. J Infect Dis. 1989;159:890–899. doi: 10.1093/infdis/159.5.890. [DOI] [PubMed] [Google Scholar]
- 3.Colling M, Nigg C, Heckly R J. Toxins of Pseudomonas pseudomallei. I. Production in vitro. J Bacteriol. 1958;76:422–426. doi: 10.1128/jb.76.4.422-426.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper D G, Zajic J E. Surface-active compounds from microorganisms. Adv Appl Microbiol. 1980;26:229–253. [Google Scholar]
- 5.Dance D A B. Melioidosis: the tip of the iceberg? Clin Microbiol Rev. 1991;4:52–60. doi: 10.1128/cmr.4.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dance D A B. Melioidosis. Rev Med Microbiol. 1990;1:143–150. [Google Scholar]
- 7.Dance D A B. Pseudomonas pseudomallei: danger in the paddy fields. Trans R Soc Trop Med Hyg. 1991;85:1–3. doi: 10.1016/0035-9203(91)90134-k. [DOI] [PubMed] [Google Scholar]
- 8.Domon B, Costello C E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj J. 1988;5:397–409. [Google Scholar]
- 9.Fujita K, Akino T, Yoshioka H. Characteristics of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect Immun. 1988;56:1385–1387. doi: 10.1128/iai.56.5.1385-1387.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerwig G J, Kamerling J P, Vliegenthart J F G. Determination of the D and L configuration of neutral monosaccharides by high-resolution capillary GLC. Carbohydr Res. 1978;62:381–396. [Google Scholar]
- 11.Heckly R J. Differentiation of exotoxin and other biologically active substances in Pseudomonas pseudomallei filtrates. J Bacteriol. 1964;88:1730–1736. doi: 10.1128/jb.88.6.1730-1736.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heckly R J, Nigg C. Toxins of Pseudomonas pseudomallei. II. Characterization. J Bacteriol. 1958;76:427–436. doi: 10.1128/jb.76.4.427-436.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hommel R K, Ratledge C. Biosynthetic mechanisms of low molecular weight surfactants and their precursor molecules. Surfactant Sci Ser. 1993;48:3–63. [Google Scholar]
- 14.Howe C, Sampath A, Spotnitz M. The Pseudomallei group: a review. J Infect Dis. 1971;124:598–606. doi: 10.1093/infdis/124.6.598. [DOI] [PubMed] [Google Scholar]
- 15.Jarvis F G, Johnson M J. A glycolipid produced by Pseudomonas aeruginosa. J Am Chem Soc. 1949;71:4124–4126. [Google Scholar]
- 16.Jones A L, Beveridge T J, Woods D E. Intracellular survival of Burkolderia pseudomallei. Infect Immun. 1996;64:782–790. doi: 10.1128/iai.64.3.782-790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kownatzki R, Tümmler B, Döring G. Rhamnolipids of Pseudomonas aeruginosa in sputum of cystic fibrosis patients. Lancet. 1987;i:1026–1027. doi: 10.1016/s0140-6736(87)92286-0. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 18.Leelarasmee A, Bovornkitti S. Melioidosis: review and update. Rev Infect Dis. 1989;11:413–425. doi: 10.1093/clinids/11.3.413. [DOI] [PubMed] [Google Scholar]
- 19.Liu P V. Survey of hemolysin production among species of pseudomonads. J Bacteriol. 1957;74:718–727. doi: 10.1128/jb.74.6.718-727.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClure C D, Schiller N L. Effects of Pseudomonas aeruginosa rhamnolipids on human monocyte-derived macrophages. J Leukocyte Biol. 1992;51:97–102. doi: 10.1002/jlb.51.2.97. [DOI] [PubMed] [Google Scholar]
- 21.McClure C D, Schiller N L. Inhibition of macrophage phagocytosis by Pseudomonas aeruginosa rhamnolipids in vitro and in vivo. Curr Microbiol. 1996;33:109–117. doi: 10.1007/s002849900084. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa Y, Matsuyama T. Chromatographic determination of optical configuration of 3-hydroxy fatty acids composing microbial surfactants. FEMS Microbiol Lett. 1993;108:99–102. [Google Scholar]
- 23.Nigg C, Heckly R J, Colling M. Toxin produced by Malleomyces pseudomallei. Proc Soc Exp Biol Med. 1955;89:17–20. doi: 10.3181/00379727-89-21700. [DOI] [PubMed] [Google Scholar]
- 24.Ochsner U A, Hembach T, Fiechter A. Production of rhamnolipid biosurfactants. Biochem Eng Biotechnol. 1995;53:90–117. doi: 10.1007/BFb0102326. [DOI] [PubMed] [Google Scholar]
- 25.Perry M B, MacLean L L, Schollaardt T, Bryan L E, Ho M. Structural characterization of the lipopolysaccharide O antigens of Burkholderia pseudomallei. Infect Immun. 1995;63:3348–3352. doi: 10.1128/iai.63.9.3348-3352.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanford J P. Pseudomonas species (including melioidosis and glanders) In: Mandell G L, Douglas R G, Bennett J E, editors. Principles and practice of infectious disease. 4th ed. New York, N.Y: John Wiley and Sons; 1995. pp. 2003–2009. [Google Scholar]
- 27.Smith M D, Wuthiekanum V, Walsh A L, White N J. Quantitative recovery of Burkholderia pseudomallei from soil in Thailand. Trans R Soc Trop Med Hyg. 1995;89:488–490. doi: 10.1016/0035-9203(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 28.Steinmetz I, Rhode M, Brenneke B. Purification and characterization of an exopolysaccharide of Burkholderia (Pseudomonas) pseudomallei. Infect Immun. 1995;63:3959–3965. doi: 10.1128/iai.63.10.3959-3965.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]