Abstract
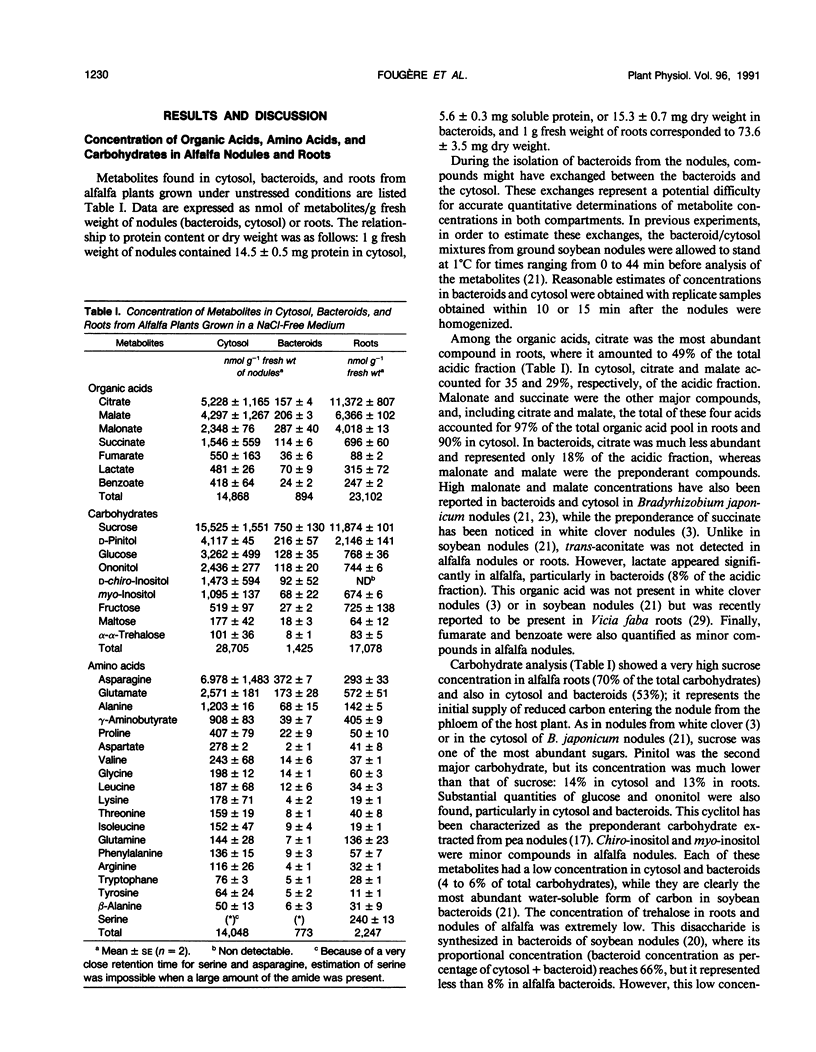
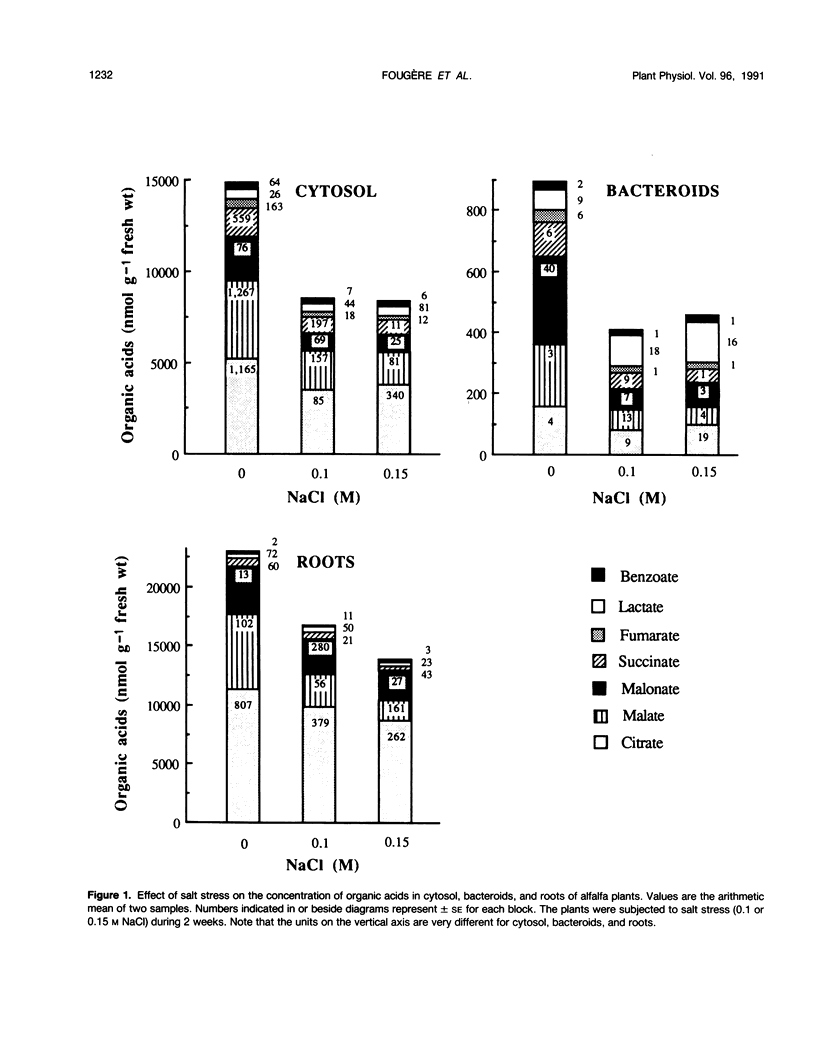
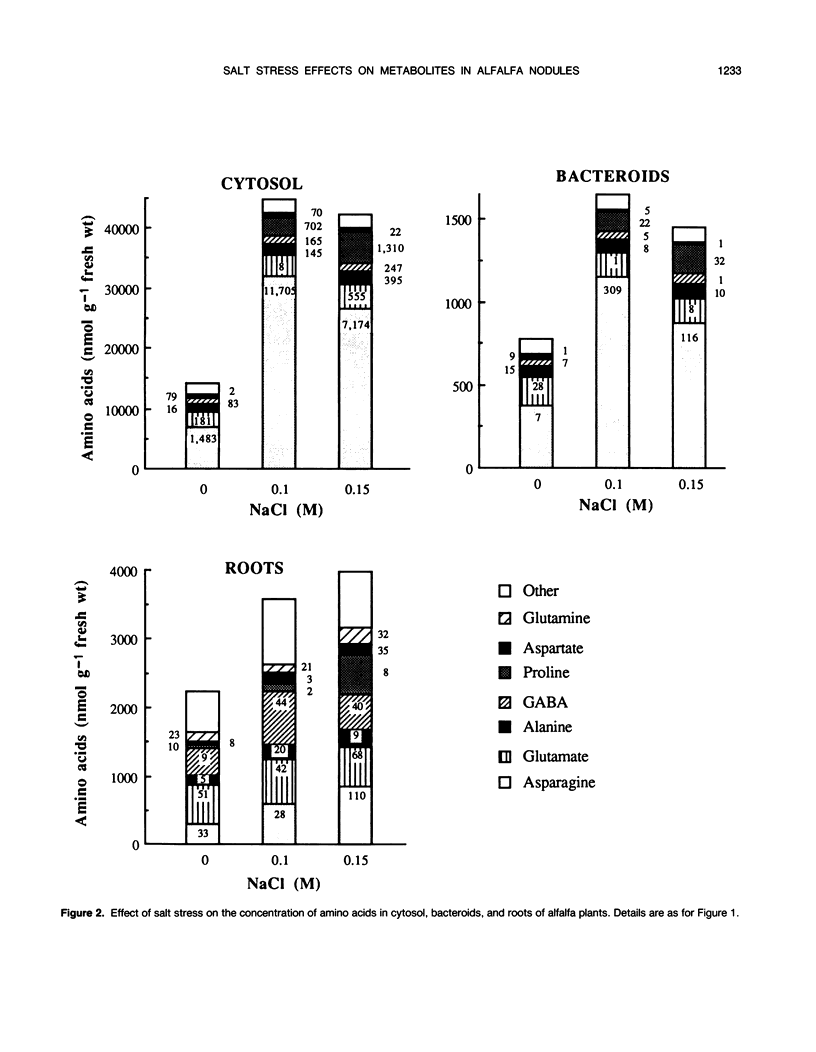
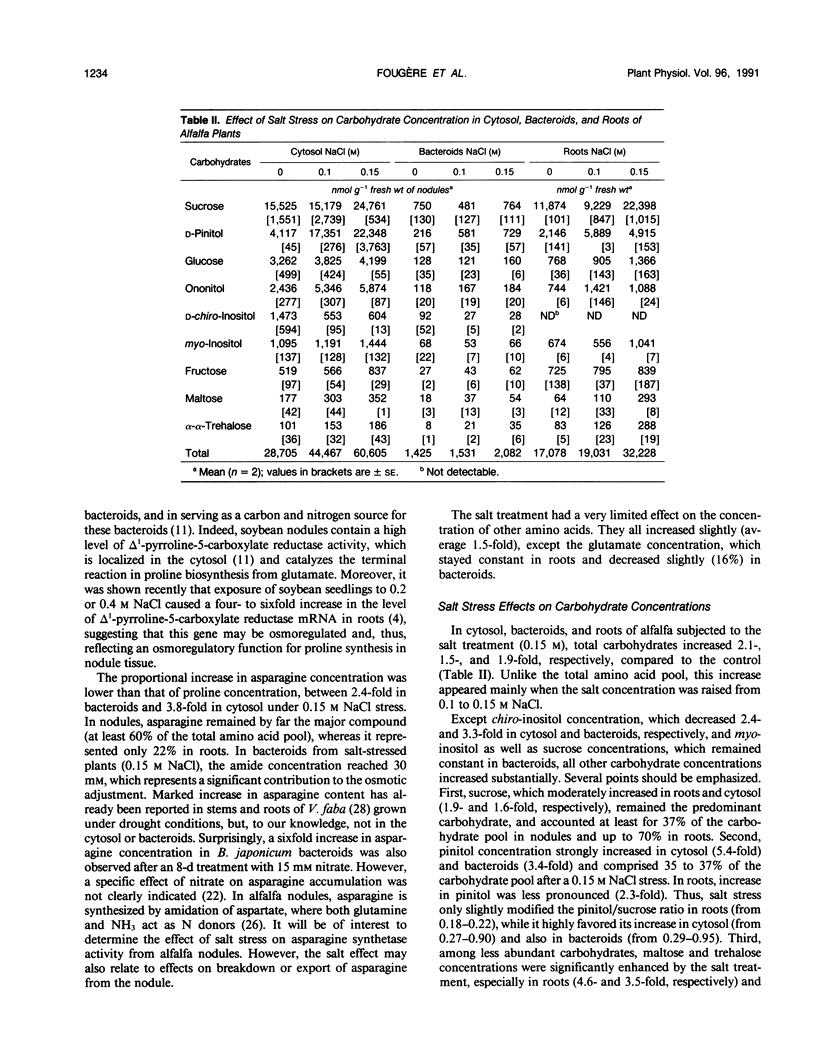
Ethanol-soluble organic acid, carbohydrate, and amino acid constituents of alfalfa (Medicago sativa) roots and nodules (cytosol and bacteroids) have been identified by gas-liquid chromatography and high performance liquid chromatography. Among organic acids, citrate was the predominant compound in roots and cytosol, with malonate present in the highest concentration in bacteroids. These two organic acids together with malate and succinate accounted for more than 85% of the organic acid pool in nodules and for 97% in roots. The major carbohydrates in roots, nodule cytosol, and bacteroids were (descending order of concentration): sucrose, pinitol, glucose, and ononitol. Maltose and trehalose appeared to be present in very low concentrations. Asparagine, glutamate, alanine, γ-aminobutyrate, and proline were the major amino acids in cytosol and bacteroids. In addition to these solutes, serine and glutamine were well represented in roots. When alfalfa plants were subjected to 0.15 m sodium chloride stress for 2 weeks, total organic acid concentration in nodules and roots were depressed by more than 40%, whereas lactate concentration increased by 11, 27, and 94% in cytosol, roots, and bacteroids, respectively. In bacteroids, lactate became the most abundant organic acid and might contribute partly to the osmotic adjustment. On the other hand, salt stress induced a large increase in the amino acid and carbohydrate pools. Within the amino acids, proline showed the largest increase, 11.3-, 12.8-, and 8.0-fold in roots, cytosol, and bacteroids, respectively. Its accumulation reflected an osmoregulatory mechanism not only in roots but also in nodule tissue. In parallel, asparagine concentration was greatly enhanced; this amide remained the major nitrogen solute and, in bacteroids, played a significant role in osmoregulation. On the contrary, the salt treatment had a very limited effect on the concentration of other amino acids. Among carbohydrates, pinitol concentration was increased significantly, especially in cytosol and bacteroids (5.4- and 3.4-fold, respectively), in which this cyclitol accounted for more than 35% of the total carbohydrate pool; pinitol might contribute to the tolerance to salt stress. However, trehalose concentration remained low in both nodules and roots; its role in osmoregulation appeared unlikely in alfalfa.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis L. C., Nordin P. Sugar and organic Acid constituents in white clover. Plant Physiol. 1983 Aug;72(4):1051–1055. doi: 10.1104/pp.72.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delauney A. J., Verma D. P. A soybean gene encoding delta 1-pyrroline-5-carboxylate reductase was isolated by functional complementation in Escherichia coli and is found to be osmoregulated. Mol Gen Genet. 1990 May;221(3):299–305. doi: 10.1007/BF00259392. [DOI] [PubMed] [Google Scholar]
- Fougère F., Le Rudulier D. Uptake of glycine betaine and its analogues by bacteroids of Rhizobium meliloti. J Gen Microbiol. 1990 Jan;136(1):157–163. doi: 10.1099/00221287-136-1-157. [DOI] [PubMed] [Google Scholar]
- Hurkman W. J., Fornari C. S., Tanaka C. K. A Comparison of the Effect of Salt on Polypeptides and Translatable mRNAs in Roots of a Salt-Tolerant and a Salt-Sensitive Cultivar of Barley. Plant Physiol. 1989 Aug;90(4):1444–1456. doi: 10.1104/pp.90.4.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl D. H., Schubert K. R., Carter M. B., Hagedorn C. H., Shearer G. Proline metabolism in N2-fixing root nodules: energy transfer and regulation of purine synthesis. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2036–2040. doi: 10.1073/pnas.85.7.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen P. I., Sydnes L. K., Landfald B., Strøm A. R. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch Microbiol. 1987 Feb;147(1):1–7. doi: 10.1007/BF00492896. [DOI] [PubMed] [Google Scholar]
- Streeter J. G. Accumulation of alpha,alpha-trehalose by Rhizobium bacteria and bacteroids. J Bacteriol. 1985 Oct;164(1):78–84. doi: 10.1128/jb.164.1.78-84.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Carbohydrate, organic Acid, and amino Acid composition of bacteroids and cytosol from soybean nodules. Plant Physiol. 1987 Nov;85(3):768–773. doi: 10.1104/pp.85.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Effect of nitrate on the organic Acid and amino Acid composition of legume nodules. Plant Physiol. 1987 Nov;85(3):774–779. doi: 10.1104/pp.85.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf D. K., Burris R. H. Organic Acid contents of soybean: age and source of nitrogen. Plant Physiol. 1981 Nov;68(5):989–991. doi: 10.1104/pp.68.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta T. C., Faris M. A., Macdowall F. D. Pathways of Nitrogen Metabolism in Nodules of Alfalfa (Medicago sativa L.). Plant Physiol. 1986 Apr;80(4):1002–1005. doi: 10.1104/pp.80.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]


