Abstract
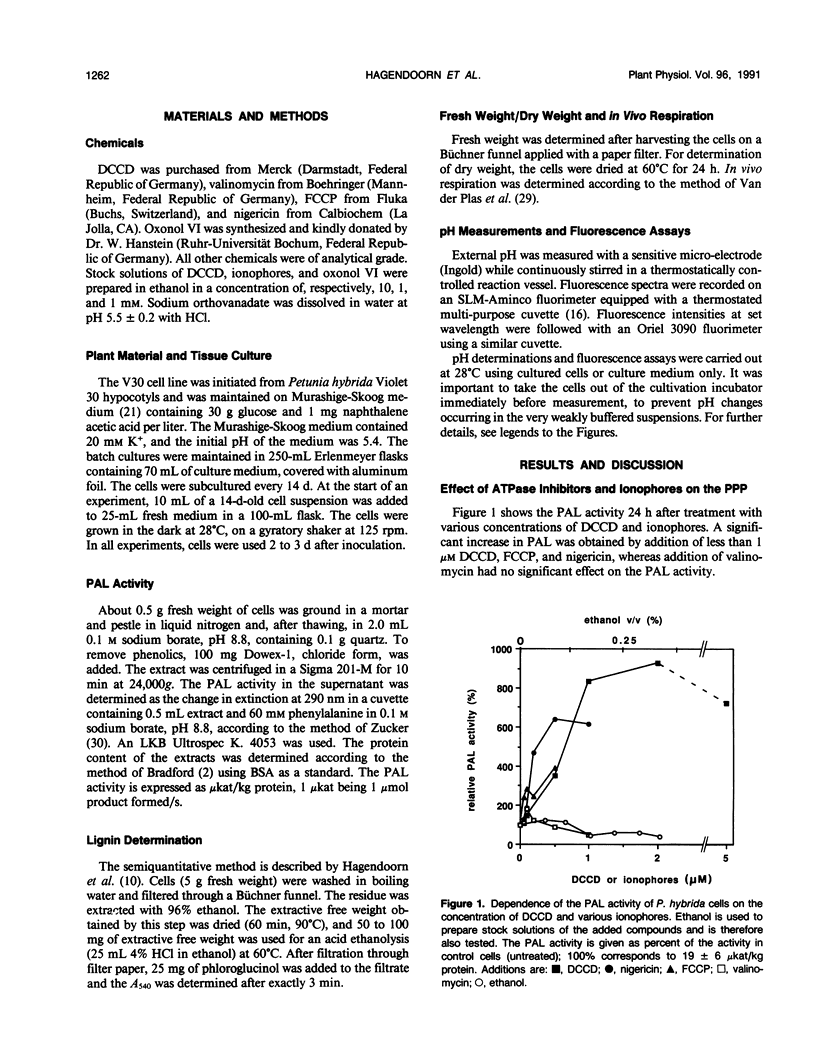
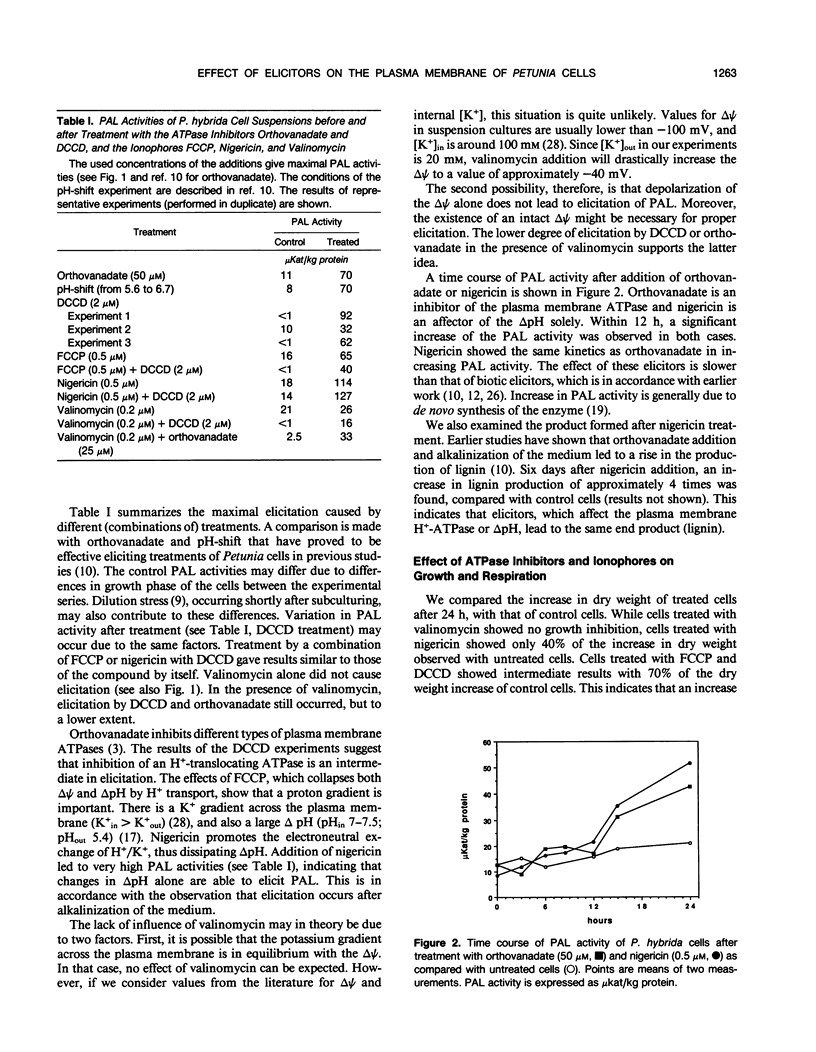
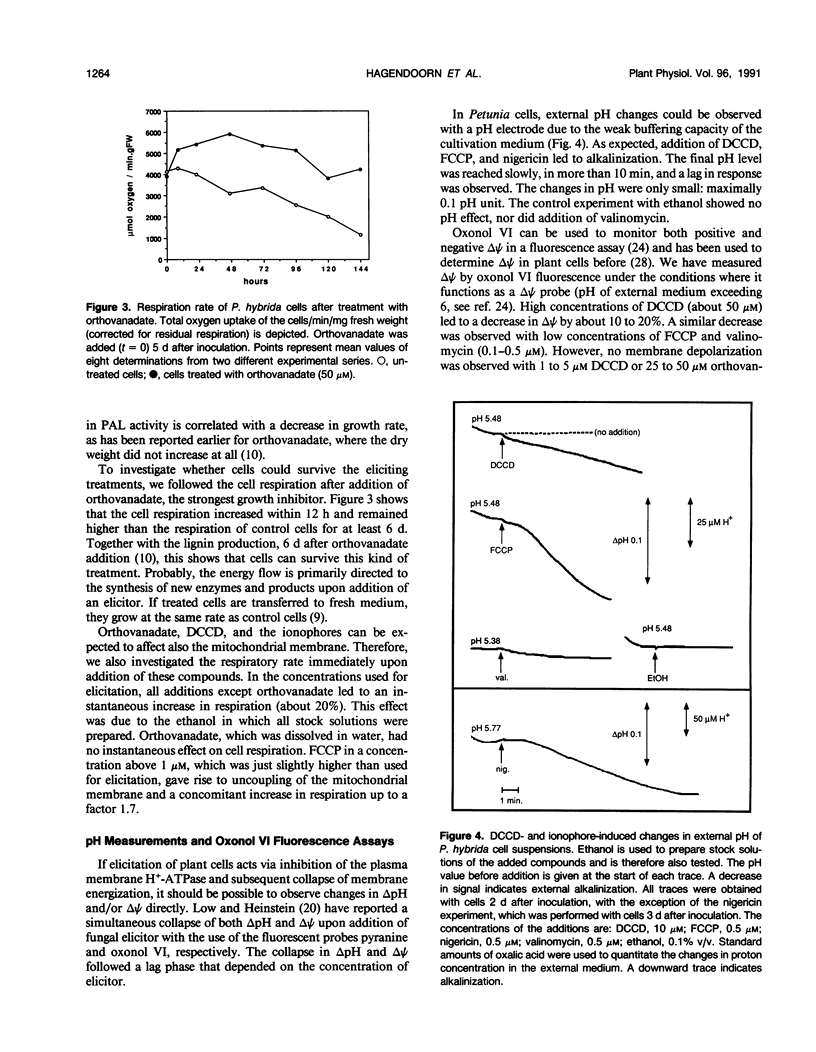
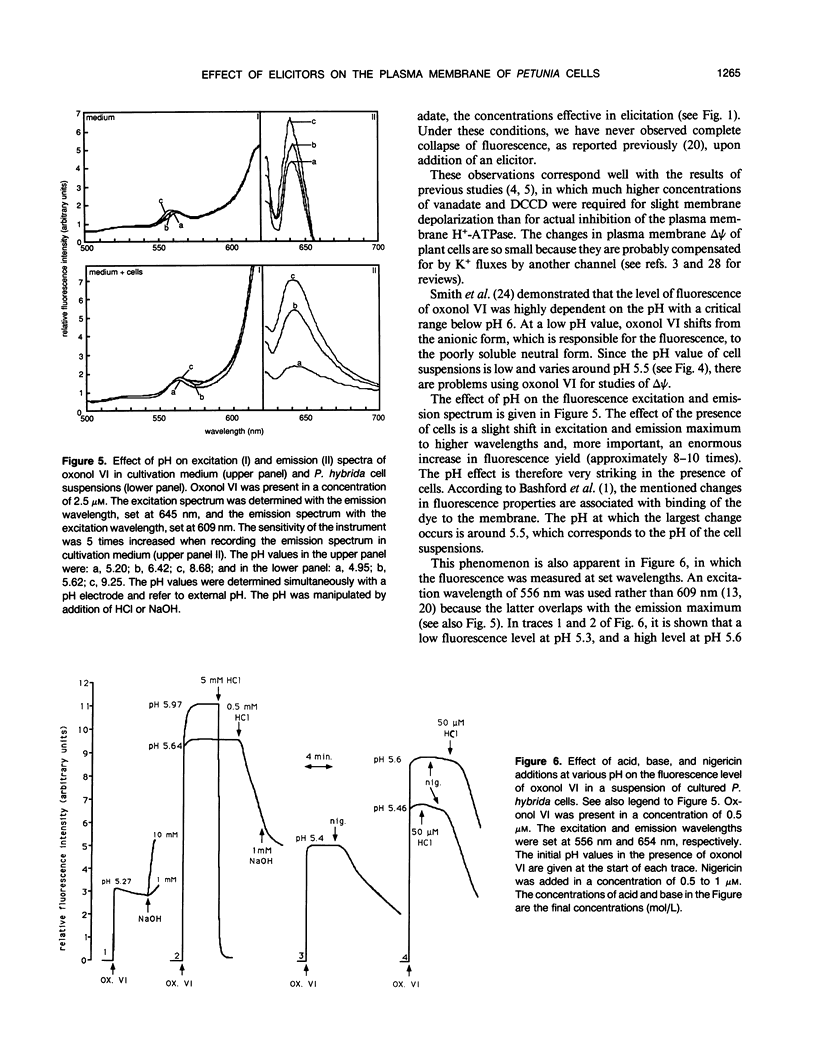
Primary processes during elicitation of the phenylpropanoid pathway (PPP) were studied in Petunia hybrida cell suspensions. We tested the hypothesis that decrease of the proton gradient across the plasma membrane activates the PPP. Induction of the PPP was determined by measuring phenylalanine ammonia lyase activity. A variety of ATPase inhibitors and ionophores were tested for the ability to elicit the PPP. The ATPase inhibitors orthovanadate and N,N′-dicyclohexylcarbodiimide and the ionophores carbonyl cyanide-4-trifluoromethoxyphenylhydrazone and nigericin were all effective elicitors. Carbonyl cyanide-4-trifluoromethoxyphenylhydrazone and nigericin elicit also when used in combination with N,N′-dicyclohexylcarbodiimide. Valinomycin had little effect on phenylalanine ammonia lyase activity. Treatment with orthovanadate or nigericin led to the formation of lignin. Alkalinization of the external medium by N,N′-dicyclohexylcarbodiimide, carbonyl cyanide-4-trifluoromethoxyphenylhydrazone, and nigericin was observed directly with the use of a sensitive pH electrode and internal acidification was deduced from the changes in emission intensity of the fluorescent probe bis[3-propyl-5-oxoisoxazol-4-yl] pentamethineoxonol. These data indicate that changes in the activity of the plasmamembrane H+-ATPase, and subsequent decrease of the proton gradient (particularly of the pH gradient) by itself are sufficient to influence phenylalanine ammonia lyase activity of P. hybrida cells and are therefore important intermediates in signal transduction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashford C. L., Chance B., Smith J. C., Yoshida T. The behavior of oxonol dyes in phospholipid dispersions. Biophys J. 1979 Jan;25(1):63–85. doi: 10.1016/S0006-3495(79)85278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cheeseman J. M., Lafayette P. R., Gronewald J. W., Hanson J. B. Effect of ATPase inhibitors on cell potential and k influx in corn roots. Plant Physiol. 1980 Jun;65(6):1139–1145. doi: 10.1104/pp.65.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel J., Ayers A. R., Albersheim P. Host-Pathogen Interactions: XII. Response of Suspension-cultured Soybean Cells to the Elicitor Isolated from Phytophthora megasperma var. sojae, a Fungal Pathogen of Soybeans. Plant Physiol. 1976 May;57(5):775–779. doi: 10.1104/pp.57.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneusel R. E., Matern U., Nicolay K. Formation of trans-caffeoyl-CoA from trans-4-coumaroyl-CoA by Zn2+-dependent enzymes in cultured plant cells and its activation by an elicitor-induced pH shift. Arch Biochem Biophys. 1989 Mar;269(2):455–462. doi: 10.1016/0003-9861(89)90129-x. [DOI] [PubMed] [Google Scholar]
- Kraayenhof R., Schuurmans J. J., Valkier L. J., Veen J. P., Van Marum D., Jasper C. G. A thermoelectrically regulated multipurpose cuvette for simultaneous time-dependent measurements. Anal Biochem. 1982 Nov 15;127(1):93–99. doi: 10.1016/0003-2697(82)90149-x. [DOI] [PubMed] [Google Scholar]
- Low P. S., Heinstein P. F. Elicitor stimulation of the defense response in cultured plant cells monitored by fluorescent dyes. Arch Biochem Biophys. 1986 Sep;249(2):472–479. doi: 10.1016/0003-9861(86)90024-x. [DOI] [PubMed] [Google Scholar]
- Ojalvo I., Rokem J. S., Navon G., Goldberg I. P NMR Study of Elicitor Treated Phaseolus vulgaris Cell Suspension Cultures. Plant Physiol. 1987 Nov;85(3):716–719. doi: 10.1104/pp.85.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. E., Ebel J. Specific binding of a fungal glucan phytoalexin elicitor to membrane fractions from soybean Glycine max. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4117–4121. doi: 10.1073/pnas.84.12.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Russ P., Cooperman B. S., Chance B. Synthesis, structure determination, spectral properties, and energy-linked spectral responses of the extrinsic probe oxonol V in membranes. Biochemistry. 1976 Nov 16;15(23):5094–5105. doi: 10.1021/bi00668a023. [DOI] [PubMed] [Google Scholar]
- Stäb M. R., Ebel J. Effects of Ca2+ on phytoalexin induction by fungal elicitor in soybean cells. Arch Biochem Biophys. 1987 Sep;257(2):416–423. doi: 10.1016/0003-9861(87)90585-6. [DOI] [PubMed] [Google Scholar]
- Zucker M. Induction of Phenylalanine Deaminase by Light and its Relation to Chlorogenic Acid Synthesis in Potato Tuber Tissue. Plant Physiol. 1965 Sep;40(5):779–784. doi: 10.1104/pp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]


