Abstract
The limited availability of organs for liver transplantation, the ultimate curative treatment for end stage liver disease, has resulted in a growing and unmet need for alternative therapies. Mesenchymal stromal cells (MSCs) with their broad ranging anti-inflammatory and immunomodulatory properties have therefore emerged as a promising therapeutic agent in treating inflammatory liver disease. Significant strides have been made in exploring their biological activity. Clinical application of MSC has shifted the paradigm from using their regenerative potential to one which harnesses their immunomodulatory properties. Reassuringly, MSCs have been extensively investigated for over 30 years with encouraging efficacy and safety data from translational and early phase clinical studies, but questions remain about their utility. Therefore, in this review, we examine the translational and clinical studies using MSCs in various liver diseases and their impact on dampening immune-mediated liver damage. Our key observations include progress made thus far with use of MSCs for clinical use, inconsistency in the literature to allow meaningful comparison between different studies and need for standardized protocols for MSC manufacture and administration. In addition, the emerging role of MSC-derived extracellular vesicles as an alternative to MSC has been reviewed. We have also highlighted some of the remaining clinical challenges that should be addressed before MSC can progress to be considered as therapy for patients with liver disease.
Keywords: chronic liver disease, mesenchymal stromal cells, extracellular vesicles, immunomodulation
Graphical Abstract
Graphical Abstract.
Significance Statement.
There is extensive published literature that supports the safety and the efficacy of mesenchymal stromal cells (MSC) as a potential therapy for liver disease. It is the immunomodulatory and the anti-inflammatory role of MSC in the setting of inflammatory liver disease that has been the focus of the contemporary research. In this article, the authors have reviewed the key translational and the clinical studies with MSC in liver disease so far. The heterogeneity between different studies and the conflicting results have been highlighted. In addition, the emerging role of extracellular vesicles and the remaining challenges in the field have also been discussed.
Introduction
The significant global disease burden associated with chronic liver disease,1,2 coupled with increasing mortality for patients on liver transplant waiting lists3,4 has fueled research efforts to seek alternative therapeutic agents. In particular, a wide range of cell therapies including regulatory T cells, hematopoietic stem cells, embryonic/pluripotent cells, and mesenchymal stromal cells (MSCs) have been studied in liver disease. These approaches all have their merits, but MSC in particular have been extensively studied due to their modest immunogenicity and capacity to modulate immune cells and inflammation for therapeutic benefit.5 There are currently 988 registered clinical trials studying MSCs search at www.clinicaltrials.gov) with at least 10 globally approved MSC therapies (Table 1).6
Table 1.
Globally approved MSC therapies.
| Name | MSC type | Indication | Country of approval/year |
|---|---|---|---|
| Alofisel | Human AT-MSC | Complex perianal fistula in Crohn’s disease | Europe (2018) |
| Prochymal (Remestemcel-L) |
Human BM-MSC | Graft versus host disease | Canada (2012) New Zealand (2012) |
| Queencell | Human AT-MSC | Subcutaneous tissue defects | South Korea (2010) |
| Cupistem | Human AT-MSC | Crohn’s fistulae | South Korea (2012) |
| Cartistem | Human BM-MSC | Knee articular cartilage defects | South Korea (2012) |
| Stemirac | Human BM-MSC | Spinal cord injury | Japan (2018) |
| Stempeucel | Human BM-MSC | Critical limb ischemia | India (2016) |
| Cellgram-AMI | Human BM-MSC | Acute myocardial infarction | South Korea (2011) |
| Temcell HS inj | Human BM-MSC | Graft versus host disease | Japan (2015) |
| Neuronata-R | Human BM-MSC | Amyotrophic lateral sclerosis | South Korea (2014) |
Abbreviations: MSC, mesenchymal stromal cell; AT-MSC, adipose tissue-derived mesenchymal stem cells; BM-MSC, bone marrow-derived mesenchymal stem cells.
Overview of MSC
MSCs are multipotential progenitor cells with an intrinsic ability to differentiate into mesodermal cell lineages.7,8 They can be isolated from a variety of sources including bone marrow,9 umbilical cord,10 adipose tissue,11 dental pulp,12 and virtually from any vascularized tissue.13 When comparing the biological properties of MSCs from different sources, some studies have reported these to be similar14-16 whilst others have reported differences in protein expression profile, cytokine production, differentiation capacity,17 surface antigen expression, and immunomodulatory activity.18,19 These differences in biological properties result from heterogeneity in culture expanded MSC population which varies with MSC manufacturing variables including donor variation,20 isolation technique, culture protocol, media used, passage number as well as tissue origin the cells.21 This poses challenges when comparing the results of different studies with MSC and predicting the therapeutic potency of the MSC product for a specific clinical application. To set minimal standard criteria, the International Society of Cellular Therapy (ISCT) decreed 3 phenotypic criteria to define human MSC in 2005.22 These include (1) plastic adherence when maintained under standard culture conditions; (2) expression of CD105, CD73, CD90, and lack of expression of CD45, CD34, CD14, or CD11b, CD79 alpha, or CD19 and HLA-DR surface molecules; and (3) the ability to differentiate into osteoblasts, adipocytes, and chondroblasts. These criteria reflect the “stemness” of MSC and not their immunomodulatory and regenerative properties. Hence, ISCT have updated the MSC definition to include further 2 criteria (1) tissue origin and (2) associated functional assays informed by intended therapeutic mode of action.23
In addition to their progenitor properties, MSCs have an ability to modulate the adaptive and innate immune systems by suppressing T-cell activation and proliferation, suppressing dendritic cell maturation, reducing B-cell activation and proliferation, inhibiting proliferation and cytotoxicity of NK cells and promoting generation of regulatory T cells.24,25 MSCs also have the ability to switch macrophages between pro- and anti-inflammatory phenotypes which is in part mediated by phagocytosis.26 Dazzi et al27 demonstrated this effect in a graft versus host disease (GvHD) model, whereby apoptosis of infused MSCs by host macrophages was found to be central to the initiation of MSC-induced immunosuppression. Indole 2,3 dioxygenase (IDO) released by the apoptotic MSCs was reported to be the key soluble factor for immunosuppressive activity. More recently, De Witte et al28 demonstrated the rapid clearance of infused MSCs was mediated via phagocytosis by monocytes. In vitro experiments confirmed that human CD14++/CD16− classical monocytes polarized toward a non-classical CD14++ CD16+ CD206+ phenotype after phagocytosis of MSC, and subsequently expressed programmed death ligand-1 and IL-10. MSC-primed monocytes also induced Foxp3+ regulatory T-cell formation in mixed lymphocyte reactions. This study demonstrated that phagocytosis of MSC induces phenotypical and functional changes in monocytes, which subsequently modulated cells of the adaptive immune system.
Initial studies reported MSCs as relatively immune privileged as they do not express class II major histocompatibility complex (MHC) and/or other co-stimulatory molecules at high levels which facilitates their allogeneic use.29 Several pre-clinical and clinical studies have however challenged the degree to which MSCs are immune-privileged, by demonstrating an immune response following allogenic MSC transplantation.29 MSCs exposed to IFN-γ have demonstrated both MHC Class I and Class II expression.30 MSC-derived extracellular vesicles including exosomes and micro vesicles have also been identified as central to their trophic effects31,32 which will be discussed later in this review.
Efficacy of MSCs in Experimental Models of Liver Injury and Disease
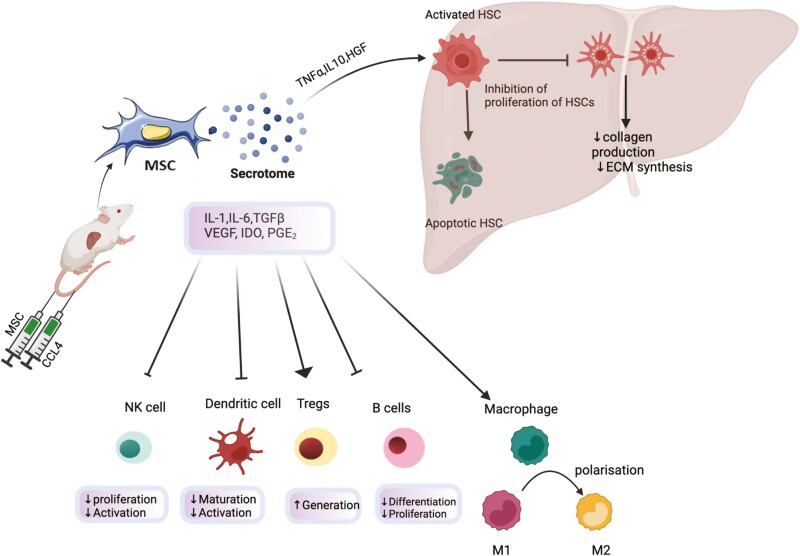
Hepatic fibrosis represents the final common pathway of chronic inflammatory liver injury and is mediated by the activation of hepatic stellate cells (HSCs), the key effectors of fibrogenesis.33,34 The MSC secretome has been linked to therapeutic benefit by inhibiting liver fibrosis due to the production of transforming growth factor beta-isoform 3 (TNF-β3), tumor necrosis factor α (TNFα), hepatocyte growth factor (HGF),35 and IL-10, all of which inhibit the proliferation of ΗSCs (Fig. 1).36,37
Figure 1.
Effects of adoptively transferred MSCs in murine models of liver disease have been reported. MSCs release various soluble factors as part of its secretome including interleukin-1 (IL-1), interleukin-6 (IL-6), transforming growth factor β(TGF-β),vascular endothelial growth factor (VEGF), indoleamine 2,3 dioxygenase (IDO), hepatocyte growth factor (HGF),Tumour necrosis factorα (TNFα). These mediators modulate hepatic fibrogenesis through inhibition of proliferation of hepatic stellate cells (HSC) as well as through inducing apoptosis of HSCs. This results in decreased synthesis of collagen and reduced synthesis of extracellular matrix(ECM). MSC induced modulation of immune cells including natural killer cells (NK), regulatory T cells (T regs) etc reduces the hepatic infliltration of inflammatory cells and thus, exerts an anti-inflammatory effect . This immunomodulation also has an indirect antifibrotic effect. Polarisation of macrophages from proinflammatory (M1) to anti-inflammatory (M2) phenotype also plays a key role in this process.
The antifibrotic effects of adoptively transferred MSCs have been reported in various murine models of liver injury and fibrosis (Table 2). Qiao et al38 reported human bone marrow (hBM-MSC)-induced inhibition and apoptosis of activated HSCs in models of murine liver fibrosis. This was associated with increased expression of pro-apoptotic genes Bax and cleaved capase-3 protein Bax, which was proposed as part of the underlying therapeutic mechanism along with inhibition of NADPH oxidase pathway. The anti-inflammatory attributes of MSCs may also play an indirect role in exerting antifibrotic effects, with candidate soluble factors released by MSC including nitric oxide (NO), prostraglandin-E2 (PGE2),39 indoleamine 2,3 dioxygenase (IDO),40 interleukin-6 (IL-6), IL-10, and HLA-G. MSC can also modulate a range of other immune cell populations including their induction of regulatory T cells. Milosavljevic et al41 investigated the role of MSCs in modulating IL-17 signaling and its subsequent effect on CCL4 induced hepatic fibrosis in mice. Decreased serum levels of profibrogenic IL-17 were observed alongside increased levels of immunosuppressive and hepato-protective interleukin-10 (IL-10) and IDO following the administration of MSC. In a study by Fathy et al42 adipose tissue-derived MSC (ASC) along with eugenol preconditioned ASC were injected in CCL4 model of liver fibrosis. Eugenol is a natural compound with various concentration-dependent pharmacological activities including inhibition of NF-ΚB activation, promotion of cell cycle arrest, reduction in inflammatory cytokines, etc.43,44 Eugenol improved self-renewal, proliferation, and migration abilities of ASCs through upregulation of c-MET, Rex1, Oct4, and nano genes. Effective homing of E-ASCs also resulted in attenuation of expression of genes involved in inflammation including inducible nitric oxide (iNOS), monocyte chemoattractant protein-1 (MCP-1), cluster differentiation 163 (CD163), and tumor necrosis factorα (TNFα). Histological examination of the fibrotic livers demonstrated a marked reduction in inflammatory cell infiltrate, reduced hepatocyte damage, and a preservation of tissue architecture in the E-ASC group in comparison with ASC only or CCL4 only groups. Similarly, Chai et al45 reported an increase in anti-inflammatory cytokines including IL-4 and IL-10 alongside mobilization of liver resident macrophages (Kupffer cells) following administration of MSC in a murine model of liver fibrosis. Addition of IL-4 antibodies into coculture of MSCs and KCs resulted in decreased KC mobilization. MSC-induced polarization of proinflammatory macrophages (M1) to anti-inflammatory (M2) phenotype was proposed as a mechanism to reduce liver fibrosis.
Table 2.
Efficacy of MSCs in murine models of liver disease.
| Murine model |
MSC source/route of infusion | Dose and time of treatment | Mediators | Effects | References |
|---|---|---|---|---|---|
| Fibrosis | Mouse AD-MSC alone or incubated with eugenol 2 × 106 cells/well Tail vein |
At week 5 4 groups (1) Control (2) CCL4 treated (3) CCL4+ MSCs (4) CCL4+ Eugenol preconditioned MSCs |
↓ Fibrotic markers (Type III collagen, HA, hydroxyproline) ↓inflammatory cytokines |
Effective homing of eugenol treated MSCs with attenuation of liver inflammation and fibrosis | Fathy et al. 42 |
| Human BM-MSCs Liver portal vein |
8 x 106 of hBM-MSCs after 11 weeks of CCL4 | Apoptosis of activated HSCs mediated through NADPH oxidase pathway |
↓ Liver fibrosis | Qiao et al. 38 |
|
| Mouse BM-MSCs and MSC-CM Tail vein |
1 × 106 after 24 hours of CCL4 and on 7th,14th and 21st day of experiment | ↓ Serum IL-17 ↓ Th17 ↑ IDO, IL-10, CD4+FoxP3+ |
↓ Liver fibrosis and ↓ Liver inflammation |
Milosavljevic et al 41 |
|
| Human UC-MSCs Tail vein |
5 × 106 7 days after DMN treatment | ↑IL-4 and IL-10 KC mobilization |
↓Liver fibrosis | Chai et al 45 |
|
| Mouse BM-MSC Tail vein |
2 doses 0.5 × 106 Day 60 and 61 |
Cytokine induced ↑expression of IDO and iNOS ↓ MMP-2 |
MSC induced ↓ fibrosis MSc effects negated in mice pre-treated with dexamethasone. |
Chen et al 56 |
|
| FHF/ ALF | Mouse BM MSC Tail vein |
In FHF: After 5 h of TAA. 200 μL MSC-CM. or 1 × 106 MSC In CCL4: 1 × 106 MSC or 200 μL twice/week for 3 weeks at 6 weeks of CCL4 infusion |
↑ Tregs ↓Th1, Th17 Down regulation of HSC and infiltrating macrophages |
Inhibition of hepato-cellular apoptosis Stimulate liver regeneration |
Huang et al 2016 57 |
| Cirrhosis | Human MSC |
24 h after induction of FHF 2 × 106 hMSCs or MSC-CM |
↓Attenuation of leukocyte migration to liver | ↓ Liver injury. Pan-lobular leukocyte invasion and hepatocellular death |
Parekkadan et al .58 |
| Mouse BM-MSCs and BMMs Tail vein |
At 8 weeks: injection of 1 × 106 MSCs or id-BMMs; or 5 × 105 MSCs and id-BMMs |
↑ mRNA of MMP-13 SDF-1,IL-10 and IL-13, PGE2 | Reduced liver fibrosis. Stronger effect in MSC-id MMs then MSC alone. Improved liver function. |
Watanabe et al 59 |
|
| Mouse ADSCs Splenic vein injection |
8-week mice injected with 1 × 105 ADSCs | ↓ Ratio of CD8+/CD4+ cells ↓Intrahepatic infiltration of CD11+ and Gr-1+ cells |
↓Liver fibrosis | Seki et al 11 |
|
| PBC | Mouse BM-MSC |
1 × 106 cells at week 16 | IFNγ↓ CD4+FoxP3+, TGFβ↑ |
↓Liver injury, inflammation, inflammatory cell infiltration | Wang et al 60 |
| Acute liver injury/ hepatitis |
Mouse and Human MSCs and MSC CM Tail vein |
↓ TNFα, IFNγ, IL-4 iNOS and IDO dependant attenuation of cytokines |
↓Liver injury | Gazdic et al 61 |
|
| Mouse AD-MSCs Tail vein |
1 × 105 ADSCs immediately after ConA injection | ↓CD11b+ Gr-1+, F4/80+ cells |
↓ALT and LDH ↓Hepatocyte necrosis |
Higashimoto et al 62 |
|
| NASH | Mouse BM-MSC i.v. tail |
1 × 106 cells at weeks 6 and 7 | ↓CD4+IFNγ,IL-6+ | ↓Steatosis, ballooning lobular inflammation and hepatic fibrogenesis | Wang et al 63 |
Abbreviations: ADMSCs, adipose tissue MSCs, NADPH, nicotinamide adenine dinucleotide phosphatase; antifibrotic factor, DMN, MMP-13; SDF-1, chemoattractant factor; IL-10 () IL-13, anti-inflammatory; PGE2, prostaglandin E2.
MSCs have also been reported to inhibit epithelial to mesenchymal transition (EMT) and exert an antifibrotic effect in liver fibrosis models. EMT refers to a mechanism where by certain resident liver epithelial cells undergo biological changes to assume a mesenchymal phenotype and contribute to the fibrogenic process in the injured liver.46,47 Some of these epithelial-derived mesenchymal cells, however, may subsequently undergo mesenchymal to epithelial transition and ultimately become hepatocyte and cholangiocytes. Balance between EMT/MET has therefore been suggested to determine the outcome of liver injury.47 There is an inherent difficulty in establishing EMT as a mechanism of liver fibrosis and its significance in the outcomes of liver injury.48 Furthermore, existing data available from murine studies that can help understand the role of EMT in liver fibrosis/repair is confounded by different models of injury and examination of different outcomes at different time points across the studies.47 Nonetheless, antifibrotic effect of human UC-MSC derived exosomes through inhibition of EMT of hepatocytes has been demonstrated in CCL4 model of liver fibrosis.49 Inactivation of (TGFβ)-1/Smad signaling pathway was shown to be involved. In an another study, MSC-induced amelioration of surgery-induced liver damage and inhibition of EMT has been reported in a pig liver resection model.50 Mechanistic analysis showed modulation of thrombospondin-1/TGF-β to play a key role. Thrombospondin-1 secreted from thrombocytes and non-parenchymal cells is linked with TGF-β production and EMT. In the pig resection model, it was proposed that MSC-derived soluble factors inhibited thrombospondin-1 secretion with resultant reduction in active TGF-β and subsequent attenuation of liver damage.50
While data from these studies along with other studies listed in Table 2 support indirect antifibrotic effects of MSCs, there are other studies that report no effect of MSC on liver fibrosis when administered at, or after, cessation of liver injury.
Carvalho et al51 injected BM-MSC at a dose of 1 × 107 cells in a model of severe chronic liver injury (15 weeks of CCL4 and 14 weeks of alcohol diet) and demonstrated no improvement in liver injury biomarkers with no difference in histological results between MSC treated and placebo group. Mannheimer et al52 reported similar findings with MSC isolated from cirrhotic rats. Hepatic injury was induced in a bimodal pattern with alcohol infused diet and injection of CCL4 for a prolonged period of time (14 weeks) but no difference in fibrosis was noted in the treated group in comparison to controls. This highlights the impact of source of MSC and duration of liver injury on therapeutic potential of MSC. Similarly, Briquet et al53 also reported no antifibrotic effects of MSC when administered after cessation of liver injury. They injected hBM-MSC, UC-MSC, and liver MSC in NOD/SCID/IL-2Rg null (NSG) mice after 4 weeks of CCL4-induced fibrosis. They reported no therapeutic effect on liver fibrosis, but this study published in 2014 was later retracted based on misuse and misrepresentation of a peer’s scientific data.
Profibrotic effects of MSC-like cells have also been reported. Genetic lineage tracing of tissue resident perivascular GLii+ cells shows them to be MSC-like cells which contribute significantly to myofibroblast generation in CCL4-nduced liver fibrosis.54 Baertschiger et al55 reported human BM-MSC introduced via intrahepatic injection in mice undergoing liver regeneration or fibrosis contribute to myofibroblast formation.
In summary, it can be concluded that the direct anti-fibrotic actions of MSCs remain controversial, with the more likely case being that any such effect is mediated indirectly through an effect on liver inflammation.
Autologous Versus Allogenic MSC Transplantation
Both autologous and allogeneic MSCs have been studied in preclinical and clinical studies, and despite the relative ease in obtaining autologous MSCs, this often comes at a high cost of preparation for a single recipient. There is also a time-critical aspect of obtaining and expanding enough cells for transfusion, raising logistical challenges for their use in treating acute diseases. It is also difficult to obtain sufficient autologous MSCs from some patients, for example, ASC from thinner patients. MSCs from elderly donors have been shown to lack in proliferation and differentiation, thus potentially possessing less regenerative properties.64 In contrast, allogeneic MSCs are usually obtained from young healthy donors, are readily available, and can be cryopreserved, stored, and quickly thawed prior to administration. This process also allows for quality assurance of MSCs and reduces their overall cost. Overall, cryopreserved allogeneic MSCs offer many advantaged compared to autologous MSCs as regards time, cost production, and quality assurance.
Emerging Role of Extracellular Vesicles
Bruno et al65 fractioned human MSC conditioned media (MSC-CM) by ultracentrifugation and demonstrated that MSC-like effects were retained within the cell-free supernatant and associated with 80 nm to 1 μm spherical moieties. These were described as microvesicles and were able to suppress murine acute renal tubular injury in vivo.65 The microvesicle fraction also suppressed apoptosis and enhanced tubular epithelial cell proliferation in vitro to a similar extent as MSC. Lai et al66 used high performance liquid chromatography (HPLC) for size exclusion and enriched a fraction comprised of particles with a hydrodynamic radius of 55-66 nm. These particles were named exosomes based on the presence of exosome associated proteins such as CD9, CD81, and Alix and resulted in a reduction in size of an infarct in a murine model of acute myocardial infarction (AMI). Similar effects have been observed in a previous study with MSC and MSC conditioned media MSC-CM67 and since then there is growing preclinical evidence to support the role of MSC-derived EVs as therapeutic agents.68
Characteristics of MSC-EV
MSC secrete different classes of EV including exosomes,69 microparticles,70 and microsomes,65 and each subset is defined by its physical, biochemical, and biogenetic characteristics.69,71 At present, exosomes are probably the best described EV particles with specific surface markers72 and contents that include mRNA, miRNA, and assorted proteins which in turn can modulate function of target cells.32,73 EV exert their effects through cell signaling,74 alterations in cell metabolism,75 and via intercellular communication.76 Pre-treatment of MSC-EV can also impact their content and subsequent therapeutic action which requires further research (Fig. 2).77
Figure 2.
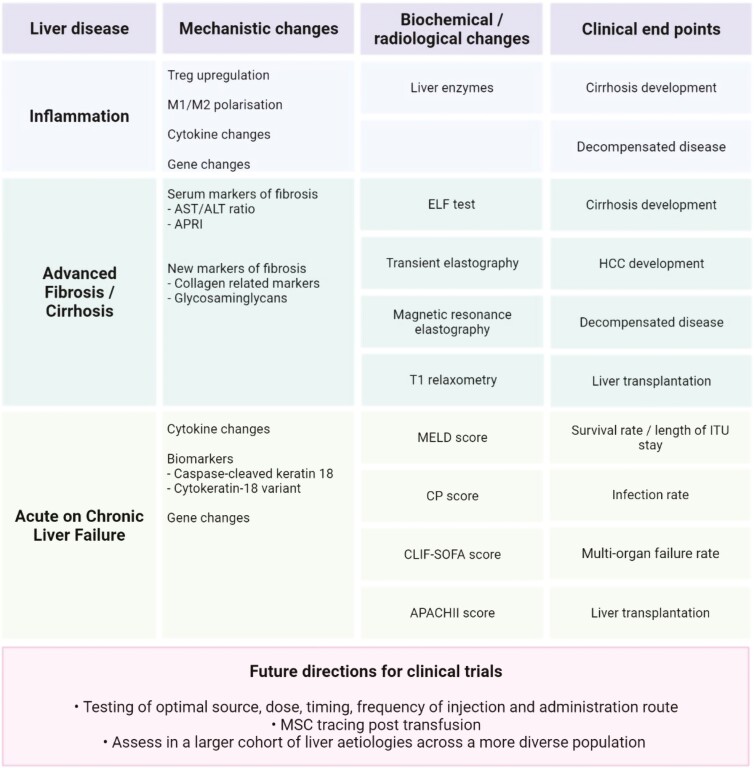
MSC and clinical trials. ALT, alanine aminotransferase; APACHII, acute physiology, and chronic health evaluation; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; CLIF-SOFA, chronic liver failure-sequential organ failure assessment; CP, Child-Pugh score; ELF, enhanced liver fibrosis test; HCC, hepatocellular carcinoma; ITU, intensive therapy unit; M1, macrophage 1; M2, macrophage 2; MELD, model for end-stage liver disease; MSC, mesenchymal stromal cell; T1, longitudinal relaxation time; T reg, regulatory T cells.
A wide array of therapeutic effects has been attributed to such mRNAs in experimental models, for example, the transfer of insulin like growth factor-1 receptor (IGF-1R) mRNA from exosomes to cisplatin-damaged proximal tubular epithelial cells sensitizes them to the reno-protective effects of locally produced IGF-1.78 In other studies, mRNA from MSC exosomes has been shown to play a role in inhibiting tumor growth,79,80 reducing cardiac fibrosis,81 stimulation of axonal growth from neurons,82 and angiogenesis.83 An in vivo study demonstrated that miRNA-223 from MSC-derived exosomes was critical in delivering MSC-induced cardio protection in sepsis,84 which was achieved through downregulation of the inflammation related genes Sema3A and stat3.85
The contents of MSC EV and their subsequent therapeutic effects can be a function of their origin; for example, bone marrow-derived MSC EV contain cystinosin (CTNS), a cystine efflux channel in the lysosomal membrane, which can reduce cystine levels when cocultured with renal tubular cells from patients with cystinosis.86 Similarly, adipose tissue-derived MSC (ADSC) contain up to four-fold higher concentration of neprilysin, an enzyme that degrades β-amyloid (Aβ) peptide in brain and which is associated with Alzheimer’s disease, than BM-MSCs. Transfer of ADSC-derived exosomes into N2a cells (neuroblastoma cell line) decreased intracellular Aβ levels.87 Due to the lack of an agreed methodology for EV preparation, the international society for extracellular vesicles (ISEV) released a position statement to define minimal criteria recommend for characterization of purified EVs88 which included semi-quantitative analysis of the EV protein composition for typical EV marker proteins such as CD9, CD63, CD81, Alix, and TSG101, size analysis and analysis of their morphology. ISEV also agreed on naming all experimentally derived vesicles as EV.
Role of MSC-EVs in Liver Injury
Recent research has shown that MSCs produce a significant amount of EVs, which has been proposed to be one of the mechanisms by which MSCs exert their various therapeutic effects in liver injury.89 In light of this, Newman et al90 proposed that MSCs can be used to take advantage of the critical role of EVs in liver cell communication by transporting a variety of macromolecules, such as connective tissue growth factor (CTGF), which facilitates the interaction between parenchymal cells and non-parenchymal cells such as hepatocytes, hepatic stellate cells (HSCs), and Kupffer cells.
Fiore et al91 demonstrated that the delivery of hepatocyte-derived EVs enhanced hepatocyte proliferation, suppressed cell death, and accelerated liver regeneration in rats following 70% hepatectomy. This was hypothesized to be caused by EVs delivering RNA to target cells through fusion with target hepatocytes, which then transferred the cargo.92 For example, hepatocyte-derived EVs provide neutral ceramidase and sphingosine kinase 2 (SK2) to hepatocytes, which induces sphingosine-1-phosphate (S1P) respectively.93 These findings suggest that MSC-derived EVs may have the potential to be a new therapeutic for both acute and chronic liver damage.89
MSC-EVs express CD40L, which activates macrophages and changes their phenotype from anti-inflammatory to pro-inflammatory,92 which may play an important role in liver regeneration and resolution of liver fibrosis. When exposed to pro-inflammatory cytokines, regional growth factors, and microbial metabolites, miR-27a, which is abundant in naïve monocytes, is known to cause M2 polarization and hence a switch to an anti-inflammatory micro-environment.91
Additionally, during liver injury, hepatic stellate cells (HSCs) and EVs produced by MSC interact resulting in the release of connective tissue growth factor (CCN2),90 which by binding integrin v3 or v5 with heparan sulfate proteoglycan ligands, can signal to endothelial cells to modulate activation.91 On the other hand, MSC-EVs affect target cells via activating the transcription factors Twist-1, miR-214, and miR-199a-5p.89 Despite numerous preclinical studies on liver fibrosis with MSC-EVs, it remains unclear whether MSCs-EV stimulate local damage liver cells.
MSC-EVs in Liver Disease Models
MSC-EVs were initially investigated as a potential therapy for accelerating liver regeneration in the model of 70% hepatectomized rats.94 Recent murine studies (listed in Table 3) have demonstrated the paracrine effects of MSC EV, with Haga et al95 demonstrating reduced hepatic injury, reduced apoptosis, and modulated cytokine expression after systemic administration of BM derived MSC-EV in models of fulminant hepatic failure. Chen et al96 reported similar anti apoptotic ability of MSC EV with improved liver function, when human menstrual blood stem cell-derived exosomes (MenSC-Ex) were injected into the tail vein of mice 24 h before D-GalN/LPS induced fulminant hepatic failure (FHF). Hepatic regeneration and anti-apoptotic effect has also been reported in a murine model of drug induced liver injury by Tan et al,97 with upregulation of Bcl-xL protein expression implicated as the underlying mechanism. Glutathione peroxidase1 (GPX1) derived from human umbilical cord MSC (hucMSC) exosomes was reported to elicit antioxidant and anti-apoptotic effects in CCL4 induced liver failure when administered as a single dose via tail vein. Similarly, Li et al89 demonstrated amelioration of CCl4 induced hepatic inflammation and liver fibrosis in mice when treated with hucMSC-EV. The underlying mechanism involved inactivation of TGF-β1/Smad signaling pathway, reduced expression of collagen I and III, and inhibition of epithelial to mesenchymal transition (EMT). In another experiment by Zhang et al.98 MSC-EV pre-treated with TNFα (T-exo) were administered after an hour of lipopolysacharride(LPS)/D-galactosamine (D-GalN) induced liver injury. T-exo were reported to reduce ALT, AST, and pro-inflammatory cytokine levels and inhibited the activation of NLRP3 inflammation associated pathway proteins and thus, promoted tissue repair.
Table 3.
Effects of MSC exosomes in murine studies.
| Source of exosome | Liver disease model |
Effects | Mediators/pathway | Ref. |
|---|---|---|---|---|
| MenSC-Ex | Fulminant hepatic failure (FHF) | Anti-apoptotic Improved liver function ↓ Necrosis and inflammation |
↓ TNFα, IL-6 and IL-1β. | Chen et al 96 |
| Murine or human BM-MSC Exosomes |
FHF | Anti-apoptotic/reduced hepatic injury Modulation of cytokine expression |
y-RNA-1 | Haga et al 95 |
| hiPSC-MSC-Exo | Hepatic ischemia-reperfusion injury | Anti-apoptotic Anti-oxidative stress response |
↓ Hepatocyte injury markers in treatment group ↓TNFα, IL-6, HMGB1. ↓In apoptosis protein (Bax, Caspase-3) and GSH, GSH-Px, and SOD higher than in control group |
Nong et al 99 |
| Human foetal tissue derived MSC-exo | CCL4-induced hepatic injury followed by xenobiotic-induced liver injury | ↓Hepatic injury ↑Hepatocyte proliferation and inhibition of apoptosis |
↑ Cell viability in treatment group. ↑ In proliferating protein pANCA and cyclin D1 Upregulation of Bcl-xL protein |
Tan et al 97 |
| huc-MSc-Exo | CCL4-induced hepatic failure | Antioxidant and anti-apoptotic effect | Induction of ERK ½ phosphorylation and inhibition of IIKB/NFkB/casp9/3 pathway Delivery of GPX-1 protein |
Yan et al 100 |
| Huc-MSCs-Exo | CCL4-induced liver fibrosis | ↓Hepatic inflammation ↓Liver fibrosis |
↓Collagen I and III ↓TGF-β1 Inactivation of TGFβ/Smad2 pathway Inhibition of EMT |
Li et al 49 |
| T-Exo and hucMSC-Ex |
LPS+D-GalN ALF |
↓ ALT, AST, proinflammatory cytokines | Inhibit NLRP3 proteins | Zang et al 98 |
| ADMSCs EXO | HCC | HCC suppression | ↑ Nk-T cell ↑ ADC ratio |
Ko et al 101 |
Abbreviations: MenSC-Ex, menstrual blood-derived exosomes; TNFα, tumor necrosis factor; BM-MSC, bone marrow MSC; hiPSC-MSC exo, human-induced pluripotent stem cell-derived MSCs Exo; HMGB-1, human mobility group box-1; GSH, glutathione; GSH-PX, glutathione peroxidase; SOD, superoxide dismutase; pANCA, proliferating cell nuclear antigen; huc-MSC exo, human umbilical cord MSC exosome; GPX-1, glutathione peroxidase 1; ERK, extracellular signal regulated kinase64; Bcl, B-cell lymphoma; EMT, epithelial-to-mesenchymal transition; CP-MSCs, chorionic plate MSCs; 9LPS, lipopolysaccharide; D-GalN, D-galactosamine; ADMSCs, adipose-derived MSCs; NK-T cells, natural killer T cells; ADC, apparent diffusion coefficient; HCC, hepatocellular carcinoma.
Data from these studies and those summarized in Table 3 suggest MSC-EVs may be effective in inhibiting hepatocyte apoptosis, support hepatocyte function, promote hepatocyte proliferation and in addition, modulate inflammatory response by preventing immune cell infiltration and/or stimulation of inflammatory cytokines. While these murine studies make a case for MSC EVs as an alternative to cell-based therapies for liver disease, further investigations to elucidate the optimal route and dose of administration, and precise mechanism of biological actions are required. It should be noted that majority of these studies were of short duration of liver injury 4-6 weeks with immediate administration of MSC EV.
Limitations of Experimental Models
Tsiapalis et al102 claimed that finding reliable, reproducible, and robust approaches for isolating and purifying therapeutic EVs and their mass manufacture at the cGMP quality for clinical application poses a significant challenge. Additionally, little is understood about EVs’ biogenesis, uptake, and cellular activity, making it unclear how they may affect therapeutic outcomes, with Maji et al,103 indicating that EV contents can vary with cell type and environmental influences. It is necessary to conduct in vivo assessments of their pharmacokinetics, biodistribution, dose-escalation, toxicity, and immunogenicity, in addition to providing evidence of the effectiveness and potency of any EV-based therapeutics. Additionally, identifying which EV subpopulations among the heterogeneous populations will be important and indeed the classification into several types is still under investigation. Additional studies are also necessary to determine the most effective therapeutic dosages and delivery methods for clinical use in the future to ensure improved EV homing and targeting of injured liver.104 The use of MSC-EVs in clinical settings will require resolution of several issues, including large-scale production and isolation methods, (ii) methods for rapid and accurate quantification and characterization of EV, (iii) precise content characterization of the cargo, (iv) pharmacokinetics, targeting and transfer mechanisms of EV to the target sites, and (v) safety profiling.
Hepatic Pathogenesis and Role of MSCs
Chronic liver injury leads to fibrosis, cirrhosis and hepatocellular carcinoma development and is caused by a range of factors including hepatitis B or C, cholestasis (primary biliary cirrhosis, primary sclerosing cholangitis), alcohol related liver disease and non-alcoholic steatohepatitis. Liver fibrosis occurs as a result of acute and/or chronic cellular injury mediated via activation of hepatic stellate cells to myofibroblasts. HSCs are the primary effector cells that lead to deposition of collagen in extra-cellular matrix (ECM), and which also interact with other immune cells, secreting cytokines and chemokines. Other mechanisms of fibrosis have also been described with ECM mechanical stiffness also activating further HSCs.105
The role of dendritic cells is less well understood; however, they can activate NK cells which in turn mediate HSC apoptosis causing downregulation of inhibitory MHC class 1 molecules. Other mechanisms include adipokines as key mediators in fibrogenesis particularly in the setting of NAFLD, where Leptin has been shown to promote HSC fibrogenesis and enhance TIMP-1 expression which is associated with increased leptin signaling. It also partially suppresses peroxisome proliferator-activated receptor-γ (PPARγ) anti-fibrogenic nuclear receptor that can reverse HSC activation and maintain HSC quiescence.106
MSC Mechanism of Action in Clinical Settings
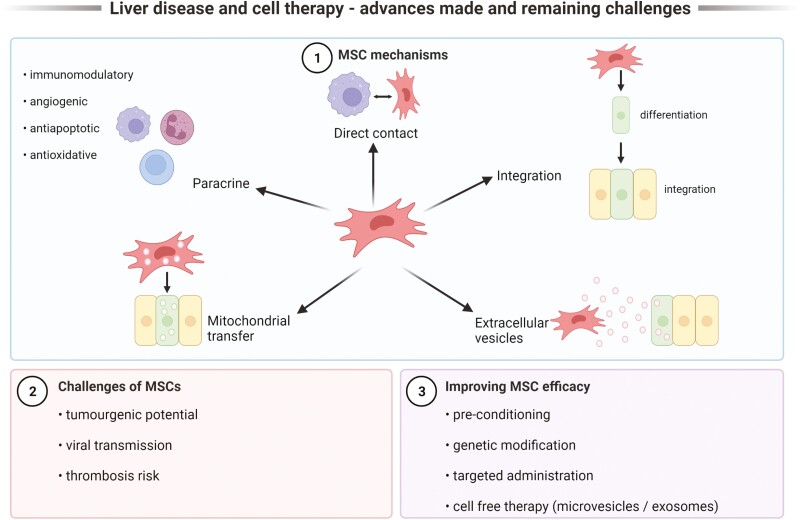
Several mechanisms of actions of MSCs have been described with Kampera et al107 summarizing the 3 main mechanisms of action as: 1. Living cell expansion and differentiation into mesodermal tissues 2. Close interactions with neighboring cells via cell-to-cell contact and release of paracrine factors and extracellular vesicles 3. Apoptotic phenomena involving MSCs and immune cells, ie, efferocytosis of cellular debris, leading to functional polarization of phagocytic cells toward inhibitory phenotypes.
Several studies in vivo have suggested that human MSCs can differentiate into hepatocyte-like cells when transplanted.108-115 Seo et al116 retrieved human adipose-derived MSCs in the liver of CCl-4-injured severe combined immunodeficiency mice after intravenous injection and differentiated to hepatocyte-like cells. Chamberlain et al117 injected human-derived MSCs into pre-immune fetal sheep in the absence of liver injury—this resulted in the generation of hepatocytes 70 days after xenotransplantation. These studies suggest that MSC hepatocyte-like derived cells may replace damaged hepatocytes in liver disease. Importantly, the in vivo signals orchestrating trans differentiation are not completely understood at present. The overall contribution of this mechanism to ameliorating liver injury and repair is likely low though.
The immunosuppressive role of MSCs has been confirmed both in pre-clinical and clinical settings, where persistent inflammation drives liver injury through infiltration with T cell, B cell and monocytes. A reduction in inflammation will reduce injury and also facilitate liver regeneration, as potentially achieved by MSCs suppression of T lymphocyte activation, proliferation and cytotoxicity.118 Other mediators of MSC suppressive effects include secretion of prostaglandin E2 that promotes IL-10 secretion by dendritic cells, increase in regulatory T cells and decrease in TNF-α, INF-γ, and IL-4; indoleamine 2,3-dioxygenase with INF-γ stimulation, leading to inhibition of T-cell proliferation.35 Selmani et al119 also demonstrated that HLA-G5 human leukocyte antigen protein was produced by MSCs, leading to a reduction in T-cell proliferation and an increase in T-cell numbers.
Chemokines and cytokines secreted by MSCs during both acute and chronic liver injury may also play a significant role in reducing inflammation and hepatocytes apoptosis. Poll et al120 demonstrated that administration of MSC medium downregulated IL-1β, TNF-α and IL-6. It increased levels of IL-10. Overall, this resulted in lower lymphocyte infiltration in the liver, reduced hepatocyte apoptosis and increased hepatocyte proliferation.
The main anti-inflammatory signals of MSCs in liver disease that have been described in the literature include secretion of epidermal growth factor,121 TNF-α secretion inhibiting stellate cell proliferation and collagen type 1 synthesis,36 promotion of hepatic stellate apoptosis through nerve growth factor secretion.122 Higashyimama et al123 also demonstrated extracellular matrix degradation via matrix metalloproteinase-9 expression. The pro-inflammatory cytokines that have been described are TGF-β 1 and 3, IL-6, monocyte chemoattractant protein-1, macrophage inflammatory protein-1 α and β.120,124,125
Of note the majority of MSC transplanted cells are entrapped in the pulmonary circulation and subsequently cleared from there126 which reduces their homing capability and engraftment within target tissues. Paracrine and apoptotic phenomena are therefore more likely to play a significant role; however, the exact mechanisms remain unknown.
Although various mechanisms for MSCs mitigating hepatic fibrosis directly have been described, the case is not fully proven, thus the MSC mechanism of mitigating fibrosis directly remains uncertain.
Therapeutic Mechanisms of MSC in the Injured Liver
The replacement of injured tissue by differentiating into different cell lineages and the modulation of immune responses are 2 fundamental mechanisms contributing to MSCs’ therapeutic potential,127 although the latter is by far the most important. According to Fan et al,128 studies conducted in vitro and in vivo have demonstrated that MSCs can modulate innate and adaptive immune systems by reducing T-cell activation and promoting T-cell development toward a regulatory phenotype. Indeed, Eom et al129 stated that MSCs release soluble proteins such as transforming growth factor-1 (TGF-1), indoleamine-pyrrole 2,3-dioxygenase (IDO), nitric oxide (NO), interleukin-10 (IL-10), a potent anti-inflammatory cytokine, prostaglandin E2 (PGE2), as well as extracellular vesicles (EVs), which have immunomodulatory functions and are therapeutic in many disease models.130
Furthermore, several studies indicate that MSCs may be important in macrophage polarization, encouraging differentiation toward the M2 phenotype both in vitro and in vivo. At the same time, MSCs can reduce NK cell proliferation and cytotoxicity.131 In addition, Protein kinase B, extracellular receptor kinase (ERK), and the mitogen-activated protein kinase (MAPK) axis all seem to be elements of various molecular mechanisms that drive MSC-EV-based therapy. Likewise, the Wnt/-catenin signaling pathway, which is important for tissue repair and cell destiny, might also be involved in EV-mediated tissue regeneration.132
Several studies have shown that MSCs can travel across vascular endothelial cells to injured tissue locations and engraft there. Eom et al129 reported that MSC homing could be divided into 2 main categories: non-systemic, which refers to the local transplantation of MSCs at the injured site, and systemic, which corresponds to the release of homing-promoting molecules from injured tissue.132 Adhesion molecules that are expressed on the MSC surface help in adhesion. Rolling, activation, firm attachment, crawling, and transendothelial migration are the 5 sequential processes that constitute MSC homing.129 According to numerous studies, hyaluronic acid (HA) may interact as a binding site for the CD44 receptor on activated endothelial cells with P-selectin on the MSC surface, promoting MSC homing and demonstrating rolling motion. At the same time, chemokines such as stromal cell-derived factor-1 (SDF-1) that are produced after damage can interact with C-X-C-motif chemokine receptors such as (CXCR4) and (CXCR7) on MSCs to activate integrin adhesiveness, which in turn can facilitate MSC migration19 MSC transportation and homing are significantly influenced by the shift to high affinity, which occurs once the chemokine attaches to its receptor and the cell membrane and binds to the integrin tail. A critical component of the firm adhesion process is the tight adherence between MSCs and vascular endothelial cells, which is promoted by the expression of VCAM1 intercellular adhesion molecule 1 (ICAM1) by activated MSCs.
After establishing firm endothelial adhesion, MSCs can help generate actin filaments and pseudopodia and subsequently promote cytoskeleton remodeling by forming intracellular linker molecules by activating the CCR2 signaling pathway.93 Because of this, MSC crawl along the inner wall of blood vessels whilst being subjected to a chemo-tactic gradient to find the best site for trans-endothelial migration.133 By secreting MMPs to complete trans-endothelial migration, this mechanism enables MSCs to damage the endothelial basement membrane. Of note, urokinase-type plasminogen activator (uPA), which breaks down ECM components, has been discovered in prominent pseudopodia of MSCs.
Clinical Applications in Liver Disease
The use of MSC in liver disease has been the focus of many clinical trials, as summarized in Table 4, with applications mainly targeting patients with advanced fibrosis/cirrhosis rather than those with active inflammation. This merits scrutiny as MSC are focused on their anti-inflammatory actions which are more relevant in settings of MSC’s major mechanisms of action are focused on their anti-inflammatory actions, which are more relevant in settings of their anti-inflammatory actions which are more relevant in inflammatory rather than fibrotic disease.
Table 4.
Clinical trials using MSCs to treat liver disease.
| Study | Year | Liver disease | Type of clinical trial | Duration of follow-up (month) | Patients, sample size (MSC/cohort) | Source of MSC | Injection route | Dose | Primary outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Mohamadnejad et al154 | 2007 | Decompensated liver cirrhosis | Case series | 12 | 4/0 | Autologous BM |
Peripheral vein | 31.7 × 106 | MELD score and creatinine |
| Kharaziha et al155 | 2009 | Liver cirrhosis | Cohort | 6 | 8/0 | Autologous BM |
Portal vein (n = 6) Peripheral vein (n = 2) |
3.0-5.0 × 107 | MELD score, prothrombin, and creatinine |
| El-Ansary et al156 | 2010 | Decompensated liver cirrhosis due to HCV or HBV | Case-control | 6 | 12/6 | Autologous BM |
Intra-splenic (n = 6) Peripheral vein (n = 6) |
1.0 × 106 | Creatinine, prothrombin time, albumin, bilirubin, and MELD score |
| Amer et al136 | 2011 | Decompensated liver cirrhosis due to HCV | Case-control | 6 | 20/20 | Autologous BM |
Intra-splenic (n = 10) Intra-hepatic (n = 10) |
2.0 × 107 | MELD score, ascites, peripheral oedema, Child-Pugh score, albumin, and fatigue |
| Peng et al146 | 2011 | ACLF caused by HBV | Case-control | 12 | 53/05 | Autologous BM |
Hepatic artery | 1.0 × 106 | MELD score, prothrombin time, albumin, and bilirubin |
| El-Ansary et al137 | 2012 | Decompensated liver cirrhosis due to HCV | Case-control | 12 | 15/10 | Autologous BM |
Peripheral vein | 1.0 × 106 | MELD score and albumin |
| Shi et al147 | 2012 | ACLF associated HBV | Case-control | 12 | 24/19 | Allogeneic UC |
Peripheral vein | 0.5 × 106 | MELD score, albumin, prothrombin time, bilirubin, ALT, and survival rates |
| Zhang et al138 | 2012 | Decompensated liver cirrhosis due to HBV | Case-control | 12 | 30/15 | Allogeneic UC |
Peripheral vein | 0.5 × 106 | MELD score, albumin, bilirubin and ascites |
| Amin et al157 | 2013 | Chronic HCV – associated liver cirrhosis | Cohort | 6 | 20/0 | Autologous BM |
Intra-splenic | 1.0 × 107 | MELD score, albumin, prothrombin time, bilirubin, AST, and ALT |
| Mohamadnejad et al134 | 2013 | Decompensated liver cirrhosis | RCT | 12 | 15/12 | Autologous BM |
Peripheral vein | 1.0 × 106 | No improvement |
| Wang et al145 | 2013 | UDCA-resistant PBC | Cohort | 12 | 7/0 | Allogeneic UC |
Peripheral vein | 0.5 × 106 | Alkaline phosphatase and GGT levels |
| Jang et al158 | 2014 | Alcohol-related liver cirrhosis | Cohort | 6 | 11/0 | Autologous BM |
Hepatic artery | 5.0 × 107 | MELD score and liver histology |
| Salama et al140 | 2014 | Chronic HCV—associated liver cirrhosis | RCT | 6 | 20/20 | Autologous BM |
Peripheral vein | 1.0 × 106 | MELD score and Child-Pugh score |
| Wang et al144 | 2014 | UDCA-resistant PBC | Cohort | 12 | 10/0 | Allogeneic | Peripheral vein | 3.0-5.0 × 105 | ALT, AST, GGT and IgM |
| Xu et al141 | 2014 | Chronic HBV-associated liver cirrhosis | RCT | 6 | 27/29 | Autologous BM |
Hepatic artery | 8.45 × 105 | MELD score improvement and reduction in IL-6, IL-17, and TNF-α levels |
| Kantarcioglu et al159 | 2015 | Liver cirrhosis | No control group | 6 | 12/0 | Autologous BM |
Peripheral vein | 1.0 × 106/kg | Partial improvement MELD score, no change in liver regeneration or fibrosis after 6 months |
| Suk et al143 | 2016 | Alcohol-related liver cirrhosis | RCT | 12 | 37/18 | Autologous BM |
Hepatic artery | 5.0 × 107 | Histologic fibrosis and Child-Pugh score |
| Shi et al151 | 2017 | Acute liver allograft rejection | RCT | 5.5 | 14/13 | Allogeneic | Peripheral vein | 1.0 × 106/kg | Liver function and graft histology, increased Treg/Th17 ratio, CD4 T-cell activation, elevated levels of TGF-b1 and PGE2 |
| Lanthier et al153 | 2017 | Decompensated alcoholic hepatitis | RCT | 1 | 28/30 | Autologous BM |
Hepatic artery | 4.7 × 107 | No improvement |
| Lin et al148 | 2017 | ACLF associated HBV | RCT | 6 | 56/54 | Allogeneic BM |
Peripheral vein | 1.0 × 106 | MELD score, bilirubin, and survival rates |
| Detry et al152 | 2017 | Liver transplant recipients | Non-RCT | 12 | 10/9 | Allogeneic BM |
Peripheral vein | 1.5-3.0 × 106 | No difference in the rate of infection or de novo cancer |
| Sakai et al160 | 2017 | Liver cirrhosis—liver disease of mixed aetiologies | Cohort | 12 | 4/0 | Autologous Adipose |
Hepatic artery | 6.6 × 105 | Improvement of liver function, HGF, and IL-6 increased |
| Zhang et al161 | 2017 | Ischemic-type biliary lesions following liver transplantation | RCT | 24 | 12/70 | Allogeneic UC |
Peripheral vein | 1 × 106/kg, 6-time | Improved liver function and survival rate |
| Fang et al142 | 2018 | Chronic HBV-associated liver cirrhosis | RCT | 12 | 50/53 | Allogeneic UC |
Peripheral vein | (4.0-4.5) × 108 | MELD score, Child-Pugh score, and liver function |
| Casiraghi et al150 | 2021 | Liver transplant recipients—liver disease of mixed aetiologies | RCT | 12 | 9/10 | Autologous BM |
Peripheral vein | 1-2 × 106/kg | Safety of MSCs in liver transplant recipients. Increase in T reg cells and tolerant NK cells. |
| Schacher et al149 | 2021 | CLD of mixed aetiologies (HCV/alcohol mainly) |
RCT | 3 | 4/5 | Allogeneic BM |
Peripheral vein | 1 × 106 | MELD score, Child Pugh score and ACLF grade |
Abbreviations: ACLF, acute-on-chronic liver failure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BM, bone marrow; CD4, cluster of differentiation 4; CLD, chronic liver disease; GGT, γ-glutamyltransferase; HBV, hepatitis B virus; HCV, hepatitis C virus; HGF, hepatocyte growth factor; IL, interleukin; IgM, immunoglobulin M; MELD, model for end-stage liver disease; MSC, mesenchymal stromal cell; NK, natural killer; PBC, primary biliary cholangitis; PGE, prostaglandin E2; RCT, randomised controlled trial; TGF, tumour growth factor; Th, T helper cells; TNF, tumour necrosis factor; T reg, regulatory T cells; UC, umbilical cord; UDCA, ursodeoxycholic acid.
Role of MSC in Human Liver Cirrhosis
Liver cirrhosis is a consequence of chronic liver injury and is characterized by profound scarring and architectural disruption. Mohamadnejad et al134 used infusions of autologous BM-derived-MSCs in 4 patients with decompensated liver cirrhosis. Results confirmed that the approach was feasible and safe, and there was also an improvement in the Model for End-Stage Liver Disease score in 3 out of the four patients at 6 months.135 An initial case–control study by Amer et al136 included 20 patients with either chronic Hepatitis C or B-induced cirrhosis that received MSC either via the intra-hepatic or intra-splenic route. Compared to the control group, there was an improvement in, the MSC group’s MELD score, ascites, and peripheral edema.
Several other studies showed similar improvements in patients with viral-related cirrhosis,137-139 and 2 larger randomized-control trials (RCTs) by Salama and Xu et al,140,141 respectively, with a total patient number of 47, also demonstrated an improvement in MELD score, Child-Pugh score, in addition to reductions in IL-6, IL-17, and TNFα levels after MSC administration. A larger controlled trial that included 50 patients with chronic hepatitis B showed similar improvements. In the treated group, there was a marked reduction in IL-6 and TNFα levels. IL-10 increased at 2 and 4 weeks post-MSC transplantation. CD4T and Treg cells were higher in the treated group than in the control group at 2 and 4 weeks, whereas CD8T and B cells were markedly reduced.142 the Similar benefit was demonstrated in the largest phase 2 trial for patients with alcohol-related cirrhosis. Here 55 patients completed the study (19 in a one-dose MSC group, 19 in a 2-dose MSC group, and 18 in the control group) and were followed up for 12 months. The one-time and 2-time MSC groups showed a reduction in the proportionate collagen area following MSC therapy with figures of 25% and 37%, respectively; however, there was no difference in fibrosis between the groups.143
Finally, 2 cohort studies by Wang et al144 investigated MSC in the setting of UDCA-resistant PBC. Although these trials used small numbers (n = 17) of patients with no control group, results demonstrated a reduction in liver biochemistry, mainly alkaline phosphatase and gamma-glutamyl transferase.144,145 While initial findings from these studies are promising, many of these studies are small in number, short in duration, and often lacking a control arm. Larger and double-blinded controlled trials are required across different liver cirrhosis etiologies with a wider geographical distribution to reliably assess the role of MSC here.
Role of MSC in Acute on Chronic Liver Failure
Acute on chronic liver failure (ACLF) is a condition characterized by systemic inflammation and organ failure and carries a poor prognosis. A case-control study by Peng et al146 included 53 patients with ACLF caused by Hepatitis B and 105 in the control arm group. The use of MSC demonstrated short-term efficacy but no effect on long-term outcomes. Hence albumin levels improved at 3-24 weeks post MSC infusion but not beyond that. Similarly, Bilirubin and PT levels improvement was limited to 4-12 weeks. The MELD score was superior to the control group at 3-36 weeks post-transplantation. There were no differences in mortality between the 2 groups over a 192-week period.146
Further studies by Shi et al147 and Lin et al148 demonstrated safety with MSC use and also an improvement in survival rates associated with an improvement in liver function and a reduction in the incidence of severe infection. In Lin et al’s paper, the rate of severe infection was 33.3% in the control arm receiving standard therapy versus 16.1% in the MSC group. Mortality rate from multi-organ failure was also lower in the MSC group—17.9% versus 37% in the control arm.148 Shi et al describe a significant improvement in PT levels over a 48-week period in those receiving MSC therapy versus the control group. This suggests the possibility of improved thrombin function in those receiving MSCs. After MSC infusion, the MELD score significantly decreased at 4, 8, and 12 weeks significantly decreased at 4, 8, and 12 weeks compared to the control group.147
The limitation in mortality benefit in Peng et al’s trial may be reflected in using a single autologous infusion of MSCs. The Shi et al and Lin et al studies used multiple allogenic infusions of MSCs over several weeks with an overall improvement in survival rates. However, autologous MSCs from patients with Hepatitis B may proliferate more slowly, and thus patients may require further transfusions to potentially improve efficacy.
In a more recent randomized placebo-controlled phases I-II trial, 4 patients with ACLF were treated with 5 infusions of BM-MSC for 3 weeks and compared to 5 patients in the control arm who received standard medical therapy and placebo saline infusion. This demonstrated the safety of MSCs; however, the 90-day survival rate was similar between both groups (20% for placebo versus 25% for BM-MSC).149
There are notable differences between the studies related to ACLF and its various etiologies. The earlier studies included only patients with Hepatitis B whilst Schacher et al’s recent study included patients with a wider range of etiologies (Hepatitis C and alcohol-related liver disease) and with more severe forms of ACLF—grades 2 and 3. This study was significantly underpowered with regard to mortality outcomes due to the small number in the intervention arm. Only one patient completed the infusion protocol due to the high ACLF mortality rates.149
Role of MSC in Liver Transplantation
Two of the most common complications in patients post-transplant are rejection and infection. Casiraghi et al150 aimed to assess the safety profile of MSC in post-transplant patients using a single pre-transplant IV infusion of MSC given to 19 patients in total (10 controls). Over a 1-year follow-up period, there were no infusion-related complications post-MSC administration. Liver graft function remained similar in both groups. Immunologically, T reg and memory T regs increased for the first 2 weeks post MSC infusion; however, their levels at 6-12 months were comparable to the control arm.150
In a pilot study by Shi et al151, 14 patients with acute liver allograft rejection were given MSC, which improved liver function and graft histology. ALT, AST, and total Bilirubin significantly reduced in the 12-week follow-up period in those patients receiving MCSs. Histologically, portal vein endothelitis and bile duct damage improved in 42.8% of patients at 4 weeks post MSC infusion. However, these patients had not responded to immunosuppressive agent dose adjustments in relation to their acute rejection.151
In contrast, though Detry et al152 demonstrated that infusion of MSC in 10 liver transplant patients (with 10 controls) did not have any impact on the rejection rate or overall graft survival. A difference between these trials is that in the first trial, MSCs were given as a therapeutic option, while in the second trial, MSCs were given a few days after transplant with the aim of withdrawing standard immunosuppression. In Detry et al’s study, immunosuppression weaning was unsuccessful in the MSC group.
Mohamadnejad et al134 infused autologous BM-MSC in 15 patients with decompensated cirrhosis, with 12 patients in the control group. There were no changes in MELD score, Child-Pugh score, serum albumin, INR, and serum transaminases between the MSC and control groups over a 12-month follow-up period.134 This contrasts with Mohamadnejad’s initial study in 2009, which suggested an improvement in both liver function and quality of life in a similar cohort of patients, although the lack of a control group is a major limitation of the initial study.
Additionally, Lantheir et al153 treated 28 patients with alcohol-associated hepatitis with autologous BM-derived CD34+ stem cells and MSC. In the control arm, 30 patients received standard supportive therapy. Overall, there was no difference in hepatocyte proliferation on liver biopsy at 4 weeks in both arms. Macrophage activation in alcohol-associated hepatitis was reported to be a positive prognostic marker in a prior study by the same group. Although the trial displayed negative results, the greater level of macrophage activation in the MSC arm may warrant further study.153
Larger and more robust randomized clinical trials, both in the pre-clinical and clinical setting, are required to provide definitive evidence of MSC efficacy. In addition, such studies need to be undertaken across a more diverse geography to ascertain if the beneficial effects are consistently observed. Further work should also delineate the impact of MSC cell source, the best delivery route, and the importance of optimizing culture conditions of MSC.
Utilizing MSCs and EV Therapy for Hepatic Disease
Various clinical trials thus far have investigated the role of MSCs in many liver diseases, the majority being in patients with Hepatitis B and C. Due to recent breakthroughs in antiviral therapy, Hepatitis C has become a curable disease; thus, the future role of MSCs in this cohort of patients is uncertain. In those with Hepatitis B, there are various effective antiviral therapies in development, which may deem it a curable disease soon. In other liver aetiologies, such as alcohol-related liver disease and NAFLD—MSCs have an anti-inflammatory and anti-fibrotic role. However, the exact anti-fibrotic mechanisms remain uncertain, although these have been postulated in the literature as described below (Fig. 3).
Figure 3.
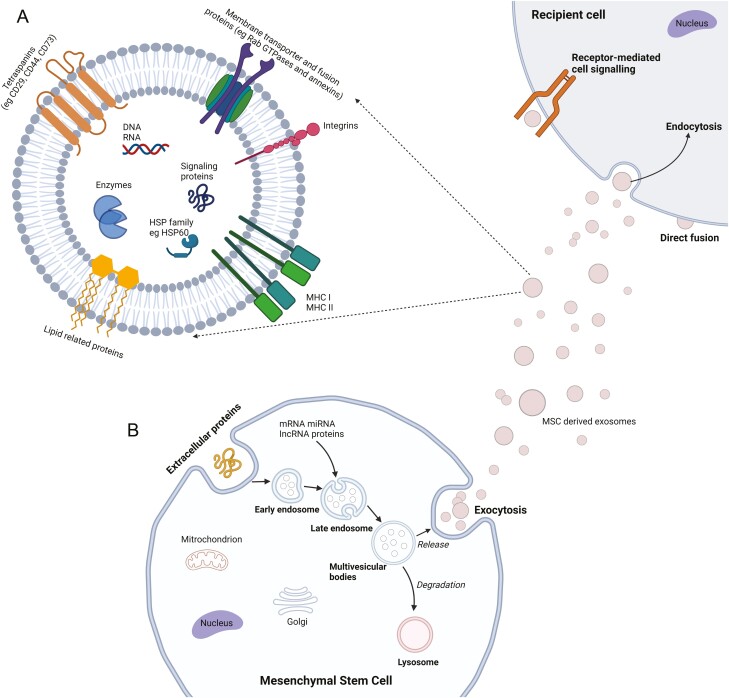
EVMs. (A) Extracellular proteins are invaginated via the cell wall to form early endosomes. Late endosomes develop into multivesicular bodies (MVB) via a selection of protein cargo. MVBs then either fuse with the plasma membrane resulting in exosome release or undergo degradation by lysosomes. The released exosomes then enter the recipient cell via 3 methods: receptor medicated cell signaling, endocytosis and direct cell fusion. (B) MSC-derived exosomes carry various proteins supporting its biogenesis—eg, proteins (eg, ALIX, TSG101), tetraspanins (eg, CD29, CD44, and CD73), membrane transporter and fusion proteins (eg, Rab GTPases and annexins), integrins, heat shock proteins (eg, HSP60, HSP70, and HSP90), and MHC classes I and II protein.
Potential Risks of MSC Therapy
Tumourigenic
Despite numerous studies reporting on the safety profile of MSCs, there have been contradictory results describing the pro and anti-tumorigenic effects of MSCs. One potential concern is the susceptibility of MSCs to undergo malignant transformation, as MSCs undergo several passages before transplantation, potentially increasing their risk for genetic mutations, both in vitro and in vivo. Murine MSCs exhibited chromosomal changes and transformation into malignant cells.162-166 Paradoxically, several studies demonstrated human MSCs becoming senescent both in vitro and in vivo.163,167,168 Dahl et al169 showed that a high passages N170, MSCs may display telomeric deletions, and a further study observed microsatellite instability and downregulation of genes involved in DNA repair in human MSCs.170
MSCs have a potential risk of promoting pre-existing tumor growth or precancerous lesions, and in vitro, when transplanted with cancer cells, MSCs have been shown to promote tumor formation by releasing various growth factors.171 In contrast, Cousin et al172 highlighted tumor growth inhibition in pancreatic cancer cells in vivo. In animal models, MSCs have shown conflicting results—both promoting173-176 and inhibiting tumor growth.177-179
Casirgaghi et al180 published a safety study in 700 patients demonstrating that no patients developed any tumors from autologous or allogeneic MSCs. This would suggest that there are fundamental differences between human and murine MSC when it comes to the risk of tumorigenicity, as human MSC have not been associated with any such events.
Viral Transmission
Autologous MSC transplantation may carry a risk of viral transmission to the recipient, as has been demonstrated in the treatment of GvHD after hemapoietic stem cell transplantation.138,181 Sundin et al182 demonstrated that MSCs can be infected with CMV and HSV-1 in vitro, but not EBV, although small subpopulations of MSC expressed CD21—receptor for EBV uptake in those patients. On the other hand, parvovirus B19 transmission to bone marrow cells in vitro carries a low pathogenicity, and patients transplanted with allogeneic B19-positive MSCs did not result in symptom development or viraemia.182
MSCs are known to reduce lymphocyte responses to bacteria, fungi, and viruses, but it’s relevance needs to be evaluated further in clinical trials . As yet, no information is available on the role of HSV and CMV transmission in vivo, but to reduce the risk of viral transmission, both recipient and MSC donors should be screened for the above viruses, as they can be fatal in immunocompromised patients.
Bridging the Gap Between Optimism and Reality
Despite the encouraging reports from murine studies and early phase clinical trials, there remain a series of challenges and issues that need to be addressed before MSC can be translated into meaningful therapeutic products for patients.
Improving Lab-Based Mechanistic Cell Therapy: From Bench to Clinic
Animal models often inadequately reflect human disease, which is compounded by species-specific differences in the immunobiology of human and murine MSC. Thus, according to Ritskes-Hoitinga et al,183 specific measures have been suggested to create in vivo systems that replicate native human tissues for cell testing to overcome the limitations of in vivo models.184 In this way, Hu et al185 illustrated that the creation of human liver disease models for use as test platforms has been made possible by using human-induced pluripotent stem cell (hiPSC) technology. When sufficient evidence demonstrates the predictive value of these models for efficacy and toxicity data, alongside well-established and validated relevant models for each disease, it will hopefully be possible to replace animal testing with human 3D in vitro liver tissue models. Humanized mouse systems can be utilized, and Bissig-Choisat et al186 conducted research using a unique NAFLD humanised mouse system, with metabolic analyses of humanized livers demonstrating similarities to those seen in people with liver disease.187
MSC Product Optimization
Potent effects of MSC therapy may only be observed in 45%-50% of the patients,188,189 which is not dissimilar to the effects observed with other cellular therapy products in development. Suboptimal clinical response is dependent on a variety of variables, including heterogeneity of the MSC, patient recipient disease heterogeneity, variable dosing, and dosing routes, and limited understanding of the host response.189,190 Inherent heterogeneity between sources of MSC is due to multiple factors including donor characteristics, tissue origin,191 and also the isolation and in vitro preparation methods.192,193 This point can be illustrated by the results of the phase III trial conducted for graft versus host disease (GVHD) using prochymal (a commercial MSC product).194 MSC used in this trial were retrieved from a single donor, expanded to passages 3 and 4 during manufacturing, and infused at 2 million cells/kg twice a week for 4 weeks to treat 240 participants. The trial did not meet its primary clinical endpoint of complete response in GVHD in comparison to placebo control, although a positive impact was noted on the gut and liver. However, these findings were in contrast to published European data,195 where a phase II trial138 to treat a similar patient population with GVHD demonstrated clinical efficacy with a response rate of 77% in patients with GVHD at the primary endpoint (day 28). In this study, MSCs were pooled from 8 donors, and variable doses (median 2.2 × 106 cell/kg) with a median of 3 doses (range 1-9) were administered. These examples highlight the need for developing standardized protocols for MSC production to allow meaningful comparisons between different studies. In addition, they suggest that careful identification of potency markers in MSC batches made from different donors is important to maximize efficacy.152
Priming of MSC
To cater for diverse immune disorders and to augment their therapeutic efficacy, priming and purification of MSC may be required196 as there are marked differences in gene expression of therapeutically effective and ineffective MSC clones, as demonstrated by Lee et al197 Among the genes expressed by effective clones, endothelin-1(EDN-1) significantly increased the efficacy of human UC-MSC against myocardial infarction. In addition, mechanistic analysis of EDN-1 showed significantly increased expression of Cadherin2 (CDH2) and vascular endothelial growth factor (VGEF). To improve the predictability of MSC effector functions, the Clinical Indications Prediction (CLIP) scale has been developed198 to predict the impact of culture conditions and donor to donor heterogeneity on the therapeutic efficacy of MSC for different disease indications.
Priming MSC in vitro with stimulatory factors is another strategy to improve their engraftment and therapeutic functions, albeit complicated by cost of goods considerations. Agents tested to date include the use of cytokines or growth factors such as TNF-γ, IL-β1, TNFα, IL-17, TGFβ-1199-202 as well as hypoxic culture conditions,135,203 modification of culture methods204 and genetic modification of MSC205 to improve potency.
Twist1 has been demonstrated to be a direct target of fibroblast growth factor (Fgf2) and interferon-gamma in MSCs, thus allowing an opportunity to alter the immunomodulatory and anti-inflammatory effects of MSCs.198,206-208 Bauer et al209 developed an in vitro assay that integrated multidimensional flow cytometry data into a measurement of MSC-mediated inhibition of T-cell activation. They identified distinct morphological subpopulations that could be predictive of MSC immunosuppressive function via activation of CD4+ and CD8+ T cells. In addition, HOXB7 overexpression was found to potentially increase MSC proliferation by inducing a fibroblast growth factor-mediated autocrine loop.210
MSCs are thawed and directly infused as the standard of care in most clinical studies. Notably, during cryopreservation, the actin filaments within the MSCs are disrupted resulting in death in the bloodstream prior to reaching their target destination.211 Nolta et al211 demonstrated that MSCs prepared with cryo-recovery and hypoxic formulation pre-infusion have better survival than unconditioned cells in immune-deficient mice (10% versus <1% in pre-conditioning). To improve in vivo survival and preserve MSC function, there needs to be a period of recovery after cryopreservation prior to injection. Reducing sugars in the post-thawing period has also been shown to improve the duration of MSC retention and functionality in vivo revascularization assays.212 Courtman et al’s phase I trial on MSCs in septic shock used freshly infused allogeneic bone marrow-derived MSCs, which were demonstrated to be safe (www.clinicaltrials.gov; NCT02421484).
In a study of ARDS, MSC direct coculture with monocyte-derived macrophages enhanced their phagocytotic activity.213 In a separate study by Le Blanc et al,214 in patients with steroid-resistant severe GvHD, 71% of patients responded to MSC therapy but with varying survival rates. Follow-up data suggested that “”responders’’ had a gut immune profile with higher levels of CD8+ lymphocytes and FoxP3+ T lymphocytes and lower levels of CD56+ and CD68+ compared to “”non-responders’’. This suggests that the ongoing gut inflammation in recipients may affect the therapeutic potential of MSCs. Further studies to explore the role of a patient’s immunogenic profile and their effect on MSCs.
Route of Administration
There is no consensus yet on the best delivery route for MSCs for clinical studies relating to liver disease, with the peripheral vein being the most widely used, followed by the hepatic artery. Some trials used more directed methods via the intra-splenic and intra-hepatic routes, but there was no difference in efficacy based on the route of administration (PV, IS, portal vein, or IH).136,155 injection could lead to cell damage by trauma, hypoxia, or NK cell-mediated MSC apoptosis, as well as adding logistical and financial consequences. In contrast, MSC systemic administration may limit biodistribution and homing effects on the target tissue.
MSC delivery has been demonstrated to trigger a prothrombotic state via activation of the complement system and coagulation cascade, known as “Instant Blood-Mediated Inflammatory Reaction” IBMIR, although thrombotic events have been reported in only a few studies.215,216 MSC infusion increases C3a and sC5b-9 levels, activating the thrombin anti-thrombin complex, resulting in a pro-coagulant state.217 Further studies are required to establish the optimum route of delivery that would improve the efficacy of MSC-based therapies.
Other clinical challenges to address include standardizing dosing regimens (single versus multiple doses) and cell therapy release assays as relevant for diseases and differing patient populations.189 Various dosing regimens have been used in clinical trials and given that most of these trials have been aimed at determining the efficacy, an optimal dose of MSC for clinical use has not been established.218,219 For example, in a study using MSC in liver cirrhosis, MSCs were injected at a dose of 1 × 107, and they were found to be effective for 6 months.139 In the same year, 2013, another study with MSC administration in liver cirrhosis found a dose of 2 × 108 to have no significant effect after 12 months compared with a placebo.134 Thus, heterogeneity between studies poses challenges in comparing the different trials.
Analytical Potency Methods
The International Society for Cellular Therapy (ISCT) identified the need for functional markers of potency and the development of release potency assays to meet regulatory requirements for advanced clinical trials.220 Three preferred analytical methods were suggested: quantitative RNA analysis of selected gene products, flow cytometry analysis of functionally relevant surface markers, and a protein-based assay of the secretome. MSC poses a certain challenge to potency analytical methods development due to the various tissue sources, heterogeneity of proposed mechanisms, and lack of reference standards. To date, only a few potency assays have been studied—robust and reproducible quantitative potency assays for MSCs are needed to accelerate the transition into clinical practice. In addition, these assays will need to be validated for analytical procedure, specificity, accuracy, and precision.
In order to improve MSC safety in those with hepatic disorders, we need to be selective in choosing the relevant patient population, ensuring patients have the right indication. Alongside this, we need to ensure that the appropriate donors are selected and that the MSCs undergo a rigorous handling process in terms of cryopreservation, storing, and thawing. The optimum delivery of MSCs in hepatic disease is yet to be defined. To reduce the risk of immediate reactions following MSC infusion, pre-medications such as steroids and antihistamines may be considered.
Conclusions
The data available thus far provides a strong foundation to robustly investigate the potential of the MSCs and overcome the remaining challenges before MSC can be used as a clinical treatment. In comparison to MSC, MSC-derived EVs, with their higher safety profile, lower immunogenicity, and safer cargo of EV contents between recipient and donor cells, also warrants further research to harness their full potential. Integrated efforts between scientific, regulatory, industrial, and clinical stakeholders to expedite the production of MSC that are optimized and tailored for the indication of use and readily available at an affordable cost are required to make a meaningful progress.
Contributor Information
Sheeba Khan, National Institute for Health Research, Biomedical Research Centre at University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham, Birmingham, West Midlands, UK; Centre for Liver and Gastrointestinal Research, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, West Midlands, UK; Liver Unit, University Hospitals Birmingham NHS Foundation Trust, Birmingham, Birmingham, West Midlands, UK.
Sara Mahgoub, National Institute for Health Research, Biomedical Research Centre at University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham, Birmingham, West Midlands, UK; Centre for Liver and Gastrointestinal Research, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, West Midlands, UK; Liver Unit, University Hospitals Birmingham NHS Foundation Trust, Birmingham, Birmingham, West Midlands, UK.
Nada Fallatah, National Institute for Health Research, Biomedical Research Centre at University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham, Birmingham, West Midlands, UK; Centre for Liver and Gastrointestinal Research, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, West Midlands, UK; Department of Laboratory Medicine, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah, Saudi Arabia.
Patricia F Lalor, National Institute for Health Research, Biomedical Research Centre at University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham, Birmingham, West Midlands, UK; Centre for Liver and Gastrointestinal Research, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, West Midlands, UK.
Philip N Newsome, National Institute for Health Research, Biomedical Research Centre at University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham, Birmingham, West Midlands, UK; Centre for Liver and Gastrointestinal Research, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, West Midlands, UK; Liver Unit, University Hospitals Birmingham NHS Foundation Trust, Birmingham, Birmingham, West Midlands, UK.
Data Availability
There are no new data associated with this article. DOI links to the translational and clinical studies appraised in this review are cited in the references section.
Conflict of Interest
All of the authors declared no potential conflicts of interest.
Funding
P.N.N., S.M., P.F.L. were supported by the Birmingham NIHR Biomedical Research Centre. This paper presents independent research supported in part by National institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS foundation Trust and the University of Birmingham. (Grant reference number: BRC-1215-20009). The views expressed are those of author(s) and not necessarily those of NIHR or the Department of Health and Social Care.
Author Contributions
S.K. and P.N. conceptualized this review. S.K. and S.M. equally contributed to writing original draft and preparation. S.M. updated and edited manuscript. N.F. helped in writing parts of the manuscript. All authors reviewed manuscript and provided critical feedback. P.N. provided overall supervision, reviewed manuscript and proof reading.
References
- 1. Asrani SK, Devarbhavi H, Eaton J, Kamath PS.. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151-171. 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 2. Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12(1):1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maria Effenberger MAK, Bettac E, Grabherr F, et al. Using infodemiology metrics to assess public interest in liver transplantation: Google trends analysis. J Med Internet Res. 2021;16(23):8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. NHSBT. Lliver transplant annual report NHSBT. 2018-2019. [Google Scholar]
- 5. Müller L, Tunger A, Wobus M, et al. Immunomodulatory properties of mesenchymal stromal cells: an update. Front Cell Dev Biol. 2021;9(9):637735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levy O, Kuai R, Siren EMJ, et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6(30):eaba6884. 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147. 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71-74. 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 9. Huang S, Xu L, Sun Y, et al. An improved protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Journal of Orthopaedic Translation. 2015;3(1):26-33. 10.1016/j.jot.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsai PC, Fu TW, Chen YMA, et al. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton’s jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009;15(5):484-495. 10.1002/lt.21715. [DOI] [PubMed] [Google Scholar]
- 11. Seki A, Sakai Y, Komura T, et al. Adipose tissue-derived stem cells as a regenerative therapy for a mouse steatohepatitis-induced cirrhosis model. Hepatology. 2013;58(3):1133-1142. 10.1002/hep.26470. [DOI] [PubMed] [Google Scholar]
- 12. Lei M, Li K, Li B, et al. Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials. 2014;35(24):6332-6343. 10.1016/j.biomaterials.2014.04.071. [DOI] [PubMed] [Google Scholar]
- 13. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313. 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 14. Najar M, Raicevic G, Boufker HI, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: combined comparison of adipose tissue, Wharton’s Jelly and bone marrow sources. Cell Immunol. 2010;264(2):171-179. [DOI] [PubMed] [Google Scholar]
- 15. Yoo KH, Jang IK, Lee MW, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259(2):150-156. 10.1016/j.cellimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 16. Mattar P, Bieback K.. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front Immunol. 2015;6:560. 10.3389/fimmu.2015.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lv F, Lu M, Cheung K MC, Leung V YL, Zhou G.. Intrinsic properties of mesemchymal stem cells from human bone marrow, umbilical cord and umbilical cord blood comparing the different sources of MSC. Curr Stem Cell Res Therapy. 2012;7(6):389-399. [DOI] [PubMed] [Google Scholar]
- 18. Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H.. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2(6):455-463. 10.5966/sctm.2012-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Secunda R, Vennila R, Mohanashankar A, et al. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology. 2015;67(5):793-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ketterl N, Brachtl G, Schuh C, et al. A robust potency assay highlights significant donor variation of human mesenchymal stem/progenitor cell immune modulatory capacity and extended radio-resistance. Stem Cell Res Therapy. 2015;6(1):236. 10.1186/s13287-015-0233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yin JQ, Zhu J, Ankrum JA.. Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng. 2019;3(2):90-104. 10.1038/s41551-018-0325-8. [DOI] [PubMed] [Google Scholar]
- 22. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 23. Viswanathan S, Shi Y, Galipeau J, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21(10):1019-1024. 10.1016/j.jcyt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 24. Nauta AJ, Fibbe WE.. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499-3506. [DOI] [PubMed] [Google Scholar]
- 25. Chen P-M, Yen M-L, Liu K-J, Sytwu H-K, Yen B-L.. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci. 2011;18(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vasandan AB, Jahnavi S, Shashank C, et al. Human mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci Rep. 2016;6(1):38308. 10.1038/srep38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galleu A, Riffo-Vasquez Y, Trento C, et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9416):eaam7828. 10.1126/scitranslmed.aam7828 [DOI] [PubMed] [Google Scholar]
- 28. de Witte SFH, Luk F, Sierra Parraga JM, et al. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells. 2018;36(4):602-615. 10.1002/stem.2779. [DOI] [PubMed] [Google Scholar]
- 29. Ryan JM, Barry FP, Murphy JM, Mahon BP.. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005;2:8. 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O.. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890-896. 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 31. Börger V, Bremer M, Ferrer-Tur R, et al. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci . 2017;18(7):1450. 10.3390/ijms18071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phinney DG, Pittenger MF.. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851-858. 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 33. Friedman SL. Liver fibrosis – from bench to bedside. J Hepatol. 2003;38 (Suppl 1):S38-53. 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 34. Iredale JP, Thompson A, Henderson NC.. Extracellular matrix degradation in liver fibrosis: biochemistry and regulation. Biochim Biophys Acta. 2013;1832(7):876-883. 10.1016/j.bbadis.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 35. Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838-3843. [DOI] [PubMed] [Google Scholar]
- 36. Parekkadan B, van Poll D, Megeed Z, et al. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;363(2):247-252. 10.1016/j.bbrc.2007.05.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang J, Bian C, Liao L, et al. Inhibition of hepatic stellate cells proliferation by mesenchymal stem cells and the possible mechanisms. Hepatol Res. 2009;39(12):1219-1228. 10.1111/j.1872-034x.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 38. Qiao H, Zhou Y, Qin X, et al. NADPH oxidase signaling pathway mediates mesenchymal stem cell-induced inhibition of hepatic stellate cell activation. Stem Cells Int. 2018;2018:1239143. 10.1155/2018/1239143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aggarwal S, Pittenger MF.. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815-1822. 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 40. Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2, 3-dioxygenase–mediated tryptophan degradation. Blood. 2004;103(12):4619-4621. 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 41. Milosavljevic N, Gazdic M, Simovic Markovic B, et al. Mesenchymal stem cells attenuate liver fibrosis by suppressing Th17 cells–an experimental study. Transpl Int. 2018;31(1):102-115. [DOI] [PubMed] [Google Scholar]
- 42. Fathy M, Okabe M, Othman E M, Saad Eldien HM, Yoshida T.. Preconditioning of adipose-derived mesenchymal stem-like cells with eugenol potentiates their migration and proliferation in vitro and therapeutic abilities in rat hepatic fibrosis. Molecules. 2020;25(9):2020. 10.3390/molecules25092020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sisakhtnezhad S, Heidari M, Bidmeshkipour A.. Eugenol enhances proliferation and migration of mouse bone marrow-derived mesenchymal stem cells in vitro. Environ Toxicol Pharmacol. 2018;57:166-174. 10.1016/j.etap.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 44. Yi J-L, Shi S, Shen Y-L, et al. Myricetin and methyl eugenol combination enhances the anticancer activity, cell cycle arrest and apoptosis induction of cis-platin against HeLa cervical cancer cell lines. Int J Clin Exp Path. 2015;8(2):1116-1127. [PMC free article] [PubMed] [Google Scholar]
- 45. Chai N-L, Zhang X-B, Chen S-W, Fan K-X, Linghu E-Q.. Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats. World J Gastroenterol. 2016;22(26):60366036. 10.3748/wjg.v22.i26.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kalluri R, Weinberg RA.. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420-1428. 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Choi SS, Diehl AM.. Epithelial-to-mesenchymal transitions in the liver. Hepatology. 2009;50(6):2007-2013. 10.1002/hep.23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zavadil J, Böttinger EP.. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764-5774. 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 49. Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22(6):845-854. 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nickel S, Vlaic S, Christ M, et al. Mesenchymal stromal cells mitigate liver damage after extended resection in the pig by modulating thrombospondin-1/TGF-β. NPJ Regener Med. 2021;6(1):84. 10.1038/s41536-021-00194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carvalho AB, Quintanilha LF, Dias JV, et al. Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem Cells. 2008;26(5):1307-1314. 10.1634/stemcells.2007-0941. [DOI] [PubMed] [Google Scholar]
- 52. Mannheimer EG, Quintanilha LF, Carvalho AB, et al. Bone marrow cells obtained from cirrhotic rats do not improve function or reduce fibrosis in a chronic liver disease model. Clin Transplant. 2011;25(1):54-60. 10.1111/j.1399-0012.2009.01191.x. [DOI] [PubMed] [Google Scholar]
- 53. Briquet A, Grégoire C, Comblain F, et al. RETRACTED: human bone marrow, umbilical cord or liver mesenchymal stromal cells fail to improve liver function in a model of CCl4-induced liver damage in NOD/SCID/IL-2Rγ (null) mice. Cytotherapy 2014;16(11):1511-1518. 10.1016/j.jcyt.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 54. Kramann R, Schneider RK, DiRocco DP, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell stem cell. 2015;16(1):51-66. 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baertschiger RM, Serre-Beinier V, Morel P, et al. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One. 2009;4(8):e6657. 10.1371/journal.pone.0006657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen X, Gan Y, Li W, et al. The interaction between mesenchymal stem cells and steroids during inflammation. Cell Death Dis. 2014;5(1):e1009. 10.1038/cddis.2013.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang B, Cheng X, Wang H, et al. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J Transl Med. 2016;14(1):45. 10.1186/s12967-016-0792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Parekkadan B, van Poll D, Suganuma K, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2(9):e941. 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Watanabe Y, Tsuchiya A, Seino S, et al. Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice. Stem Cells Transl Med. 2019;8(3):271-284. 10.1002/sctm.18-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang D, Zhang H, Liang J, et al. Effect of allogeneic bone marrow-derived mesenchymal stem cells transplantation in a polyI:C-induced primary biliary cirrhosis mouse model. Clin Exp Med. 2011;11(1):25-32. 10.1007/s10238-010-0105-6. [DOI] [PubMed] [Google Scholar]
- 61. Gazdic M, Simovic Markovic B, Vucicevic L, et al. Mesenchymal stem cells protect from acute liver injury by attenuating hepatotoxicity of liver natural killer T cells in an inducible nitric oxide synthase- and indoleamine 2,3-dioxygenase-dependent manner. J Tissue Eng Regen Med. 2018;12(2):e1173-e1185. 10.1002/term.2452. [DOI] [PubMed] [Google Scholar]
- 62. Higashimoto M, Sakai Y, Takamura M, et al. Adipose tissue derived stromal stem cell therapy in murine C on A-derived hepatitis is dependent on myeloid-lineage and CD 4+ T-cell suppression. Eur J Immunol. 2013;43(11):2956-2968. 10.1002/eji.201343531. [DOI] [PubMed] [Google Scholar]
- 63. Wang H, Wang D, Yang L, et al. Compact bone-derived mesenchymal stem cells attenuate nonalcoholic steatohepatitis in a mouse model by modulation of CD4 cells differentiation. Int Immunopharmacol. 2017;42:67-73. 10.1016/j.intimp.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 64. Maredziak M, Marycz K, Tomaszewski KA, Kornicka K, Henry BM.. The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells. Stem Cells Int. 2016;2016:2152435. 10.1155/2016/2152435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053-1067. 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214-222. 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 67. Timmers L, Lim SK, Arslan F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2008;1(2):129-137. 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 68. Akyurekli C, Le Y, Richardson RB, et al. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev Rep. 2015;11(1):150-160. 10.1007/s12015-014-9545-9. [DOI] [PubMed] [Google Scholar]
- 69. Duijvesz D, Luider T, Bangma CH, Jenster G.. Exosomes as biomarker treasure chests for prostate cancer. Eur Urol. 2011;59(5):823-831. 10.1016/j.eururo.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 70. Kim SJ, Moon GJ, Cho YH, et al. Circulating mesenchymal stem cells microparticles in patients with cerebrovascular disease. PLoS One. 2012;7(5):e37036. 10.1371/journal.pone.0037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Théry C, Ostrowski M, Segura E.. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581-593. 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 72. Wubbolts R, Leckie RS, Veenhuizen PT, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278(13):10963-10972. 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 73. Chen TS, Lai RC, Lee MM, et al. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38(1):215-224. 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Taylor DD, Gerçel-Taylor C.. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer. 2005;92(2):305-311. 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yang E, Wang X, Gong Z, et al. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Targeted Therapy. 2020;5(1):242. 10.1038/s41392-020-00359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mittelbrunn M, Sánchez-Madrid F.. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13(5):328-335. 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Psaraki A, Ntari L, Karakostas C, Korrou-Karava D, Roubelakis MG.. Extracellular vesicles derived from mesenchymal stem/stromal cells: the regenerative impact in liver diseases. Hepatology.n/a(n/a). [DOI] [PubMed] [Google Scholar]
- 78. Tomasoni S, Longaretti L, Rota C, et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22(5):772-780. 10.1089/scd.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Katakowski M, Buller B, Zheng X, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335(1):201-204. 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ono M, Kosaka N, Tominaga N, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63. [DOI] [PubMed] [Google Scholar]
- 81. Feng Y, Huang W, Wani M, Yu X, Ashraf M.. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9(2):e88685. 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang Y, Chopp M, Liu XS, et al. Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Mol Neurobiol. 2017;54(4):2659-2673. 10.1007/s12035-016-9851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liang X, Zhang L, Wang S, Han Q, Zhao RC.. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129(11):2182-2189. 10.1242/jcs.170373. [DOI] [PubMed] [Google Scholar]
- 84. Wang X, Gu H, Qin D, et al. Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci Rep. 2015;5(1):13721. 10.1038/srep13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang X, Huang W, Yang Y, et al. Loss of duplexmiR-223 (5p and 3p) aggravates myocardial depression and mortality in polymicrobial sepsis. Biochim Biophys Acta. 2014;1842(5):701-711. 10.1016/j.bbadis.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Iglesias DM, El-Kares R, Taranta A, et al. Stem cell microvesicles transfer cystinosin to human cystinotic cells and reduce cystine accumulation in vitro. PLoS One. 2012;7(8):e42840. 10.1371/journal.pone.0042840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Katsuda T, Tsuchiya R, Kosaka N, et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3:1197. 10.1038/srep01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li X, Chen R, Kemper S, Brigstock DR.. Extracellular vesicles from hepatocytes are therapeutic for toxin-mediated fibrosis and gene expression in the liver. Front Cell Dev Biol. 2019;7:368. 10.3389/fcell.2019.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Newman LA, Muller K, Rowland A.. Circulating cell-specific extracellular vesicles as biomarkers for the diagnosis and monitoring of chronic liver diseases. Cell Mol Life Sci. 2022;79(5):232. 10.1007/s00018-022-04256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fiore EJ, Domínguez LM, Bayo J, García MG, Mazzolini GD.. Taking advantage of the potential of mesenchymal stromal cells in liver regeneration: Cells and extracellular vesicles as therapeutic strategies. World J Gastroenterol. 2018;24(23):2427-2440. 10.3748/wjg.v24.i23.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhou L, Shen M, Fan X, Liu Y, Yang L.. Pathogenic and potential therapeutic roles of exosomes derived from immune cells in liver diseases. Front Immunol. 2022;13:810300. 10.3389/fimmu.2022.810300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Németh K, Varga Z, Lenzinger D, et al. Extracellular vesicle release and uptake by the liver under normo- and hyperlipidemia. Cell Mol Life Sci. 2021;78(23):7589-7604. 10.1007/s00018-021-03969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Herrera MB, Fonsato V, Gatti S, et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14(6b):1605-1618. 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Haga H, Yan IK, Takahashi K, Matsuda A, Patel T.. Extracellular vesicles from bone marrow-derived mesenchymal stem cells improve survival from lethal hepatic failure in mice. Stem Cells Transl Med. 2017;6(4):1262-1272. 10.1002/sctm.16-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen L, Xiang B, Wang X, Xiang C.. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Research & Therapy. 2017;8(1):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tan CY, Lai RC, Wong W, et al. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5(3):76. 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang S, Jiang L, Hu H, et al. Pretreatment of exosomes derived from hUCMSCs with TNF-α ameliorates acute liver failure by inhibiting the activation of NLRP3 in macrophage. Life Sci. 2020;246:117401. 10.1016/j.lfs.2020.117401. [DOI] [PubMed] [Google Scholar]
- 99. Nong K, Wang W, Niu X, et al. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell–derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy. 2016;18(12):1548-1559. 10.1016/j.jcyt.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 100. Yan Y, Jiang W, Tan Y, et al. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther. 2017;25(2):465-479. 10.1016/j.ymthe.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ko SF, Yip HK, Zhen YY, et al. Adipose-derived mesenchymal stem cell exosomes suppress hepatocellular carcinoma growth in a rat model: apparent diffusion coefficient, natural killer T-cell responses, and histopathological features. Stem Cells Int. 2015;2015:853506. 10.1155/2015/853506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tsiapalis D, O’Driscoll L.. Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells. 2020;9(4):991. 10.3390/cells9040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Maji S, Matsuda A, Yan IK, Parasramka M, Patel T.. Extracellular vesicles in liver diseases. Am J Physiol Gastrointest Liver Physiol. 2017;312(3):G194-G200. 10.1152/ajpgi.00216.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gowen A, Shahjin F, Chand S, Odegaard KE, Yelamanchili SV.. Mesenchymal stem cell-derived extracellular vesicles: challenges in clinical applications. Front Cell Dev Biol. 2020;8:149. 10.3389/fcell.2020.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lee UE, Friedman SL.. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25(2):195-206. 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ikejima K, Okumura K, Kon K, Takei Y, Sato N.. Role of adipocytokines in hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22(Suppl 1):S87-S92. 10.1111/j.1440-1746.2007.04961.x. [DOI] [PubMed] [Google Scholar]
- 107. Krampera M, Le Blanc K.. Mesenchymal stromal cells: putative microenvironmental modulators become cell therapy. Cell Stem Cell. 2021;28(10):1708-1725. 10.1016/j.stem.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 108. Lee KD, Kuo TK, Whang-Peng J, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40(6):1275-1284. 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- 109. Li J, Tao R, Wu W, et al. 3D PLGA scaffolds improve differentiation and function of bone marrow mesenchymal stem cell-derived hepatocytes. Stem Cells Dev. 2010;19(9):1427-1436. 10.1089/scd.2009.0415. [DOI] [PubMed] [Google Scholar]
- 110. Ong SY, Dai H, Leong KW.. Inducing hepatic differentiation of human mesenchymal stem cells in pellet culture. Biomaterials. 2006;27(22):4087-4097. 10.1016/j.biomaterials.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 111. Banas A, Teratani T, Yamamoto Y, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46(1):219-228. 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 112. Piryaei A, Valojerdi MR, Shahsavani M, Baharvand H.. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Rev Rep. 2011;7(1):103-118. 10.1007/s12015-010-9126-5. [DOI] [PubMed] [Google Scholar]
- 113. Aurich I, Mueller LP, Aurich H, et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007;56(3):405-415. 10.1136/gut.2005.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Campard D, Lysy PA, Najimi M, Sokal EM.. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134(3):833-848. 10.1053/j.gastro.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 115. Aurich H, Sgodda M, Kaltwasser P, et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2009;58(4):570-581. 10.1136/gut.2008.154880. [DOI] [PubMed] [Google Scholar]
- 116. Seo MJ, Suh SY, Bae YC, Jung JS.. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328(1):258-264. 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- 117. Chamberlain J, Yamagami T, Colletti E, et al. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology. 2007;46(6):1935-1945. 10.1002/hep.21899. [DOI] [PubMed] [Google Scholar]
- 118. Uccelli A, Moretta L, Pistoia V.. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726-736. 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 119. Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212-222. 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 120. van Poll D, Parekkadan B, Cho CH, et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47(5):1634-1643. 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 121. Natarajan A, Wagner B, Sibilia M.. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci USA. 2007;104(43):17081-17086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lin N, Hu K, Chen S, et al. Nerve growth factor-mediated paracrine regulation of hepatic stellate cells by multipotent mesenchymal stromal cells. Life Sci. 2009;85(7-8):291-295. 10.1016/j.lfs.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 123. Higashiyama R, Inagaki Y, Hong YY, et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45(1):213-222. 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- 124. Salazar KD, Lankford SM, Brody AR.. Mesenchymal stem cells produce Wnt isoforms and TGF-beta1 that mediate proliferation and procollagen expression by lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2009;297(5):L1002-L1011. 10.1152/ajplung.90347.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Boomsma RA, Geenen DL.. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7(4):e35685. 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683-692. 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Driscoll J, Patel T.. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J Gastroenterol. 2019;54(9):763-773. 10.1007/s00535-019-01599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Fan XL, Zhang Y, Li X, Fu QL.. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77(14):2771-2794. 10.1007/s00018-020-03454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Eom YW, Kang SH, Kim MY, Lee JI, Baik SK.. Mesenchymal stem cells to treat liver diseases. Ann Transl Med. 2020;8(8):563. 10.21037/atm.2020.02.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ezquer F, Huang YL, Ezquer M.. New perspectives to improve mesenchymal stem cell therapies for drug-induced liver injury. Int J Mol Sci . 2022;23(5):2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. He C, Yang Y, Zheng K, et al. Mesenchymal stem cell-based treatment in autoimmune liver diseases: underlying roles, advantages and challenges. Ther Adv Chronic Dis. 2021;12:2040622321993442. 10.1177/2040622321993442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Amadeo F, Trivino Cepeda K, Littlewood J, et al. Mesenchymal stromal cells: what have we learned so far about their therapeutic potential and mechanisms of action?. Emerg Top Life Sci. 2021;5(4):549-562. 10.1042/ETLS20210013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Harting MT, Srivastava AK, Zhaorigetu S, et al. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells. 2018;36(1):79-90. 10.1002/stem.2730. [DOI] [PubMed] [Google Scholar]
- 134. Mohamadnejad M, Alimoghaddam K, Bagheri M, et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33(10):1490-1496. 10.1111/liv.12228. [DOI] [PubMed] [Google Scholar]
- 135. Gonzalez-King H, García NA, Ontoria-Oviedo I, et al. Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells. 2017;35(7):1747-1759. [DOI] [PubMed] [Google Scholar]
- 136. Amer ME, El-Sayed SZ, El-Kheir WA, et al. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011;23(10):936-941. 10.1097/MEG.0b013e3283488b00. [DOI] [PubMed] [Google Scholar]
- 137. El-Ansary M, Abdel-Aziz I, Mogawer S, et al. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev Rep. 2012;8(3):972-981. 10.1007/s12015-011-9322-y. [DOI] [PubMed] [Google Scholar]
- 138. Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27(Suppl 2):112-120. 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 139. Amin MA, Sabry D, Rashed LA, et al. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transplant. 2013;27(4):607-612. 10.1111/ctr.12179. [DOI] [PubMed] [Google Scholar]
- 140. Salama H, Zekri AR, Medhat E, et al. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther. 2014;5(3):70. 10.1186/scrt459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Xu L, Gong Y, Wang B, et al. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J Gastroenterol Hepatol. 2014;29(8):1620-1628. 10.1111/jgh.12653. [DOI] [PubMed] [Google Scholar]
- 142. Fang X, Liu L, Dong J, et al. A study about immunomodulatory effect and efficacy and prognosis of human umbilical cord mesenchymal stem cells in patients with chronic hepatitis B-induced decompensated liver cirrhosis. J Gastroenterol Hepatol. 2018;33(4):774-780. 10.1111/jgh.14081. [DOI] [PubMed] [Google Scholar]
- 143. Suk KT, Yoon JH, Kim MY, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64(6):2185-2197. 10.1002/hep.28693. [DOI] [PubMed] [Google Scholar]
- 144. Wang L, Han Q, Chen H, et al. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev. 2014;23(20):2482-2489. 10.1089/scd.2013.0500. [DOI] [PubMed] [Google Scholar]
- 145. Wang L, Li J, Liu H, et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28(Suppl 1):85-92. 10.1111/jgh.12029. [DOI] [PubMed] [Google Scholar]
- 146. Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54(3):820-828. 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 147. Shi M, Zhang Z, Xu R, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1(10):725-731. 10.5966/sctm.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lin BL, Chen JF, Qiu WH, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66(1):209-219. 10.1002/hep.29189. [DOI] [PubMed] [Google Scholar]
- 149. Schacher FC, Martins Pezzi da Silva A, Silla L, Álvares-da-Silva MR.. Bone marrow mesenchymal stem cells in acute-on-chronic liver failure grades 2 and 3: a phase I-II randomized clinical trial. Can J Gastroenterol Hepatol. 2021;2021:3662776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Casiraghi F, Perico N, Podestà MA, et al. Third-party bone marrow-derived mesenchymal stromal cell infusion before liver transplantation: a randomized controlled trial. Am J Transplant. 2021;21(8):2795-2809. 10.1111/ajt.16468. [DOI] [PubMed] [Google Scholar]
- 151. Shi M, Liu Z, Wang Y, et al. A pilot study of mesenchymal stem cell therapy for acute liver allograft rejection. Stem Cells Transl Med. 2017;6(12):2053-2061. 10.1002/sctm.17-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Detry O, Vandermeulen M, Delbouille MH, et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: A phase I-II, open-label, clinical study. J Hepatol. 2017;67(1):47-55. 10.1016/j.jhep.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 153. Lanthier N, Lin-Marq N, Rubbia-Brandt L, et al. Autologous bone marrow-derived cell transplantation in decompensated alcoholic liver disease: what is the impact on liver histology and gene expression patterns?. Stem Cell Res Ther. 2017;8(1):88. 10.1186/s13287-017-0541-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10(4):459-466. [PubMed] [Google Scholar]
- 155. Kharaziha P, Hellström PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21(10):1199-1205. 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 156. El-Ansary M, Mogawer S, Abdel-Aziz I, Abdel-Hamid S.. Phase I trial: mesenchymal stem cells transplantation in end stage liver disease. Stem Cell. 2010;1(2):22-33. [Google Scholar]
- 157. Amin MA, Sabry D, Rashed LA, et al. Short‐term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transplant. 2013;27(4):607-612. [DOI] [PubMed] [Google Scholar]
- 158. Jang YO, Kim YJ, Baik SK, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int. 2014;34(1):33-41. 10.1111/liv.12218. [DOI] [PubMed] [Google Scholar]
- 159. Kantarcıoğlu M, Demirci H, Avcu F, et al. Efficacy of autologous mesenchymal stem cell transplantation in patients with liver cirrhosis. Turk J Gastroenterol. 2015;26(3):244-250. 10.5152/tjg.2015.0074. [DOI] [PubMed] [Google Scholar]
- 160. Sakai Y, Takamura M, Seki A, et al. Phase I clinical study of liver regenerative therapy for cirrhosis by intrahepatic arterial infusion of freshly isolated autologous adipose tissue-derived stromal/stem (regenerative) cell. Regen Ther. 2017;6:52-64. 10.1016/j.reth.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Zhang YC, Liu W, Fu BS, et al. Therapeutic potentials of umbilical cord-derived mesenchymal stromal cells for ischemic-type biliary lesions following liver transplantation. Cytotherapy. 2017;19(2):194-199. 10.1016/j.jcyt.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 162. Zhou YF, Bosch-Marce M, Okuyama H, et al. Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res. 2006;66(22):10849-10854. 10.1158/0008-5472.CAN-06-2146. [DOI] [PubMed] [Google Scholar]
- 163. Miura M, Miura Y, Padilla-Nash HM, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24(4):1095-1103. 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 164. Li H, Fan X, Kovi RC, et al. Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Res. 2007;67(22):10889-10898. 10.1158/0008-5472.CAN-07-2665. [DOI] [PubMed] [Google Scholar]
- 165. Aguilar S, Nye E, Chan J, et al. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells. 2007;25(6):1586-1594. 10.1634/stemcells.2006-0762. [DOI] [PubMed] [Google Scholar]
- 166. Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25(2):371-379. 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 167. Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67(19):9142-9149. 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 168. Kim J, Kang JW, Park JH, et al. Biological characterization of long-term cultured human mesenchymal stem cells. Arch Pharm Res. 2009;32(1):117-126. 10.1007/s12272-009-1125-1. [DOI] [PubMed] [Google Scholar]
- 169. Dahl J, Duggal S, Coulston N, et al. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous seum or fatal bovine serum. Int J Dev Biol. 2008;52:1033-1042. 10.1387/ijdb.082663jd. [DOI] [PubMed] [Google Scholar]
- 170. Oliveira PH, Boura JS, Abecasis MM, da Silva CL, Cabral JMS.. An appraisal of genetic stability in human mesenchymal stem cells. In: 1st Portuguese Biomedical Engineering Meeting. Lisbon, Portugal; 2011:1-3. 10.1109/ENBENG.2011.6026040. [DOI] [Google Scholar]
- 171. Zhu W, Xu W, Jiang R, et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80(3):267-274. 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 172. Cousin B, Ravet E, Poglio S, et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One. 2009;4(7):e6278. 10.1371/journal.pone.0006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557-563. 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 174. Ramasamy R, Lam EW, Soeiro I, et al. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21(2):304-310. 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 175. Bian ZY, Fan QM, Li G, Xu WT, Tang TT.. Human mesenchymal stem cells promote growth of osteosarcoma: involvement of interleukin-6 in the interaction between human mesenchymal stem cells and Saos-2. Cancer Sci. 2010;101(12):2554-2560. 10.1111/j.1349-7006.2010.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kucerova L, Matuskova M, Hlubinova K, Altanerova V, Altaner C.. Tumor cell behaviour modulation by mesenchymal stromal cells. Mol Cancer. 2010;9(1):129. 10.1186/1476-4598-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006;203(5):1235-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Dasari VR, Kaur K, Velpula KK, et al. Upregulation of PTEN in glioma cells by cord blood mesenchymal stem cells inhibits migration via downregulation of the PI3K/Akt pathway. PLoS One. 2010;5(4):e10350. 10.1371/journal.pone.0010350. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 179. Secchiero P, Zorzet S, Tripodo C, et al. Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin’s lymphoma xenografts. PLoS One. 2010;5(6):e11140. 10.1371/journal.pone.0011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Casiraghi F, Remuzzi G, Abbate M, Perico N.. Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem Cell Rev Rep. 2013;9(1):65-79. 10.1007/s12015-011-9345-4. [DOI] [PubMed] [Google Scholar]
- 181. Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579-1586. 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 182. Sundin M, Lindblom A, Örvell C, et al. Persistence of human parvovirus B19 in multipotent mesenchymal stromal cells expressing the erythrocyte P antigen: implications for transplantation. Biol Blood Marrow Transplant. 2008;14(10):1172-1179. 10.1016/j.bbmt.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Ritskes-Hoitinga M, Leenaars C, Beumer W, et al. Improving translation by identifying evidence for more human-relevant preclinical strategies. Animals (Basel). 2020;10(7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Haugabook SJ, Ferrer M, Ottinger EA.. In vitro and in vivo translational models for rare liver diseases. Biochim Biophys Acta Mol Basis Dis. 2019;1865(5):1003-1018. 10.1016/j.bbadis.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 185. Hu C, Zhao L, Duan J, Li L.. Strategies to improve the efficiency of mesenchymal stem cell transplantation for reversal of liver fibrosis. J Cell Mol Med. 2019;23(3):1657-1670. 10.1111/jcmm.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Bissig-Choisat B, Alves-Bezerra M, Zorman B, et al. A human liver chimeric mouse model for non-alcoholic fatty liver disease. JHEP Rep. 2021;3(3):100281. 10.1016/j.jhepr.2021.100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Meier RP, Müller YD, Morel P, Gonelle-Gispert C, Bühler LH.. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence?. Stem Cell Res. 2013;11(3):1348-1364. 10.1016/j.scr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 188. Caplan AI. Cell-based therapies: the nonresponder. Stem cells translational medicine. 2018;7(11):762-766. 10.1002/sctm.18-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Pittenger MF, Discher DE, Péault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Galipeau J, Sensébé L.. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824-833. 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Hass R, Kasper C, Böhm S, Jacobs R.. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling. 2011;9(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. De Wolf C, Van De Bovenkamp M, Hoefnagel M.. Regulatory perspective on in vitro potency assays for human mesenchymal stromal cells used in immunotherapy. Cytotherapy. 2017;19(7):784-797. 10.1016/j.jcyt.2017.03.076. [DOI] [PubMed] [Google Scholar]
- 193. Iftimia-Mander A, Hourd P, Dainty R, Thomas RJ.. Mesenchymal stem cell isolation from human umbilical cord tissue: understanding and minimizing variability in cell yield for process optimization. Biopreserv Biobanking. 2013;11(5):291-298. 10.1089/bio.2013.0027. [DOI] [PubMed] [Google Scholar]
- 194. Martin P, Uberti J, Soiffer R, et al. Prochymal improves response rates in patients with steroid-refractory acute graft versus host disease (SR-GVHD) involving the liver and gut: results of a randomized, placebo-controlled, multicenter phase III trial in GVHD. Biol Blood Marrow Transplant. 2010;16(2):S169-S170. 10.1016/j.bbmt.2009.12.057. [DOI] [Google Scholar]
- 195. Galipeau J. The mesenchymal stromal cells dilemma—does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road?. Cytotherapy. 2013;15(1):2-8. 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 196. Lee B-C, Kang K-S.. Functional enhancement strategies for immunomodulation of mesenchymal stem cells and their therapeutic application. Stem Cell Res Therapy. 2020;11(1):397. 10.1186/s13287-020-01920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Lee EJ, Hwang I, Kim G-H, et al. Endothelin-1 augments therapeutic potency of human mesenchymal stem cells via CDH2 and VEGF signaling. Mol Therapy Methods Clin Dev. 2019;13:503-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Boregowda SV, Krishnappa V, Haga CL, Ortiz LA, Phinney DG.. A clinical indications prediction scale based on TWIST1 for human mesenchymal stem cells. EBioMedicine. 2016;4:62-73. 10.1016/j.ebiom.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Chinnadurai R, Copland IB, Patel SR, Galipeau J.. IDO-independent suppression of T cell effector function by IFN-γ–licensed human mesenchymal stromal cells. J Immunol. 2014;192(4):1491-1501. 10.4049/jimmunol.1301828. [DOI] [PubMed] [Google Scholar]
- 200. Su W, Wan Q, Huang J, et al. Culture medium from TNF-α-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J Allergy Clin Immunol. 2015;136(2):423-432.e8. 10.1016/j.jaci.2014.12.1926. [DOI] [PubMed] [Google Scholar]
- 201. Sivanathan KN, Rojas-Canales DM, Hope CM, et al. Interleukin-17A-induced human mesenchymal stem cells are superior modulators of immunological function. Stem Cells. 2015;33(9):2850-2863. [DOI] [PubMed] [Google Scholar]
- 202. Li D, Liu Q, Qi L, et al. Low levels of TGF-β1 enhance human umbilical cord-derived mesenchymal stem cell fibronectin production and extend survival time in a rat model of lipopolysaccharide-induced acute lung injury. Mol Med Rep. 2016;14(2):1681-1692. 10.3892/mmr.2016.5416. [DOI] [PubMed] [Google Scholar]
- 203. Saller MM, Prall WC, Docheva D, et al. Increased stemness and migration of human mesenchymal stem cells in hypoxia is associated with altered integrin expression. Biochem Biophys Res Commun. 2012;423(2):379-385. 10.1016/j.bbrc.2012.05.134. [DOI] [PubMed] [Google Scholar]
- 204. McKee C, Chaudhry GR.. Advances and challenges in stem cell culture. Colloids Surf B. 2017;159:62-77. 10.1016/j.colsurfb.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 205. Garcia KO, Ornellas FL, Matsumoto P, et al. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front Aging Neurosci. 2014;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Haga CL, Phinney DG.. MicroRNAs in the imprinted DLK1-DIO3 region repress the epithelial-to-mesenchymal transition by targeting the TWIST1 protein signaling network. J Biol Chem. 2012;287(51):42695-42707. 10.1074/jbc.M112.387761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Lai WT, Krishnappa V, Phinney DG.. Fibroblast growth factor 2 (Fgf2) inhibits differentiation of mesenchymal stem cells by inducing Twist2 and Spry4, blocking extracellular regulated kinase activation, and altering Fgf receptor expression levels. Stem Cells. 2011;29(7):1102-1111. 10.1002/stem.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Phinney DG. Twist, epithelial-to-mesenchymal transition, and stem cells. Stem Cells. 2011;29(1):3-4. 10.1002/stem.553. [DOI] [PubMed] [Google Scholar]
- 209. Klinker MW, Marklein RA, Lo Surdo JL, Wei CH, Bauer SR.. Morphological features of IFN-γ-stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proc Natl Acad Sci USA. 2017;114(13):E2598-E2607. 10.1073/pnas.1617933114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Foppiani EM, Candini O, Mastrolia I, et al. Impact of HOXB7 overexpression on human adipose-derived mesenchymal progenitors. Stem Cell Res Ther. 2019;10(1):101. 10.1186/s13287-019-1200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Nolta JA, Galipeau J, Phinney DG.. Improving mesenchymal stem/stromal cell potency and survival: Proceedings from the International Society of Cell Therapy (ISCT) MSC preconference held in May 2018, Palais des Congrès de Montréal, Organized by the ISCT MSC Scientific Committee. Cytotherapy. 2020;22(3):123-126. 10.1016/j.jcyt.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 212. Moya A, Paquet J, Deschepper M, et al. Human mesenchymal stem cell failure to adapt to glucose shortage and rapidly use intracellular energy reserves through glycolysis explains poor cell survival after implantation. Stem Cells. 2018;36(3):363-376. 10.1002/stem.2763. [DOI] [PubMed] [Google Scholar]
- 213. Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154-162. 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Gavin C, Boberg E, Von Bahr L, et al. Tissue immune profiles supporting response to mesenchymal stromal cell therapy in acute graft-versus-host disease—a gut feeling. Stem Cell Research & Therapy. 2019;10(1):334. 10.1186/s13287-019-1449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Jung JW, Kwon M, Choi JC, et al. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med J. 2013;54(5):1293-1296. 10.3349/ymj.2013.54.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Wu Z, Zhang S, Zhou L, et al. Thromboembolism induced by umbilical cord mesenchymal stem cell infusion: a report of two cases and literature review. Transplant Proc. 2017;49(7):1656-1658. 10.1016/j.transproceed.2017.03.078. [DOI] [PubMed] [Google Scholar]
- 217. Coppin L, Sokal E, Stéphenne X.. Thrombogenic risk induced by intravascular mesenchymal stem cell therapy: current status and future perspectives. Cells. 2019;8(10):1160. 10.3390/cells8101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Zhao L, Chen S, Shi X, Cao H, Li L.. A pooled analysis of mesenchymal stem cell-based therapy for liver disease. Stem Cell Res Therapy. 2018;9(1):72. 10.1186/s13287-018-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Ma X-R, Tang Y-L, Xuan M, et al. Transplantation of autologous mesenchymal stem cells for end-stage liver cirrhosis: a meta-analysis based on seven controlled trials. Gastroenterol Res Practice. 2015;2015:1-10. 10.1155/2015/908275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Galipeau J, Krampera M, Barrett J, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18(2):151-159. 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no new data associated with this article. DOI links to the translational and clinical studies appraised in this review are cited in the references section.