Abstract
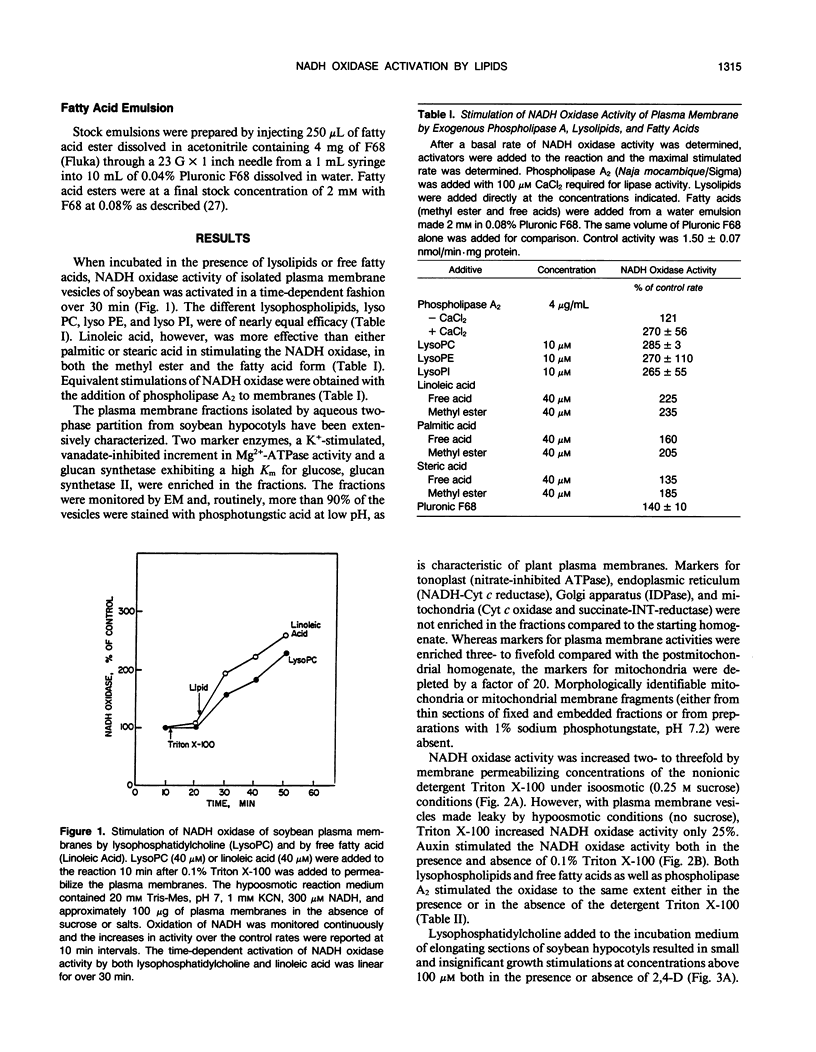
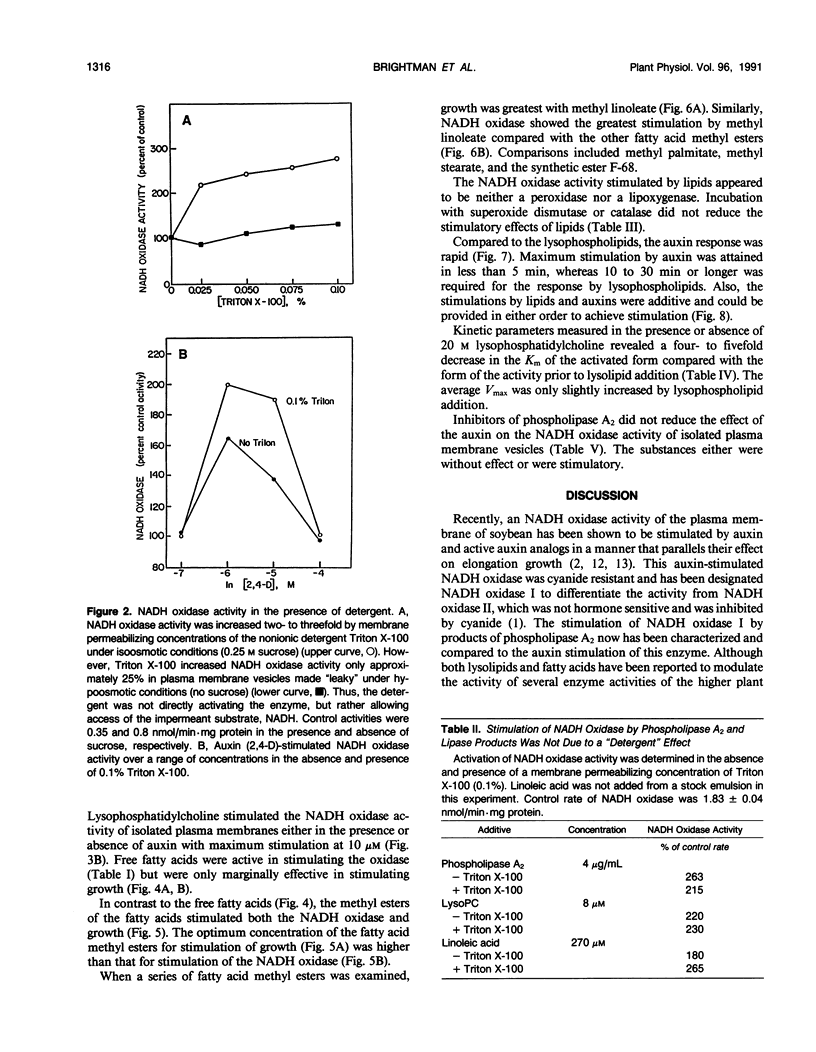
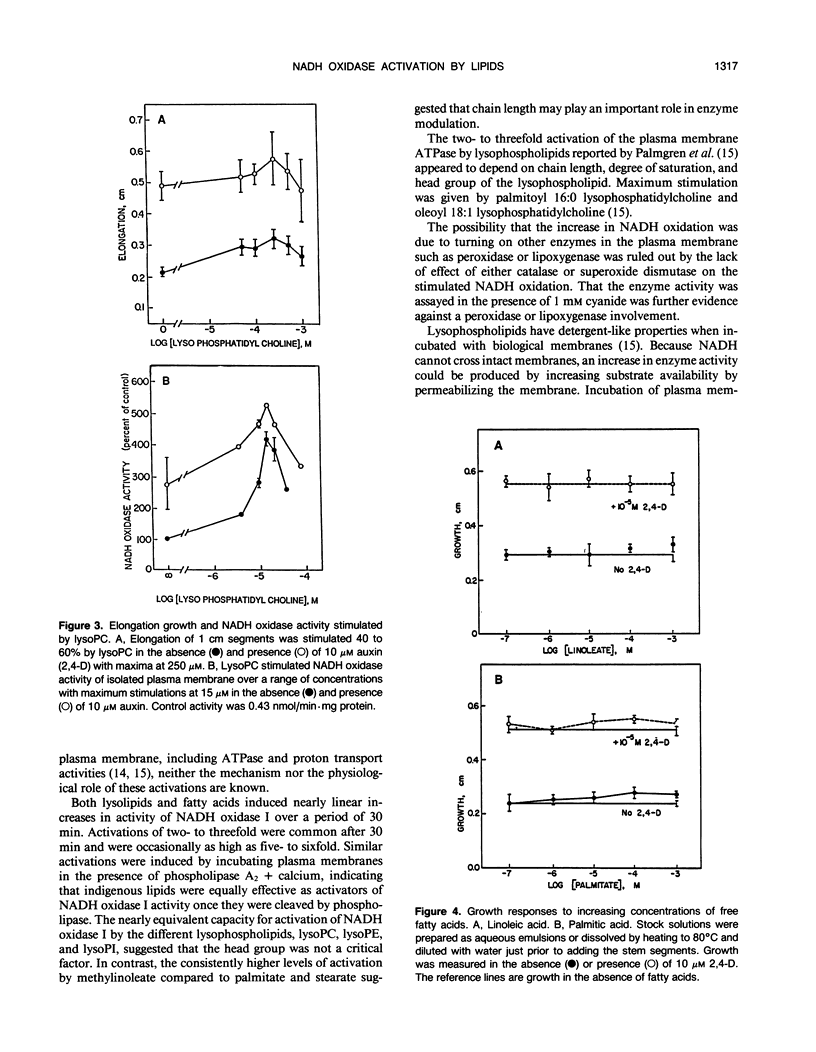
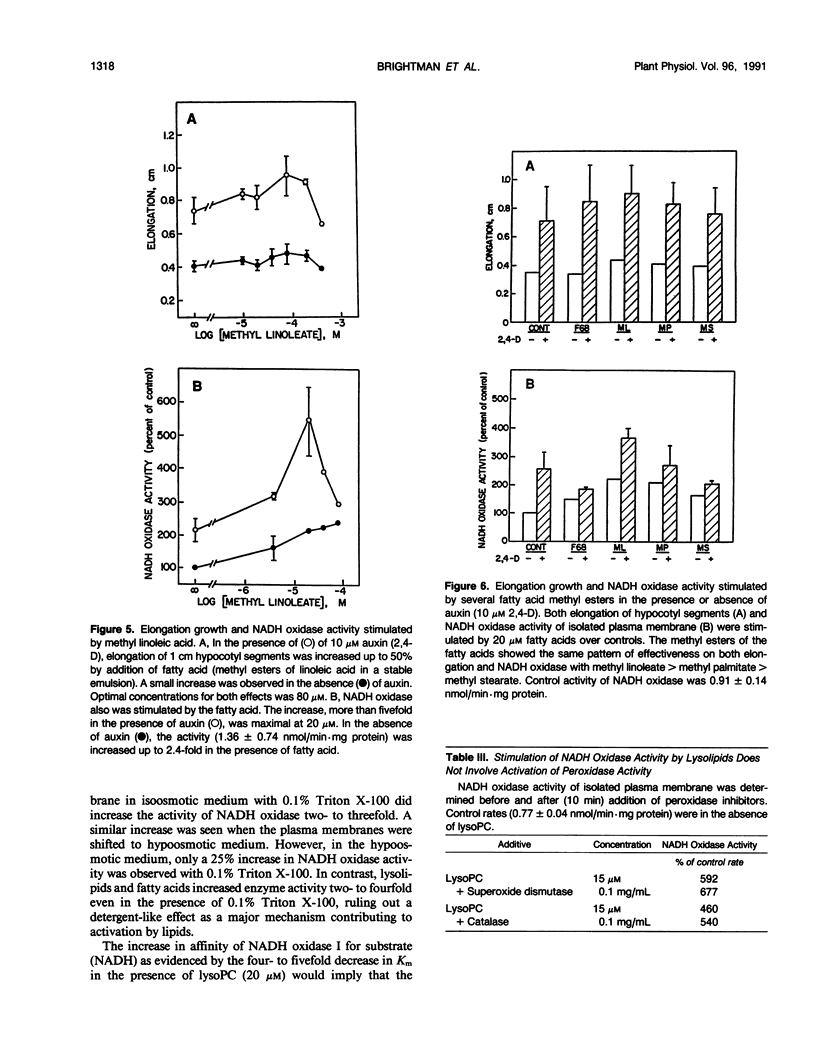
An auxin-stimulated NADH oxidase activity (NADH oxidase I) of plasma membrane vesicles, highly purified by aqueous two-phase partition from soybean (Glycine max Merr.) hypocotyls was activated by lysophospholipids and fatty acids, both products of phospholipase A action. The activation of NADH oxidase activity occurred slowly, suggesting a mechanism whereby the lipids acted to stabilize the enzyme in a more active configuration. In contrast to activation by lipids, the activation by auxin was rapid. The average Km of the NADH oxidase after activation by lipids was four- to fivefold less than the Km before activation. The Vmax was unchanged by activation. The increases occurred in the presence of detergent and thus were not a result of exposure of latent active sites. Also, the activation did not result from activation of a peroxidase or lipoxygenase. Fatty acid esters, where growth promoting effects have been reported, also activated the auxin-stimulated oxidase. However, the auxin stimulation of NADH oxidase I did not appear to be obligatorily mediated by phospholipase A, nor did inhibitors of phospholipase A2 block the stimulation of the oxidase by auxins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brightman A. O., Barr R., Crane F. L., Morré D. J. Auxin-Stimulated NADH Oxidase Purified from Plasma Membrane of Soybean. Plant Physiol. 1988 Apr;86(4):1264–1269. doi: 10.1104/pp.86.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im W. B., Blakeman D. P., Davis J. P. Effect of lysophosphatidylcholine on K+ transport in rat heavy gastric membranes enriched with (H+-K+)-ATPase. Biochem Biophys Res Commun. 1987 Jul 31;146(2):840–848. doi: 10.1016/0006-291x(87)90607-3. [DOI] [PubMed] [Google Scholar]
- Kauss H., Jeblick W. Influence of Free Fatty Acids, Lysophosphatidylcholine, Platelet-Activating Factor, Acylcarnitine, and Echinocandin B on 1,3-beta-d-Glucan Synthase and Callose Synthesis. Plant Physiol. 1986 Jan;80(1):7–13. doi: 10.1104/pp.80.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. A., O'Hara D. S., Mitch W. E., Smith T. W. Identification of NaK-ATPase inhibitors in human plasma as nonesterified fatty acids and lysophospholipids. J Biol Chem. 1986 Sep 5;261(25):11704–11711. [PubMed] [Google Scholar]
- Kirschbaum B. B., Bosmann H. B. Lysolecithin enhancement of glycoprotein: glycosyl transferase activity. FEBS Lett. 1973 Aug 15;34(2):129–132. doi: 10.1016/0014-5793(73)80773-2. [DOI] [PubMed] [Google Scholar]
- Klucis E., Polya G. M. Calcium-independent activation of two plant leaf calcium-regulated protein kinases by unsaturated fatty acids. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1041–1047. doi: 10.1016/s0006-291x(87)80175-4. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G., Scherer G. F. Phospholipid-stimulated protein kinase in plants. J Biol Chem. 1989 Oct 25;264(30):18052–18059. [PubMed] [Google Scholar]
- Palmgren M. G., Sommarin M. Lysophosphatidylcholine stimulates ATP dependent proton accumulation in isolated oat root plasma membrane vesicles. Plant Physiol. 1989 Jul;90(3):1009–1014. doi: 10.1104/pp.90.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny D., Stowe B. B. Relationship of lipid metabolism to the respiration and growth of pea stem sections. Plant Physiol. 1966 Feb;41(2):360–365. doi: 10.1104/pp.41.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOWE B. B. Growth promotion in pea epicotyl sections by fatty acid esters. Science. 1958 Aug 22;128(3321):421–423. doi: 10.1126/science.128.3321.421. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Enyedi A., Nyers A., Gárdos G. The function and regulation of the calcium pump in the erythrocyte membrane. Ann N Y Acad Sci. 1982;402:329–348. doi: 10.1111/j.1749-6632.1982.tb25753.x. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B., Skibsted U. Hysteretic activation of the Ca2+ pump revealed by calcium transients in human red cells. Biochim Biophys Acta. 1983 May 5;730(2):295–305. doi: 10.1016/0005-2736(83)90346-2. [DOI] [PubMed] [Google Scholar]
- Scherer G. F. 1-Alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet activating factor) stimulates plant H+ transport in vitro and growth. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1160–1167. doi: 10.1016/0006-291x(85)91258-6. [DOI] [PubMed] [Google Scholar]
- Schmalzing G., Kutschera P. Modulation of ATPase activities of human erythrocyte membranes by free fatty acids or phospholipase A2. J Membr Biol. 1982;69(1):65–76. doi: 10.1007/BF01871243. [DOI] [PubMed] [Google Scholar]
- Shier W. T., Trotter J. T., 3rd Stimulation of liver microsomal sialyltransferase activity by lysolecithin. FEBS Lett. 1976 Feb 15;62(2):165–168. doi: 10.1016/0014-5793(76)80044-0. [DOI] [PubMed] [Google Scholar]
- Stowe B. B. Growth Promotion in Pea Stem Sections. I. Stimulation of Auxin and Gibberellin Action by Alkyl Lipids. Plant Physiol. 1960 Mar;35(2):262–269. doi: 10.1104/pp.35.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann A. C. Free fatty acids and (Na+,K+)-ATPase: effects on cation regulation, enzyme conformation, and interactions with ethanol. Arch Biochem Biophys. 1984 Sep;233(2):354–361. doi: 10.1016/0003-9861(84)90456-9. [DOI] [PubMed] [Google Scholar]
- Swoboda G., Fritzsche J., Hasselbach W. Effects of phospholipase A2 and albumin on the calcium-dependent ATPase and the lipid composition of sarcoplasmic membranes. Eur J Biochem. 1979 Mar 15;95(1):77–88. doi: 10.1111/j.1432-1033.1979.tb12941.x. [DOI] [PubMed] [Google Scholar]
- Taverna R. D., Hanahan D. J. Modulation of human erythrocyte Ca2+/Mg2+ ATPase activity by phospholipase A2 and proteases. A comparison with calmodulin. Biochem Biophys Res Commun. 1980 May 30;94(2):652–659. doi: 10.1016/0006-291x(80)91282-6. [DOI] [PubMed] [Google Scholar]


