Abstract
Chondromyxoid fibroma (CMF) is a rarely documented benign osseous neoplasm, particularly with respect to its incidence in the lumbar spinal region. CMF predominantly manifests in vertebral bodies, exhibiting atypical emergence in ancillary anatomical sites. The present report describes, to the best of our knowledge, the second documented instance of CMF originating from the lumbar facet joint. The present case provides an example of CMF in the lumbar facet joint precipitating spinal canal stenosis, thereby engendering neurological manifestations in the lower extremities due to neoplastic proliferation through the intervertebral foramen. The present therapeutic intervention entailed surgical excision of the neoplasm concomitant with facet joint arthrodesis, with the objective of achieving comprehensive neoplasm eradication, ameliorating the symptomatology and safeguarding the spinal structural integrity of the patient. The present study aimed to illustrate the clinical implications of this rare neoplasm, thereby elucidating the diagnostic quandaries and therapeutic complexities associated with CMF in the lumbar facet joint. In addition, the present study aimed to augment the existing knowledge for the diagnosis and clinical management of CMF.
Keywords: chondromyxoid fibroma, lumbar facet joint, diagnosis, surgical treatment
Introduction
Primary neoplasms of the vertebral column are rare pathological entities. The most prevalent types of primary osseous tumors that manifest in the vertebral column include osteosarcomas, giant cell tumors, osteoblastomas, chondrosarcomas and osseous cysts. These tumors predominantly exhibit proclivity for localization within the anterior and posterior vertebral bodies, pedicles and laminae of the spinal architecture. The incidence of primary neoplasms originating from the facet joint is particularly low, owing primarily to its cartilaginous composition (1). Documented instances of primary neoplasms in this region are sporadic and principally comprise of giant cell tumors, osteoblastomas and chondrosarcomas (2).
Chondromyxoid fibroma (CMF) is an uncommon benign osseous neoplasm, which accounts for only a small percentage (<5%) of all diagnosed bone tumors recorded worldwide (3). Notably, CMF demonstrates a predilection for pediatric and young adult populations and is customarily localized within the metaphyseal regions of elongated bones, notably in the femur and tibia. The characteristic symptomatology associated with CMF includes pain, localized edema, and restricted joint mobility, with pain often exacerbating either nocturnally or after physical exertion (3). The neoplasm characteristically follows an indolent growth trajectory (4) (Table I).
Table I.
An introduction to chondromyxoid fibroma.
| Aspect | Information | (Refs.) |
|---|---|---|
| Epidemiology | - Chondromyxoid fibroma is an extraordinarily uncommon benign osseous neoplasm | (3) |
| - It chiefly afflicts individuals within the second to third decades of life; however, instances have been cited across a broader age spectrum, including pediatric and geriatric populations | (3) | |
| - There is no significant sex predilection | (2) | |
| - Commonly, chondromyxoid fibroma localizes to the metaphyseal region of elongated bones such as the femur and tibia, although manifestations in axial skeletal structures including the vertebrae, pelvis and cranium have been documented | (4) | |
| Diagnostic Methods | - Radiological evaluations predominantly rely on computed tomography and magnetic resonance imaging to elucidate the anatomical locale, dimensional attributes and morphological characteristics of the neoplasm | (5) |
| - Immunohistochemical investigations represent an indispensable diagnostic technique, consistently revealing positive immunoreactivity for S-100 protein in neoplastic cells | (6) | |
| - Histopathological scrutiny via tissue biopsy stands as the gold standard for definitively corroborating a diagnosis of chondromyxoid fibroma | (6) | |
| Pathological Features | - Histologically, chondromyxoid fibroma frequently exhibits a heterogenous architecture, encompassing both mucinous and fibrous compartments; the former replete with mucoid substrate and the latter characterized by fibrous stroma | (6) |
| - Neoplastic cells manifest an irregular morphology, frequently assuming either a fibroblastic or spindle-shaped appearance, with mitotic figures remaining an uncommon feature | (5) | |
| - Immunohistochemical assays corroborate the frequent expression of S-100 protein within chondromyxoid fibroma, thereby facilitating its differential diagnosis from other neoplastic entities | (4) | |
| Treatment Strategies | - Surgical extirpation remains the primary therapeutic paradigm, and the ideal approach encompasses complete neoplastic resection; postoperative histological analysis substantiates the extent of excision | (7) |
| - Curettage warrants consideration in scenarios where comprehensive resection is unfeasible, albeit at the expense of an elevated risk for neoplastic recurrence | (8) | |
| - Spinal arthrodesis may become requisite when the surgical intervention compromises vertebral stability, thereby ensuring biomechanical integrity | (7) | |
| - Radiation and chemotherapeutic modalities are generally precluded from the treatment regimen due to the tumor's benign proclivity | (8) |
The incidence of CMF within the spinal architecture is relatively atypical. Among the few documented cases, the predominant manifestation is in the thoracic spine, rather than the cervical or lumbar spine, and it is almost universally confined to the vertebrae (5,6). Characteristic radiographic features include: i) Demonstrable osteolytic degeneration accompanied by ‘moth-eaten’ alterations in the vertebral body, potentially with concomitant soft tissue masses, as revealed by computed tomography (CT); and ii) hypointense lesions sharply demarcated from the surrounding physiological tissues, as well as intratumoral cystic compartments and hemorrhagic foci, as revealed by magnetic resonance imaging (MRI) (2) (Table I).
Pathologically, CMF is characterized by distinct histological and cytological features. Histomorphologically, CMF predominantly features mucoid regions interspersed with fibrous structures, wherein the mucoid compartments comprise of cystic formations laden with mucin and fibrous compartments consisting of bundled fibrous connective tissue. Cytomorphologically, CMF is predominantly characterized by atypical fibroblasts with a relatively uniform cellular architecture, and infrequent presence of mitotic figures. Although categorically benign, CMF induces considerable osseous erosion and degradation under specific conditions, such as tumor location, growth rate and size. In addition, inflammation or other biological responses can influence the pathological process of tumor induction. This destructive potential is principally attributable to the inherent growth characteristics of the tumor, which include intraosseous dissolution and localized erosion, particularly in the surrounding osseous structures. Such degradative processes are principally mediated by the secretion of mucinous substances and other bioactive factors from the tumor, culminating in the attenuation of neighboring osseous tissues (5,6). In addition, the structural composition of the tumor, replete with cystic regions and fibrous compartments, may contribute to its predilection for invasive propagation into adjacent anatomical structures, especially within the osseous framework (5,6). Immunohistochemically, cells inherent to this neoplasm frequently exhibit positive immunoreactivity for cartilage-specific molecular markers, notably S-100 protein (5,6). Therefore, distinguishing CMF from other osseous pathologies such as chondrosarcoma, giant cell tumor, fibrous dysplasia, osteochondroma and aneurysmal bone cyst is imperative for an accurate diagnosis (7). Surgical intervention remains the principal therapeutic modality. In alignment with therapeutic protocols established for long-bone neoplasms, complete surgical resection is mandatory for vertebral CMFs. In scenarios where complete excision is contraindicated or impracticable, curettage is an acceptable alternative (8). After complete resection, perturbations in spinal stability should occur, and vertebral fusion procedures should be indicated (8) (Table I).
Case report
A 57-year-old female patient was admitted for clinical intervention pertaining to chronic lumbosacral radicular pain for 3 years that was exacerbated over the preceding month. Since 2019, the patient had manifested inexplicable neuropathic symptoms in the right lower extremity. In November 2022, acute exacerbation of neuropathic discomfort and ambulatory impediments were reported. Initial clinical examination revealed an antalgic gait pattern, a mild levoscoliosis of the lumbar spine, palpable tenderness over the spinous processes and paraspinal musculature adjacent to the L4 and L5 vertebrae, heightened tenderness inferior to the lumbar region and restricted spinal mobility in both the sagittal and coronal planes. Forward flexion exacerbated the radicular symptoms in the right lower extremity. Quantitative muscle strength evaluation revealed the following distinctions between the left and right lower limbs: Tibialis anterior (4/3), extensor hallucis longus (3/4) and extensor digitorum longus (3/4). Hypoesthesia was identified in the right hip region, posterolateral thigh, lateral calf, dorsolateral aspect of the foot and plantar surface. CT demonstrated localized osseous cystic degradation in the right inferior facet of the L5 vertebra, accompanied by a nodular lesion of slightly higher density extending into the right intervertebral foramen between L5 and S1, raising suspicions of tumor pathologies such as osteoblastoma (Fig. 1). MRI delineated an irregular nodular formation in the aforementioned intervertebral foramen, characterized by low signal intensity on T1-weighted images and demarcated boundaries, measuring ~0.8x1.6 cm (Fig. 2). Neural impingement of the right nerve root was also observed. The differential diagnoses included: i) A lesion in the right inferior facet joint of L5 (possibly osteoblastoma); ii) intraspinal joint tissue protrusion; and iii) lumbar spinal canal stenosis.
Figure 1.
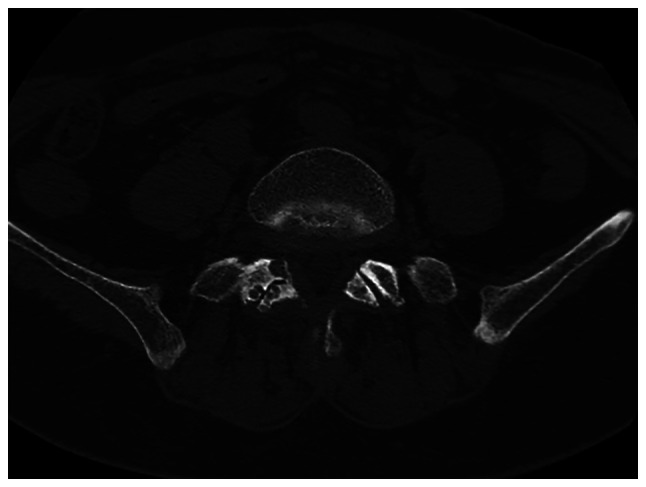
Computed tomography delineated localized cystic osseous degradation in the right inferior articular process of L5, wherein a nodular entity of slightly elevated radiodensity protrudes into the adjacent L5-S1 intervertebral foramen. The lesion exhibited distinct boundaries and dimensions ~0.9x1.8 cm.
Figure 2.
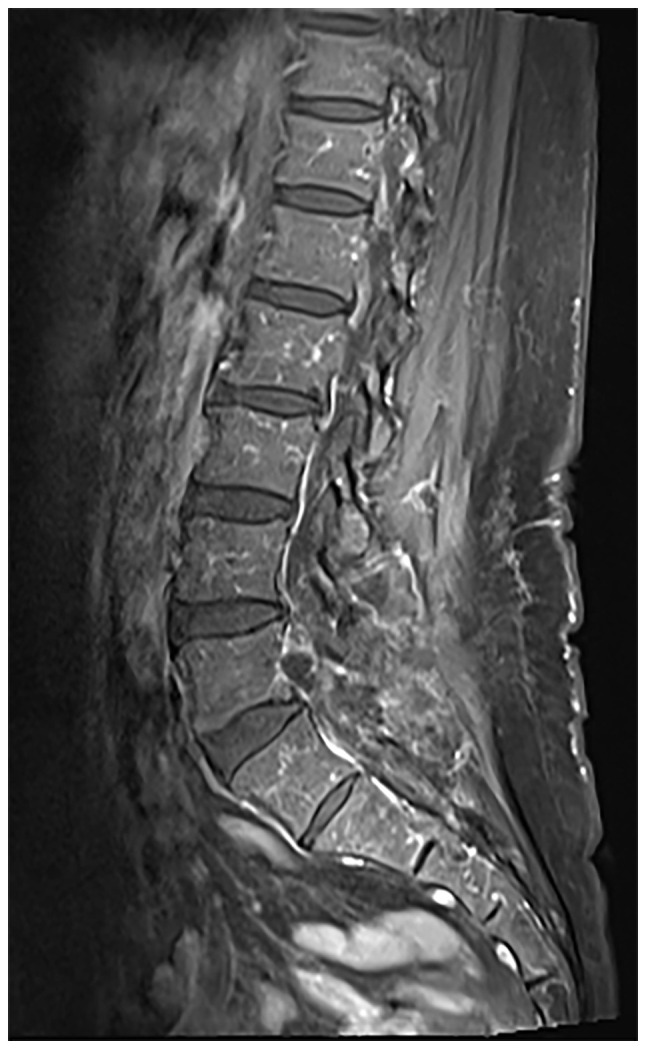
Magnetic resonance imaging revealed an irregular nodular lesion within the right L5-S1 intervertebral foramen, manifesting a slightly hypointense signal on contrast-enhanced T1-weighted sequences, accompanied by relatively demarcated borders and dimensions of ~0.8x1.6 cm. Evident compression of the right nerve root was observed.
After induction of general anesthesia, a posterior lumbar surgical approach was implemented, including the excision of the appendicular lesion, resection of the intraspinal pathology, posterolateral bone graft fusion involving the facet joint and internal stabilization utilizing pedicle screw-rod fixation. Intraoperatively, an ultrasonic osteotome facilitated complete resection of the right inferior facet joint of L5, revealing localized cystic osseous degeneration and an adjacent soft tissue nodule extending into the right intervertebral foramen between L5 and S1 with dimensions of ~1.0x1.5 cm (Fig. 3). The soft tissue nodule exerted compressive forces on the right dural sac and nerve root, necessitating its complete removal.
Figure 3.
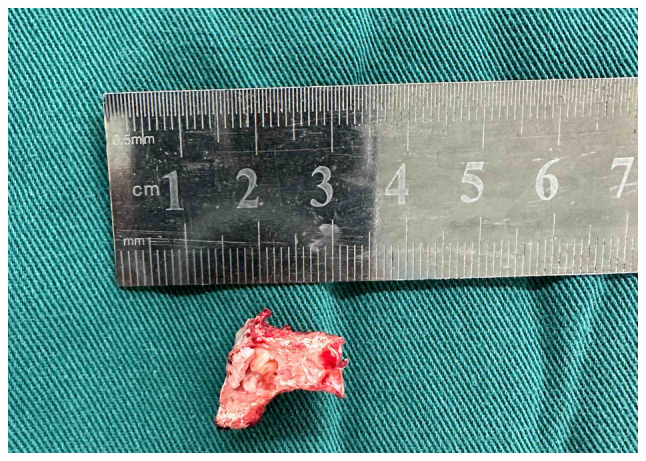
Intraoperative visualization after complete resection of the articular eminence clearly shows the morphological features of the tumor.
The postoperative course was devoid of complications, and the limb kinematics and sensory functions were within normal limits. Follow-up CT imaging validated the surgical resection of the right inferior facet joint and adjacent vertebral lamina of L5, while confirming the secure placement of the posterior pedicle screw fixation from L5 to S1, devoid of notable malalignment or hardware failure. Histopathological examination confirmed the diagnosis of CMF, which was characterized by ambiguous tumor margins and local infiltrative patterns (Fig. 4). Additionally, immunohistochemical assays revealed the expression of the S100 protein (Fig. 5).
Figure 4.
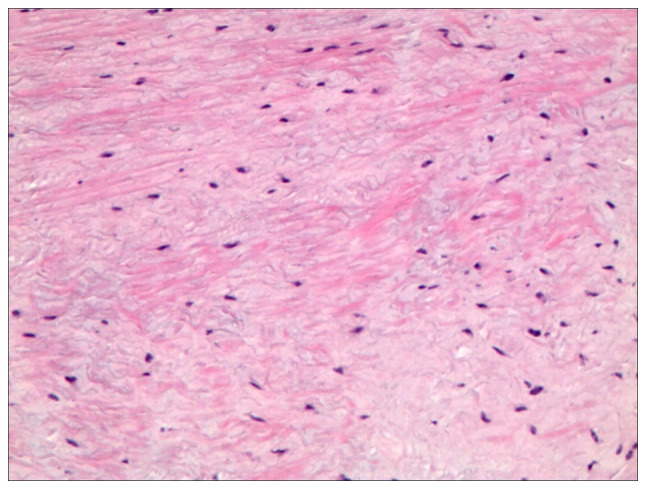
Benign chondroid neoplasm displaying cellular constituents with either stellate or spindle morphology, arrayed in a leaf-like pattern within a copious extracellular mucinous or cartilaginous matrix (H&E; magnification, x100).
Figure 5.
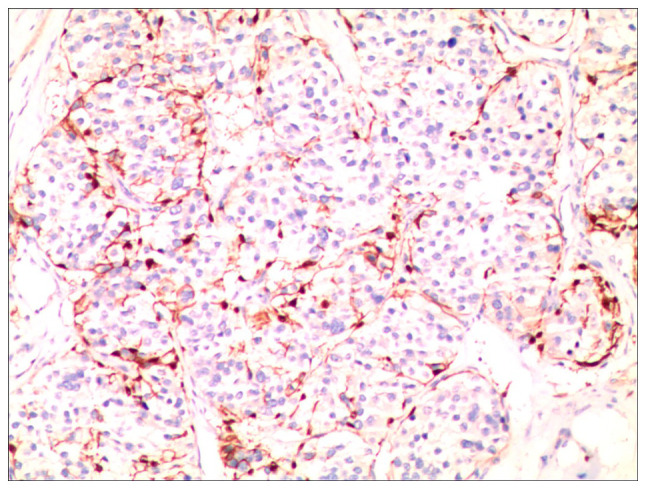
Immunohistochemical assays demonstrate expression of S100 protein (immunohistochemistry staining, magnification, x100).
During the 6 month postoperative review, the patient remained asymptomatic, with no clinical or radiographic evidence suggestive of tumor recurrence or spinal biomechanical instability.
The following is a description of the H&E staining method. Initially, tissue samples from the patient's surgery were obtained and immediately fixed in 10% buffered formalin for 12 to 24 h at room temperature. After fixation, the tissues were dehydrated by immersion in gradually increasing concentrations of ethanol and then cleared and embedded in molten paraffin wax. This was achieved using an automated tissue processor. The paraffin-embedded tissue was sliced into thin sections, typically ranging from 3-5 µm in thickness. These sections were placed on glass slides. Using the standard procedure for H&E staining, the sections were first immersed in hematoxylin dye at room temperature for 2-5 min, which stained cell nuclei blue or purple. Subsequently, the sections were immersed in eosin dye for 1-2 min at room temperature, which stains cytoplasm and collagen fibers pink to red. After staining, the sections required multiple rinses to remove excess dye. The sections were then covered with clear glass to protect the sample and examined using a light microscope.
The following is a description of immunohistochemistry staining method. Tissue samples of chondromyxoid fibroma were obtained from the surgical specimen of the patient and were fixed in 10% buffered formalin, typically for 12 to 24 h at room temperature. Similar to H&E staining, the fixed tissues were dehydrated, cleared and embedded in molten paraffin wax. Paraffin-embedded tissue was sliced into thin sections, typically ranging from 3-5 µm in thickness. The EliVision two-step method (Fuzhou Maixin Biotech Co., Ltd.) was used for immunohistochemistry staining. The tissue sections were placed in a 90˚C dry oven for 15 min for deparaffinization and were then transferred to an automated immunohistochemistry staining instrument (Roche Benchmark XT; Roche Diagnostics) for automatic staining. After removing the sections from the automated immunohistochemistry staining instrument, they were rinsed with running water for 10 min. Subsequently, the sections were sequentially placed in 75, 85, 95 and 100% ethanol for 3, 3, 5 and 5 min, respectively. After air-drying naturally, the sections were briefly placed in xylene for 3 sec, removed and neutral resin was added to the sections. Cover slips were placed on top, and after the neutral resin on the sections dried they were observed under an optical microscope (Fig. 5). The primary antibody used was S-100 ready-to-use [1:500; cat. nos. (01) 06970905811800; Fuzhou Maixin Biotech. Co., Ltd.], which was used at 4˚C for 10-12 h. The secondary antibody used was enzyme-labeled anti-mouse/rabbit IgG polymer ready-to-use [cat. no. (01) 06931988317979; Fuzhou Maixin Biotech. Co., Ltd.], which was used for 30 min at room temperature. The immunohistochemistry staining steps were strictly performed following the instructions provided in the reagent kit.
Discussion
The current report described a case of CMF originating from the right inferior articular process of the L5 vertebra, which subsequently extended through the corresponding intervertebral foramen and culminated in lumbar spinal canal stenosis and neural impingement. A review of extant case studies allowed us to ascertain that, to the best of our knowledge, the present case was the second documented occurrence of CMF localized to the lumbar facet joint. The first case was primarily associated with lumbosacral discomfort confined to the tumorous lesion site within the facet joint (9). Hence, the present report reports a rare manifestation of CMF within the lumbar facet joint, with clinical ramifications extending to neural compression and resultant neuropathic symptomatology due to neoplastic invasion of the intervertebral foramen.
Based on the clinical history of the patient, the anatomical location of the lesion and pertinent medical literature, differential diagnosis of the present study tended initially towards the potential existence of an osseous cystic lesion within the facet joint. Additional diagnostic considerations included intervertebral disc herniation and ligamentum flavum calcification. The accurate diagnosis of CMF within the facet joint presents considerable hurdles for clinicians and radiologists, and pathological examination is not exempt from similar challenges. Therefore, documenting cases such as the current one is imperative and unequivocal, and will substantially contribute to the body of literature instrumental in refining the diagnostic precision of CMF.
To manage this uncommon clinical entity, the present study implemented a surgical strategy involving comprehensive lesion resection coupled with articular process fusion and autologous bone graft implantation. Notably, the patient showed significant symptom amelioration as early as the immediate postoperative period, and follow-up assessments revealed no indications of neoplastic recurrence or spinal biomechanical destabilization.
Given the limited literature pertaining to CMF, particularly within the spinal facet joints, our intent to disseminate this case was dualistic. First, the present study endeavored to augment the diagnostic acumen of clinicians for neoplastic entities localized in this specific anatomical domain. Second, the present study aimed to act as a catalyst for the initiation of robust research trajectories encompassing molecular pathogenesis, targeted therapeutic interventions, immunomodulatory approaches and comprehensive treatment algorithms tailored for CMF. Such concerted academic efforts are expected to facilitate the evolution of improved diagnostic methodologies and efficacious therapeutic regimens for patients presenting with CMFs at disparate anatomical loci.
Acknowledgements
Not applicable.
Funding Statement
Funding: This case report was supported by the Natural Science Foundation of Gansu Province (grant nos. 21YF1FA179, 23JRRA538 and 21JR7RA007) and the Army Special Training Program (grant no. 2022YXKY003).
Availability of data and materials
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Authors' contributions
BK, CY and SL conceived the study, participated in the study design and drafted the manuscript. BK, CY, SL, HW and WY contributed to the image collection. XL, QD, GC, HW and WY participated in the study design and oversaw the manuscript drafting process. All authors read and approved the final manuscript. BK, CY and SL confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
The study was conducted with the consent of the patient and their relatives, who signed an informed consent form and agreed to publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kelley SP, Ashford RU, Rao AS, Dickson RA. Primary bone tumours of the spine: A 42-year survey from the Leeds Regional Bone Tumour Registry. Eur Spine J. 2007;16:405–409. doi: 10.1007/s00586-006-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erlemann R. Imaging and differential diagnosis of primary bone tumors and tumor-like lesions of the spine. Eur J Radiol. 2006;58:48–67. doi: 10.1016/j.ejrad.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe HL, Lichtenstein L. Chondromyxoid fibroma of bone; a distinctive benign tumor likely to be mistaken especially for chondrosarcoma. Arch Pathol (Chic) 1948;45:541–551. [PubMed] [Google Scholar]
- 4.Zou MX, Lv GH, Li J, Wang XB. Acute cauda equina syndrome secondary to chondromyxoid fibroma of the lumbar spine. Spine J. 2016;16:e587–e588. doi: 10.1016/j.spinee.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Schajowicz F, Gallardo H. Chondromyxoid fibroma (fibromyxoid chondroma) of bone. A clinico-pathological study of thirty-two cases. J Bone Joint Surg Br. 1971;53:198–216. [PubMed] [Google Scholar]
- 6.Rahimi A, Beabout JW, Ivins JC, Dahlin DC. Chondromyxoid fibroma: A clinicopathologic study of 76 cases. Cancer. 1972;30:726–736. doi: 10.1002/1097-0142(197209)30:3<726::aid-cncr2820300321>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Wu CT, Inwards CY, O'Laughlin S, Rock MG, Beabout JW, Unni KK. Chondromyxoid fibroma of bone: A clinicopathologic review of 278 cases. Hum Pathol. 1998;29:438–446. doi: 10.1016/s0046-8177(98)90058-2. [DOI] [PubMed] [Google Scholar]
- 8.Gherlinzoni F, Rock M, Picci P. Chondromyxoid fibroma. The experience at the Istituto Ortopedico Rizzoli. J Bone Joint Surg Am. 1983;65:198–204. doi: 10.2106/00004623-198365020-00008. [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez-González R, De Reina L, Saab A, Jiménez-Heffernan J, García-Uría J. Chondromyxoid fibroma of the lumbar spine: Case report and literature review. Eur Spine J. 2012;21 (Suppl 4):S458–S462. doi: 10.1007/s00586-011-2078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.


