Abstract
Eribulin is widely used to treat metastatic breast cancer (BC). Higher neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are associated with higher mortality in several cancer types. However, the association between BC prognosis and peripheral immune status remains controversial. In the present study, the relative effects of NLR and PLR on survival in patients with metastatic BC were quantified and their clinical prognostic value was evaluated. This retrospective study included 156 patients with metastatic BC who received eribulin monotherapy at Saitama Medical University International Medical Center. Clinicopathological features were examined (peripheral blood findings and biochemical liver and kidney function test results) and univariate and multivariate analyses were conducted of the overall survival (OS). The 156 patients treated with eribulin had a median follow-up duration of 18.3 months. Before eribulin treatment, patients with absolute lymphocyte counts (ALC) >1,500/µl, NLR <3.0, and PLR <150 had significantly longer OS than those with lower ALC, and higher NLR and PLR (median OS, 25.5 vs. 15.5 months; P<0.01; 20.3 vs. 13.6 months, P<0.01; and 29.2 vs. 14.8 months; P<0.001, respectively). Patients with anemia [hemoglobin (Hb) <10 g/dl] or liver dysfunction [albumin-bilirubin (ALBI) grade 2/3] had significantly shorter OS than those without (P<0.001, respectively). Multivariate analysis revealed low ALBI grade (P<0.001), high Hb (P<0.01) and low PLR (P<0.05) as independent factors of longer OS after eribulin administration. Low PLR, anemia and liver dysfunction might be factors associated with prolonged OS in patients with metastatic BC on eribulin therapy, which could be clinically useful, as their evaluation requires neither new equipment nor invasive testing.
Keywords: BC, ALC, NLR, PLR, ALBI score, eribulin
Introduction
Eribulin, a synthetic analog of halichondrin B and non-taxane microtubule dynamics inhibitor (1), is a U.S. Food and Drug Administration-approved drug with proven clinical efficacy and acceptable adverse events in patients with metastatic breast cancer (MBC) (2-4).
MBC has numerous treatment options, but treatment selection can be challenging because patient response can vary for the same treatment. In the treatment of MBC, for prolonging the overall response and progression-free survival (PFS), patient groups showing efficacy can be identified; however, clear indicators to identify patient groups with prolonged overall survival (OS) among those with MBC undergoing eribulin therapy are lacking. Various studies have been conducted to identify predictors of eribulin efficacy in patients with MBC. Higher neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have been reported to be associated with higher mortality in several cancer types (5-8). High absolute lymphocyte count (ALC), PLR and low NLR are predictors of survival in patients with BC treated with eribulin (9-12). However, the global phase 3 EMBRACE trial suggested that NLR is a general prognostic factor for improved OS, and ALC is an independent predictor of OS, in eribulin-treated patients (10). However, the association between peripheral immune status and MBC prognosis is controversial.
Liver function is assessed according to the Child-Pugh (C-P) system. This system was originally developed to predict the prognosis of patients being treated for portal hypertension and variceal bleeding associated with cirrhosis (13,14). However, the C-P classification is not statistically constructed owing to concerns regarding subjective and confounding factors, such as hepatic coma, ascites and albumin (Alb). Previously, a new liver function assessment tool, Alb-bilirubin (ALBI) grading, consisting only of Alb and total bilirubin (TBil), has been proposed (15,16). Compared with the C-P score, the ALBI score is a more appropriate assessment as it only uses objective parameters (Alb and TBil).
In the present study, the relative effect of NLR and PLR on survival was quantified in patients with recurrent/stage IV BC and the clinical significance of NLR, PLR and other clinicopathologic factors (including liver and kidney function tests) on the prognostic value was evaluated.
Materials and methods
Patients
In the present study, 156 patients with recurrent/stage IV BC disease who received eribulin monotherapy as any treatment line at Saitama Medical University International Medical Center, from April 2011 to March 2021, were enrolled. In all patients, metastases were confirmed through diagnostic radiography using computed tomography, whole-body bone scintigraphy, or 2-[(18)F]-fluoro-2-deoxy-D-glucose positron emission tomography. Patients with any type of simultaneous metastatic cancer were excluded. Clinicopathological factors and clinical outcome data were retrospectively extracted from the medical records. Patients received eribulin at a dose of 1.4 mg/m2 on days one and eight of a 21-day cycle. The dose was reduced, or the treatment postponed when toxicity developed, and treatment cessation was decided based on confirmed disease progression or intolerable toxicity.
Histological assessment
For the pathological examination of the tumors, the surgically resected specimens were fixed in 10% formalin. The tissues were embedded on paraffin wax blocks. The paraffin blocks were cut to 3-4 µm sections for hematoxylin-eosin (H&E) and immunohistochemical staining at room temperature. To determine the histologic tumor type, H&E staining of sections of the tumor specimens was performed. The expression statuses of estrogen receptors (ER), progesterone receptors (PR), human epidermal growth factor type 2 (HER2), and Ki67 labeling Index were assessed by immunohistochemical staining. The following monoclonal antibodies were used for immunohistochemistry: ER (SP1; cat. no. 790-4324, prediluted); PR (1E2; cat. no. 790-2223, prediluted), HER2 (4B5; cat. no. 790-2991, prediluted, all from Roche Tissue Diagnostics; Roche Diagnostics, Ltd.) and Ki-67 (MIB-1; cat. no. M7240, ready-to-use; Dako; Agilent Technologies, Inc.). Images were captured using a light microscope (Olympus Corporation). According to ASCO/CAP guideline (17), ER and PgR positivity was determined when ≥1% of the nuclei in the tumor were stained using immunohistochemistry. According to ASCO/CAP guideline (18-20), HER2 positivity was defined as an immunohistochemical score of 3+ or a positive result on fluorescence in situ hybridization. According to the expression of ER, PR, HER2 and Ki67, patients were divided into the triple-negative (TN) or non-TN groups, including Luminal A or B, Luminal HER2 and HER2 types.
Blood sampling and evaluation of predictive factors
On the same day or just before the start of the first cycle of eribulin administration, whole blood samples and baseline data for blood-based parameters were obtained. The cutoff values for the baseline ALC, NLR, and PLR were set at 1,500/µl (10,11), 3.0 (9,10,21) and 150 (22,23), respectively.
ALBI grading was used to assess the liver function, with ALBI scores and ALBI grades defined as follows: [log10 bilirubin (µmol/l) x 0.66] + [Alb (g/l) x (-)0.085] (grades 1, 2, 3=≤-2.60, >-2.60 to -1.39, and >-1.39, respectively) (14).
Statistical analysis
Unless otherwise noted, data are presented as percentage (%) or the mean. The relationship between OS and all clinical factors was evaluated using univariate Cox proportional hazards regression models. Candidate predictors with P<0.05 in the univariate analysis were included in the multivariate analysis.
OS for each group was calculated from the date of the start of the first cycle of eribulin administration to the date of death from any cause or the date of the last follow-up. OS was calculated using the Kaplan-Meier method, and patient subgroups were compared using the log-rank test. All statistical analyses were performed using EZR version 1.52 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (24), which is a graphical user interface for R version 3.1.2 (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of the R commander designed to add statistical functions frequently used in biostatistics.
Results
Characteristics of patients and tumors
The clinicopathological characteristics of the 156 patients included in the present study are Listed in Table I. Tumors were mainly ductal (87.8%) or lobular (5.7%) invasive carcinomas, while 41 (26.3%) were those of TN disease. At the time of eribulin administration, 127 patients (81.4%) had visceral metastases, of which 98 (62.8%) had liver metastases. The mean time from confirmation of metastasis to start of eribulin administration, mean time to treatment failure of eribulin, and median follow-up duration for the 156 patients were 24.0, 6.0 and 18.3 months, respectively (Table II). Of the patients treated with eribulin, 44.23% ultimately received a reduced dose (Table II). Tumor subtypes were classified as TN (ER, PR, HER2-negative) and non-TN, including luminal (ER-positive, PR-positive, HER2-negative); luminal HER2 (ER-positive, PR-PR-positive or negative, HER2-positive), and HER2 (ER-negative, PR-negative, HER2-positive) types, with proportions of 26.3 and 73.7%, respectively (Table I).
Table I.
Patient characteristics.
| Variable | Total number (n=156) | Percentage, % |
|---|---|---|
| Median age, years (range) | 57.1 (29-84) | |
| <70 | 132 | 84.6 |
| >70 | 24 | 15.4 |
| Histology | ||
| aIDC | 137 | 87.8 |
| bILC | 9 | 5.7 |
| Others | 10 | 6.4 |
| Viceral metastases | 127 | 81.4 |
| Liver metastases | 98 | 62.8 |
| cHER2 status | ||
| Negative | 128 | 82.1 |
| Positive | 26 | 16.7 |
| Unknown | 2 | 1.3 |
| dER status | ||
| Negative | 55 | 35.3 |
| Positive | 101 | 64.7 |
| ePR status | ||
| Negative | 97 | 62.2 |
| Positive | 59 | 37.8 |
| Triple (HER2/ER/PR) negative | 41 | 26.3 |
| Mean of baseline fWBC, µl (range) | 5,770 (2,550-13,100) | |
| Mean of baseline gANC, µl (range) | 3,776 (1,238-10,461) | |
| Mean of baseline hHb, g/dl (range) | 12.1 (7.4-16.0) | |
| <10 | 15 | 9.6 |
| >10 | 141 | 90.4 |
| Mean of baseline platelet, µl (range) | 255,000 (82,000-653,000) | |
| Mean of baseline iALC, µl (range) | 1434 (439-6,255) | |
| <1,500 | 94 | 60.3 |
| >1,500 | 62 | 39.7 |
| jNLRs | 3.0 | |
| <3.0 | 102 | 65.4 |
| >3.0 | 54 | 34.6 |
| kPLRs | 204.4 | |
| <150 | 59 | 37.8 |
| >150 | 97 | 62.2 |
| lALBI grade | ||
| 1 | 49 | 31.4 |
| 2, 3 | 40 | 25.6 |
| N/A | 67 | 42.9 |
| meGFR | ||
| <60 | 13 | 8.3 |
| >60 | 135 | 86.5 |
| nCRP | ||
| <0.3 | 49 | 31.4 |
| >0.3 | 40 | 25.6 |
| oN/A | 67 | 42.9 |
aIDC, invasive ductal carcinoma;
bILC, invasive lobular carcinoma;
cHER2, Human epidermal growth factor receptor-2;
dER, Estrogen receptor;
ePR, Progesterone receptor;
fWBC, white blood cell count;
gANC, absolute neutrophil count;
hHb, hemoglobin;
iALC, Absolute lymphocyte count;
jNLRs, neutrophil-to-lymphocyte ratios;
kPLRs, platelet-to-lymphocyte ratios;
lALBI, albumin-bilirubin;
meGFR, estimated Glomerular Filtration;
nCRP, C-reactive protein;
oN/A, not applicable.
Table II.
Eribulin administration.
| Variable | Total number (n=156) | Percentage,% |
|---|---|---|
| Median follow-up time | 18.3 (1.6-76.1) | |
| Time to eribulin administration for aMBC | 24.0 (0-114) | |
| Time to treatment failure | 6.0 (1-68) | |
| Eribulin dose reduction level | ||
| 0 | 87 | 55.77 |
| -1 | 53 | 33.97 |
| -2 | 16 | 10.26 |
aMBC, metastatic breast cancer.
Prognostic factors
As demonstrated in Table III, before eribulin administration, OS was not affected by age, subtype, or renal function decline, but patients with anemia (hemoglobin (Hb) <10 g/dl or liver dysfunction (ALBI grade 2 or 3) had significantly shorter OS than those without it (P<0.001, respectively).
Table III.
Univariate and multivariate analyses for the overall survival.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Variable | aHR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | <70 | 1.144 (0.6474-2.023) | 0.6425 | ||
| Liver metastases | Positive | 1.342 (0.8895-2.026) | 0.168 | ||
| bER status | Negative | 1.224 (0.8137-1.841) | 0.332 | ||
| cHER2 status | Positive | 0.6051 (0.3369-1.087) | 0.0927 | ||
| Subtype | dTN | 1.202 (0.7716-1872) | 0.416 | ||
| eALBI grade | 2, 3 | 2.575 (1.589-4.784) | <0.001 | 2.895 (1.602-5.234) | <0.001 |
| feGFR | <60 | 1.359 (0.7231-2.555) | 0.3406 | ||
| gHb | <10 | 4.029 (2.186-7.427) | <0.001 | 2.785 (1.329-5.836) | <0.01 |
| hCRP | Positive | 3.004 (1.971-4.578) | <0.001 | 1.629 (0.7797-3.403) | 0.19430 |
| iNLRs | >3.0 | 1.909 (1.28-2.847) | <0.01 | 1.311 (0.6768-2.538) | 0.42250 |
| jALC | <1,500 | 1.709 (1.133-2.578) | <0.05 | 1.020 (0.5063-2.055) | 0.95570 |
| kPLRs | >150 | 2.214 (1.443-3.396) | <0.001 | 2.124 (1.157-3.898) | <0.05 |
aHR, Hazard ratio;
bER, Estrogen receptor;
cHER2, Human epidermal growth factor receptor-2;
dTN, triple negative;
eALBI, albumin-bilirubin;
feGFR, estimated Glomerular Filtration;
gHb, hemoglobin;
hCRP, C-reactive protein;
iNLRs, neutrophil-to-lymphocyte ratios;
jALC, Absolute lymphocyte count;
kPLRs, platelet-to-lymphocyte ratios; CI, confidence interval.
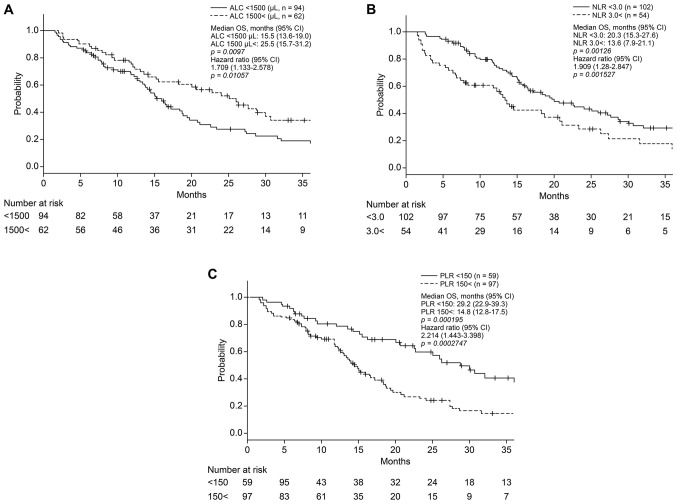
As revealed in Fig. 1A, before eribulin administration, patients with ALC >1,500/µl had significantly longer OS than those with lower ALC (median OS, 25.5 vs. 15.5 months; P<0.01). Patients with NLR <3.0 and PLR <150 had significantly longer OS than those with higher NLR and PLR [median OS, 20.3 vs. 13.6 months (P<0.01); 29.2 vs. 14.8 months (P<0.001); respectively] (Fig. 1B and C).
Figure 1.
(A) OS based on absolute lymphocyte count. Patients with a higher ALC had a significantly longer OS compared with those with lower ALC (median OS, 25.5 vs. 15.5 months, respectively; P=0.0097. (B) OS based on the NLR. Patients with a lower NLR had a significantly longer OS compared with those with a higher NLR (median OS, 20.3 vs. 13.6 months, respectively; P=0.00126). (C) OS based on PLR. Patients with a lower PLR had a significantly longer OS compared with those with higher PLR (median OS, 29.2 vs. 14.8 months, respectively; P=0.000195. OS, overall survival; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
As shown in Table III, there were no significant differences in OS based on age and subtypes such as ER/HER2. Multivariate analysis demonstrated that high ALBI grade (P<0.001) was an independent predictor of shorter OS after eribulin treatment, regardless of the presence or absence of liver metastases. Furthermore, high Hb (P<0.01), and low PLRs (P<0.05) were independent factors that predicted longer OS after eribulin administration (Table III).
Discussion
While it has become clear that the tumor microenvironment significantly affects cancer cells and contributes to the formation of cancer-specific properties (25-27), it is interesting to examine its impact on the systemic immune environment. In the present retrospective study, the baseline blood-based clinical parameters were evaluated and PLR as a predictor of OS in patients with MBC treated with eribulin was confirmed. Additionally, high degrees of anemia (Hb <10 g/dl) and liver dysfunction (ALBI grade 2 or 3) were significantly associated with impaired OS in patients with MBC treated with eribulin. The present study is the first one, to the best of the authors' knowledge, to have comprehensively analyzed the peripheral blood parameters and ratios in patients with MBC treated with eribulin and revealed relevant predictors of OS. Predictors related to systemic immune response and hepatic reserve in the treatment of BC have not been considered in clinical practice.
Eribulin induces the remodeling of the tumor vasculature and reoxygenation in patients with advanced BC and decreases the levels of transforming growth factor-beta (TGF-β), which is typically associated with hypoxic conditions (28). A retrospective analysis reported decreased expression of programmed cell death-1 (PD-1), programmed cell death ligand-1 (PD-L1), and forkhead box P3 (FoxP3) and increased infiltrating CD8+ T cell levels in eribulin responders (29). As PD-L1, FoxP3 and TGF-β have potent immunosuppressive effects, eribulin may have exerted an immunomodulatory effect mediated through vascular remodeling. Therefore, the analysis of the present study focused on peripheral immunological biomarkers and organ reserve associated with the efficacy of eribulin.
MBC treatment aims to maintain quality of life and prolong OS, unlike numerous drugs that only prolong PFS. In a post hoc analysis using the EMBRACE trial data (10), a high ALC (≥1,500/µl) was a significant and independent predictor of prolonged OS in patients treated with eribulin but not in those receiving a different treatment of physician's choice, suggesting that NLRs may not be a specific predictor of OS for eribulin but a general prognostic factor. The present results suggested that a high PLR (≥150) is a stronger prognostic factor for TN and non-TN BC than NLR and ALC, which are characteristic of TNBC. However, this finding does not imply that a high PLR (≥150) is a significant and independent predictor of shorter OS during eribulin treatment (data not shown).
Liver metastasis reduces the response rate and worsens the prognosis of patients who receive immune-checkpoint inhibitor therapy (30-32), partly because lactic acid induces PD-1 expression by Treg cells in liver metastatic lesions (33). Liver tumors induce the loss of systemic tumor-specific effector T cells by activation of Treg cells and/or hepatic macrophages (34,35). There is evidence that as a biomarker, ALBI grade can be used to optimize patient selection and treatment planning in hepatocellular carcinoma (HCC) (36). In particular, changes in ALBI grade after hepatic resection are independently associated with decreased OS and recurrence-free survival (37). In the present study, the hepatic reserve capacity due to liver metastasis and drug-induced liver injury from previous line treatment in BC by ALBI grade was evaluated, and it was demonstrated that liver dysfunction (ALBI grade 2 or 3) affects the OS. Liu et al (38) revealed that the PALBI grade, which incorporates platelet counts as an indicator of the severity of portal hypertension into the ALBI grade, could be more clinically feasible because of its superior prognostic power in HCC compared with the ALBI grade. In HCC, the effect of reduced platelet counts associated with portal hypertension should be considered but in the present study, a high PLR (≥150) may have been an independent prognostic factor that reflected a stronger immune response. However, the interpretation of the present study is limited by the fact that it is retrospective and that there were missing values in the ALBI score data.
In conclusion, blood-based parameters and ratios are easily calculated and are feasible adjunctive tools to predict prognosis, relapse rates and treatment efficacy in various types of cancers (39-42). In the present study, PLR and Hb were closely correlated with prognosis in patients with MBC who received eribulin therapy. Thus, low PLR and anemia may be useful surrogate markers for OS in patients with MBC. Blood count parameters can be easily measured without the need for new equipment or invasive testing. Further prospective studies are needed to confirm these preliminary results and to investigate the correlations between MBC characteristics or subtypes and PLR.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
HS, AF, KM, SK, AN, YI, AA, MO, HI, AO and TS provided the clinical data included in the text and confirm the authenticity of all the raw data. HS, AF, and KM wrote the manuscript draft. SK and MO contributed to the conception of the work and interpreted and revised the laboratory test results included in the present study. AO, HI, and TS revised the manuscript critically and modified the text. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
All procedures performed in the present study were in accordance with the ethical standards of committee of Saitama Medical University International Medical Center (approval no. 19-224; Hidaka, Japan) and/or national research committee and in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The requirement of informed consent was waived given the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
Toshiaki Saeki received research grants from Eisai Co., Ltd.; Taiho Pharmaceutical Co., Ltd.; and Chugai Pharmaceutical Co., Ltd., and received personal fees from Taiho Pharmaceutical Co. and Chugai Pharmaceutical Co. Akihiko Osaki received research grants from AstraZeneca K.K., Eisai Co., Ltd., MSD K.K., Ono Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Daiichi Sankyo Co., Ltd., Taiho Pharmaceutical Co., Ltd., Sawai Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Novartis Pharma K.K., Hamamatsu Photonics K.K., Parexel International Inc., and Fuji Pharma Co. Akihiko Osaki also received personal fees from AstraZeneca K.K., Kyowa Hakko Kirin Co., Ltd, Daiichi Sankyo Co., Ltd, Chugai Pharmaceutical Co., Ltd., and Novartis Pharma K.K. The authors declare that they have no competing interests.
References
- 1.Towle MJ, Salvato KA, Budrow J, Wels BF, Kuznetsov G, Aalfs KK, Welsh S, Zheng W, Seletsky BM, Palme MH, et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–1021. [PubMed] [Google Scholar]
- 2.Jain S, Vahdat LT. Eribulin mesylate. Clin Cancer Res. 2011;17:6615–6622. doi: 10.1158/1078-0432.CCR-11-1807. [DOI] [PubMed] [Google Scholar]
- 3.Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Diéras V, Delozier T, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Velikova G, Olivo MS, He Y, Dutcus CE, Cortes J. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015;33:594–601. doi: 10.1200/JCO.2013.52.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, Uchida E. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: A systematic review and meta-analysis. Ann Surg Oncol. 2016;23:646–654. doi: 10.1245/s10434-015-4869-5. [DOI] [PubMed] [Google Scholar]
- 6.Tan D, Fu Y, Su Q, Wang H. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Med (Baltim) 2016;95(e3837) doi: 10.1097/MD.0000000000003837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng J, Cai J, Li H, Zeng K, He L, Fu H, Zhang J, Chen L, Yao J, Zhang Y, Yang Y. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: A meta-analysis and systematic review. Cell Physiol Biochem. 2017;44:967–981. doi: 10.1159/000485396. [DOI] [PubMed] [Google Scholar]
- 8.Prodromidou A, Andreakos P, Kazakos C, Vlachos DE, Perrea D, Pergialiotis V. The diagnostic efficacy of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in ovarian cancer. Inflamm Res. 2017;66:467–475. doi: 10.1007/s00011-017-1026-6. [DOI] [PubMed] [Google Scholar]
- 9.Miyagawa Y, Araki K, Bun A, Ozawa H, Fujimoto Y, Higuchi T, Nishimukai A, Kira A, Imamura M, Miyoshi Y. Significant association between low baseline neutrophil-to-lymphocyte ratio and improved progression-free survival of patients with locally advanced or metastatic breast cancer treated with eribulin but not with nab-paclitaxel. Clin Breast Cancer. 2018;18:400–409. doi: 10.1016/j.clbc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Miyoshi Y, Yoshimura Y, Saito K, Muramoto K, Sugawara M, Alexis K, Nomoto K, Nakamura S, Saeki T, Watanabe J, et al. High absolute lymphocyte counts are associated with longer overall survival in patients with metastatic breast cancer treated with eribulin-but not with treatment of physician's choice-in the EMBRACE study. Breast Cancer. 2020;27:706–715. doi: 10.1007/s12282-020-01067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araki K, Ito Y, Fukada I, Kobayashi K, Miyagawa Y, Imamura M, Kira A, Takatsuka Y, Egawa C, Suwa H, et al. Predictive impact of absolute lymphocyte counts for progression-free survival in human epidermal growth factor receptor 2-positive advanced breast cancer treated with pertuzumab and trastuzumab plus eribulin or nab-paclitaxel. BMC Cancer. 2018;18(982) doi: 10.1186/s12885-018-4888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takamizawa S, Shimoi T, Satomi-Tsushita N, Yazaki S, Okuya T, Kojima Y, Sumiyoshi-Okuma H, Nishikawa T, Tanioka M, Sudo K, et al. Neutrophil-to-lymphocyte ratio as a prognostic factor for patients with metastatic or recurrent breast cancer treated using capecitabine: A retrospective study. BMC Cancer. 2022;22(64) doi: 10.1186/s12885-021-09112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 14.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 15.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiraoka A, Michitaka K, Kumada T, Izumi N, Kadoya M, Kokudo N, Kubo S, Matsuyama Y, Nakashima O, Sakamoto M, et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: The need for a more detailed evaluation of hepatic function. Liver Cancer. 2017;6:325–336. doi: 10.1159/000479984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR, Chavez-MacGregor M, Perlmutter J, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38:1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 18.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 19.Farshid G, Dhatrak D, Gilhotra A, Koszyca B, Nolan J. The impact of 2018 ASCO-CAP HER2 testing guidelines on breast cancer HER2 results. An audit of 2132 consecutive cases evaluated by immunohistochemistry and in situ hybridization. Mod Pathol. 2020;33:1783–1790. doi: 10.1038/s41379-020-0555-7. [DOI] [PubMed] [Google Scholar]
- 20.Li A, Bai Q, Kong H, Zhou S, Lv H, Zhong S, Li M, Bi R, Zhou X, Yang W. Impact of the updated 2018 American Society of Clinical Oncology/College of American Pathologists Guideline for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Arch Pathol Lab Med. 2020;144:1097–1107. doi: 10.5858/arpa.2019-0369-OA. [DOI] [PubMed] [Google Scholar]
- 21.Asano Y, Kashiwagi S, Onoda N, Noda S, Kawajiri H, Takashima T, Ohsawa M, Kitagawa S, Hirakawa K. Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple-negative breast cancer. Ann Surg Oncol. 2016;23:1104–1110. doi: 10.1245/s10434-015-4934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asano Y, Kashiwagi S, Onoda N, Noda S, Kawajiri H, Takashima T, Ohsawa M, Kitagawa S, Hirakawa K. Platelet-lymphocyte ratio as a useful predictor of the therapeutic effect of neoadjuvant chemotherapy in breast cancer. PLoS One. 2016;11(e0153459) doi: 10.1371/journal.pone.0153459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuello-López J, Fidalgo-Zapata A, López-Agudelo L, Vásquez-Trespalacios E. Platelet-to-lymphocyte ratio as a predictive factor of complete pathologic response to neoadjuvant chemotherapy in breast cancer. PLoS One. 2018;13(e0207224) doi: 10.1371/journal.pone.0207224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016(6058147) doi: 10.1155/2016/6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gysler SM, Drapkin R. Tumor innervation: Peripheral nerves take control of the tumor microenvironment. J Clin Invest. 2021;131(e147276) doi: 10.1172/JCI147276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda S, Saeki T, Takeuchi H, Shigekawa T, Yamane T, Kuji I, Osaki A. In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: A comparison to bevacizumab. Br J Cancer. 2016;114:1212–1218. doi: 10.1038/bjc.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goto W, Kashiwagi S, Asano Y, Takada K, Morisaki T, Fujita H, Takashima T, Ohsawa M, Hirakawa K, Ohira M. Eribulin promotes antitumor immune responses in patients with locally advanced or metastatic breast cancer. Anticancer Res. 2018;38:2929–2938. doi: 10.21873/anticanres.12541. [DOI] [PubMed] [Google Scholar]
- 30.Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, Fizazi K, Tangen CM, Rosenthal M, Petrylak DP, Hussain M, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34:1652–1659. doi: 10.1200/JCO.2015.65.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki A, Nakamura Y, Mishima S, Kawazoe A, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. 2019;22:793–802. doi: 10.1007/s10120-018-00922-8. [DOI] [PubMed] [Google Scholar]
- 32.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Sosman JA, Atkins MB, Leming PD, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 2019;5:1411–1420. doi: 10.1001/jamaoncol.2019.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, Kamada T, Irie T, Okumura G, Kono H, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 2022;40:201–218.e9. doi: 10.1016/j.ccell.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, Daud A, Bluestone JA. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol. 2020;5(eaba0759) doi: 10.1126/sciimmunol.aba0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye L, Liang R, Zhang J, Chen C, Chen X, Zhang Y, Wang G, Yang Y, Chen G. Postoperative albumin-bilirubin grade and albumin-bilirubin change predict the outcomes of hepatocellular carcinoma after hepatectomy. Ann Transl Med. 2019;7(367) doi: 10.21037/atm.2019.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demirtas CO, D'Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: Evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021;3(100347) doi: 10.1016/j.jhepr.2021.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu PH, Hsu CY, Hsia CY, Lee YH, Chiou YY, Huang YH, Lee FY, Lin HC, Hou MC, Huo TI. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. J Gastroenterol Hepatol. 2017;32:879–886. doi: 10.1111/jgh.13608. [DOI] [PubMed] [Google Scholar]
- 39.Zhang P, Zong Y, Liu M, Tai Y, Cao Y, Hu C. Prediction of outcome in breast cancer patients using test parameters from complete blood count. Mol Clin Oncol. 2016;4:918–924. doi: 10.3892/mco.2016.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stotz M, Liegl-Atzwanger B, Posch F, Mrsic E, Thalhammer M, Stojakovic T, Bezan A, Pichler M, Gerger A, Szkandera J. Blood-based biomarkers are associated with disease recurrence and survival in gastrointestinal stroma tumor patients after surgical resection. PLoS One. 2016;11(e0159448) doi: 10.1371/journal.pone.0159448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, Chen J, Liu X, Wang SK. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31(305) doi: 10.1007/s12032-014-0305-0. [DOI] [PubMed] [Google Scholar]
- 42.Deng Q, He B, Liu X, Yue J, Ying H, Pan Y, Sun H, Chen J, Wang F, Gao T, et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13(66) doi: 10.1186/s12967-015-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.