Abstract
In Plasmodium falciparum malaria, large proportions of resident macrophages and circulating monocytes and leukocytes contain massive amounts of the malarial pigment, hemozoin. Previous studies have shown that important functions (e.g., the generation of the oxidative burst, the ability to repeat phagocytosis, and protein kinase C activity) were severely impaired in hemozoin-loaded monocytes. Expression of membrane antigens directly involved in the immune response and in the phagocytic process, and/or under protein kinase C control, in hemozoin-loaded human monocytes was studied. Expression of major histocompatibility complex (MHC) class II after gamma interferon stimulation was blocked in hemozoin-loaded monocytes at the protein expression and gene transcription levels but was preserved in control monocytes loaded with opsonized latex beads or anti-D(Rho)-immunoglobulin G (IgG)-opsonized human erythrocytes. Expression of CD54 (intracellular adhesion molecule 1) and CD11c (p150,95 integrin) was also decreased in hemozoin-loaded monocytes. Expression of MHC class I, CD16 (low-affinity Fc receptor for aggregated IgG), CD32 (low-affinity Fc receptor for aggregated IgG), CD64 (high-affinity receptor for IgG), CD11b (receptor for complement component iC3b [CR3]), CD35 (receptor for complement components C3b and C4b [CR1]), and CD36 (non-class-A scavenger receptor) was not specifically affected by hemozoin loading. These results suggest that hemozoin loading may contribute to the impairment of the immune response and the derangement of antigen presentation reported in previous studies of P. falciparum malaria.
During the 48-h intraerythrocytic life cycle of Plasmodium falciparum, a large portion of host hemoglobin is degraded (19). However, the parasite is unable to catabolyze heme, which aggregates to form an insoluble polymer called malarial pigment or hemozoin (HE) (19, 20, 42). Crude, unpurified HE, as is present within the food vacuole (the parasite’s digestive organelle), contains large amounts of ferriprotoporphyrin IX, a globin related to host hemoglobin, and a variety of lipids and proteins of host and parasitic origin (6, 20, 48).
In vitro and in vivo studies have shown that monocytes and resident macrophages ingest HE or HE-containing parasitized erythrocytes (1, 16, 47). Large proportions of resident macrophages and circulating monocytes and leukocytes are loaded with HE in malaria (8, 16, 27). HE may persist, apparently unchanged, in macrophages for several months (16). In vitro studies have shown that HE-fed monocytes are viable but functionally impaired. They are unable to digest HE or to repeat phagocytosis (40), to generate the oxidative burst upon appropriate stimulation (36) or to kill ingested bacteria, fungi, or tumor cells (15). In addition, membrane translocation and activity of protein kinase C (PKC) were precociously and severely impaired (39). Moreover, HE-fed human and murine monocytes/macrophages were found to release large amounts of tumor necrosis factor alpha (29, 31, 41), nitric oxide (30, 45), macrophage-inhibitory protein 1α and macrophage-inhibitory protein 1β (41), and reduced amounts of interleukin-6 (IL-6) (31).
Several studies have shown impaired immune responsiveness in P. falciparum malaria (see references 46 and 50 for reviews). Altered cellular responses to blood-stage Plasmodium antigens, reduced T-cell proliferation, and alterations of lymphocyte functions were observed in acute malaria (see references 21 and 22 for reviews). Other studies also suggested depression of macrophage function (23, 25) and defects in antigen presentation (49, 50).
Derangement of macrophage functions and the immune response in malaria, widespread HE presence in phagocytes, inhibition of PKC in HE-loaded monocytes, and PKC involvement in the expression of a number of surface molecules have prompted the present work, which was aimed at studying the expression of surface molecules selected according to their roles in antigen presentation and the T-cell-dependent immune response (major histocompatibility complex [MHC] classes I and II), in cell adhesion (CD54, intracellular adhesion molecule 1 [ICAM-1], and CD11c [p150,95 integrin]), and in phagocytosis (CD16, a low-affinity Fc receptor for aggregated immunoglobulin G [IgG]; CD32, a low-affinity Fc receptor for aggregated IgG; CD64, a high-affinity receptor for IgG; CD11b, a receptor for complement component iC3b [CR3]; CD35, a receptor for complement components C3b and C4b [CR1]; and CD36, a non-class-A scavenger receptor) (24, 28).
MATERIALS AND METHODS
Preparation of human monocytes.
Peripheral blood mononuclear cells (PBMC) were separated as previously indicated (7) from freshly collected platelet-poor buffy coats discarded from blood samples from healthy adult donors of both sexes. Separated cells were washed once with lukewarm phosphate-buffered saline (PBS) supplemented with 10 mM glucose (PBS-G) and resuspended at 5 × 106 cells/ml in ice-cold RPMI 1640 medium supplemented with 23 mM NaHCO3 and 25 mM HEPES, pH 7.4 (RMBH). Dynabeads M450 Pan B and Pan T (Dynal) were added to the cells in a 4:1 ratio for 20 min at 4°C. B and T lymphocytes were removed as specified by the manufacturer. The remaining monocytes were washed twice in RMBH and resuspended in AIM V cell culture medium (Gibco) at 106 cell/ml. For fluorescence-activated cell sorter analysis, monocytes were separated from washed PBMC by a second separation on hyperosmotic Ficoll (Sigma). The original method of Recalde (33) was modified as follows. A total of 5 × 108 PBMC were resuspended in 30 ml of PBS-G and kept for 10 min at 37°C. Thereafter, 150 μl of a 9% NaCl solution was added and cells were reincubated for 12 min at 37°C. An aliquot of 300 μl of a 9% NaCl solution was added twice. Cells were incubated for 12 min after each addition. Finally, cells were resuspended with 60 ml of hyperosmotic PBS containing 2.5 mg of NaCl/ml and separated on 30 ml of Ficoll containing 3 mg of NaCl/ml by centrifugation at 700 × g for 25 min. The monocyte layer was collected, washed with PBS-G at 37°C, and resuspended in AIM V medium at 106 cells/ml. Purified cells were >90% monocytes as assessed by CD14 expression.
Preparation and opsonization of HE.
HE was prepared from P. falciparum cultures (strain FCR-3) by osmotic shock and four washes with ice-cold distilled water and opsonized without any further purification immediately before phagocytosis with an equal volume of fresh human serum for 30 min at 37°C as previously indicated (40). HE was quantified according its heme content by a luminescence method (38).
Phagocytosis of opsonized HE, opsonized human erythrocytes, and opsonized latex beads by adherent monocytes.
Phagocytosis of fresh-serum-opsonized HE, of human erythrocytes opsonized with anti-D(Rho) IgG (40), and of fresh-serum-opsonized latex beads was initiated by mixing 10 erythrocytes per monocyte, an equivalent amount of HE in terms of heme content (for details of heme quantification, see reference 38), or 1 μl of opsonized latex bead (0.1-μm average diameter; Sigma) suspension (latex beads, RMBH, and fresh human serum, 1:1:1 [vol/vol/vol]/106 monocytes. Suspensions were briefly centrifuged (150 × g for 5 s at room temperature) to improve contact between the erythrocytes, HE or latex beads, and monocytes. To avoid the attachment of monocytes after centrifugation and during the whole incubation period, cells were kept in suspension at 5 × 106 cells/5 ml of AIM V medium in 6-cm-diameter Teflon-bottom dishes (Heraeus) in a humidified incubator (95% air, 5% CO2) at 37°C. Stimulation with 200 U of human recombinant gamma interferon (IFN-γ) (a gift of G. Garotta, Roche, Basel, Switzerland)/ml was performed 12 h after the start of phagocytosis, if not indicated otherwise. On average, ≥90% of the monocytes phagocytosed HE or latex beads, and ≥80% of monocytes phagocytosed opsonized erythrocytes, as assessed by microscopic inspection. Control cells were kept under similar conditions without phagocytosis and with and without addition of IFN-γ.
Flow cytometry analysis.
Before phagocytosis and 48 h after the beginning of phagocytosis, monocytes were harvested by aspiration. Residual adherent monocytes were scraped off without noticeable alterations. After three washings with PBS, an indirect immunofluorescence test was performed by reacting 106 cells in suspension with appropriate dilutions of purified monoclonal antibody (MAb) or hybridoma cell culture supernatants for 30 min at 4°C. MAbs used in this study were as follows: NL07 (2), anti-CD36; W6/32 (American Type Culture Collection), anti-MHC class I; OKMI (Ortho Diagnostics), anti-CD11b; 2.9 (9), anti-MHC class II-DR, -DP, and -DQ; MC105 (The Binding Site), anti-CD11c; 3C10 (American Type Culture Collection), anti-CD14; 3G8 (Immunotech), anti-CD16; IV.3 (American Type Culture Collection), anti-CD32; CD54 (Serotec), anti-CD54; 32.2 (Medarex), anti-CD64; and CB04 (26), anti-CD35. Controls were class-matched irrelevant MAbs. Bound antibody was revealed by fluoroscein isothiocyanate (FITC)-conjugated F(ab′)2 goat anti-mouse Ig (Technogenetics). Cells were then analyzed on a FACScan flow cytometer (Becton Dickinson), by PC-LYSYS software.
Expression of specific mRNA for MHC class I and class II antigens.
Forty-eight hours after the start of phagocytosis and 24 h after addition of 200 U of IFN-γ/ml, monocytes were harvested and sedimented by centrifugation. The supernatant was discarded and total RNA was isolated from 5 × 106 monocytes by RNAzol B extraction as specified by the manufacturer (Biotech X Laboratories) and quantified by optical density (OD) measurement. The cDNA synthesis from 15 ng of total cellular RNA from each extract was performed with 25 ng of random primers (Gibco BRL), 200 U of Moloney murine leukemia virus reverse transcriptase (Gibco BRL), and 20 U of RNase inhibitor (Boehringer Mannheim). Reverse transcription was terminated after 60 min of incubation at 37°C by a 95°C treatment of samples for 5 min. Subsequently, PCR was carried out in the same tube used for reverse transcription, adding 20 pmol of each oligonucleotide primer and 5 U of Ampli Taq DNA polymerase (Perkin-Elmer) in a final volume of 100 μl. The volume was adjusted with PCR buffer. Oligonucleotide primers were designed for conserved sequences coding for the human MHC class I B (sense oligonucleotide, 5′-ACA GTG CCC AGG GCT CTG AT-3′; antisense oligonucleotide, 5′-AGA GGC TCT TGA AGT CAC AA-3′) and class II B (sense oligonucleotide, 5′-GAT TGG ACC TTC CAG ACC CTG-3′; antisense oligonucleotide, 5′-ACT TGG GTG CTC CAC TTG GCA-3′) chains from the DR1 haplotype and were synthesized by C. Bernd, Institute of Pathological and Clinical Biochemistry, Humboldt University, Berlin, Germany. Samples were subjected to 25 cycles consisting of 45 s at 95°C, 45 s at 48°C, and 60 s at 70°C and to an additional extension step of 5 min at 70°C in a Biometra Uni Thermoblock. Portions of 18 μl of the PCR products were electrophoresed on a 3% agarose gel (NuSieve GTG; Biozym) in Tris-borate-EDTA buffer containing ethidium bromide. Bands were detected by ethidium bromide-dependent fluorescence. The PCR products of MHC class I and class II genes had 118 and 81 bp, respectively. The quantity of PCR products for MHC class I and class II obtained was dependent on the amount of MHC-specific mRNA in the cells when total mRNA extract was employed in the range of 7.5 to 60 ng for reverse transcription and PCR.
RESULTS
HE phagocytosis abrogates the IFN-γ effect on MHC class II expression.
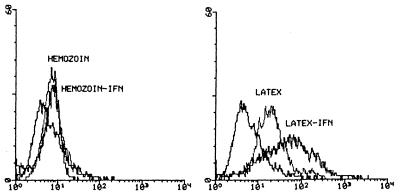
Flow cytometry analysis showed that the constitutive expression of MHC class II antigens on the surfaces of suspended human monocytes was very low in HE-fed and in unfed monocytes examined 48 h after the start of phagocytosis. Expression of class II antigens in HE-fed monocytes was not increased after IFN-γ stimulation (200 U/ml was added for 36 h 12 h after the start of phagocytosis) (Fig. 1, left panel). By contrast, phagocytosis of serum-opsonized latex beads enhanced expression of MHC class II antigens 3.6-fold without IFN-γ stimulation and 33-fold with IFN-γ stimulation (200 U/ml was added for 36 h 12 h after the start of phagocytosis) (Fig. 1, right panel). Similar results were obtained when IFN-γ stimulation was applied 5 h after the start of phagocytosis (not shown). Ingestion of serum-opsonized HE and latex beads did not modify the physical characteristics of suspended monocytes, as indicated by the constancy of the forward-scatter and side-scatter data (not shown).
FIG. 1.
Expression of MHC class II antigens in human monocytes after phagocytosis of serum-opsonized HE or latex beads with or without IFN-γ stimulation. Suspended human monocytes were allowed to phagocytose HE (left panel) or latex beads (right panel) in Teflon-bottom dishes kept in a humidified incubator at 37°C. Twelve hours after the start of phagocytosis, 200 U of IFN-γ/ml was added to a part of each dish. Forty-eight hours after the start of phagocytosis, monocytes were harvested by aspiration. After being washed, monocytes were immunostained with anti-MHC class II MAb and bound MAb was revealed by FITC-conjugated F(ab′)2 goat anti-mouse Ig. Flow cytometry analysis of surface antigens was performed on a FACScan flow cytometer (Becton Dickinson). The first peak at the left in both panels represents MHC class II antigen expression in unstimulated monocytes incubated for 48 h without phagocytosis. The y axis represents cell number and the x axis represents fluorescence intensity. This was one of four similar experiments.
MHC class I and class II mRNA contents of monocytes were compared by reverse transcriptase PCR (RT-PCR). The amount of PCR products for MHC classes I and II reflected the amount of specific cellular RNA when total cellular RNA was used in the range of 7.5 to 60 ng in the RT-PCR. Increases in fluorescence intensity of PCR products for MHC class I (118 bp) and class II (84 bp) were observed in the above range with increasing amounts of total RNA extract (Fig. 2). For each of the following RT-PCRs, 15 ng of total RNA extract was employed. This allowed us to compare the amounts of PCR products for MHC class I and class II from differently treated cells and to extrapolate them to differences in cellular content of MHC class I and class II mRNA at the time of RNA extraction.
FIG. 2.
Quantification of MHC class I and class II mRNA by RT-PCR. Increasing amounts (7.5 to 60 ng, quantified by OD, as indicated under the lanes) of total RNA extracted from human monocytes were employed in reverse transcription with Moloney murine leukemia virus reverse transcriptase for 1 h, followed by PCR in the same tube (100-μl final volume, 25 cycles). RT-PCR was performed in duplicate with each of the indicated total RNA amounts. Specific primers for conserved regions in MHC class I and class II genes were utilized to obtain PCR products of 118 and 81 bp, respectively. PCR products were separated on a 3% agarose gel and stained with ethidium bromide. DNA size standards are indicated on the right.
Similar to the expression of protein product in HE-fed monocytes examined 48 h after the start of phagocytosis, the steady-state level of MHC class II mRNA was very low and almost unresponsive to IFN-γ stimulation. In unfed monocytes, the expression of MHC class II mRNA did not change over 48 h but was remarkably enhanced after IFN-γ stimulation (Fig. 3, upper panel). Based on the dependency of output of class II-specific products on the amount of RNA used in the RT-PCR (Fig. 2), we can estimate by OD quantification that the amount of class II mRNA was at least three times lower in IFN-γ-stimulated, HE-loaded monocytes than in IFN-γ-stimulated control cells. Compared to that in time-matched unfed monocytes, expression of MHC class II mRNA was slightly increased 48 h after phagocytosis of opsonized latex beads or opsonized erythrocytes and was remarkably increased after IFN-γ stimulation (Fig. 3, lower panel). Thus, HE phagocytosis apparently hindered upregulation of MHC class II expression at the mRNA and protein levels. The remarkable differences in the IFN-γ response observed in HE-fed and latex-bead-fed cells may exclude nonspecific effects due to lysosomal occupancy related to the phagocytic process and may indicate a specific effect of HE. Expression of MHC class I mRNA was not modified after phagocytosis of any of the phagocytic targets used here and did not respond to IFN-γ stimulation (Fig. 3). Also, the amounts of extracted total RNA did not differ significantly in differently treated cells. Contrary to expectations, the stimulation of unfed monocytes with 200 U of IFN-γ/ml for 2, 6, 12, or 24 h did not modify the level of MHC class I-specific mRNA (data not shown). Consequently, in the present experimental system, class I mRNA can be considered an internal standard for RT-PCR. The higher expression of class I mRNA in untreated control cells and the relatively lower expression of class I mRNA in IFN-γ-treated control cells (shown in the upper panel of Fig. 3) was detected occasionally and was probably caused by usage of a larger amount of RNA in untreated control cells due to imprecise quantification via OD.
FIG. 3.
(Upper panel) steady-state levels of MHC class I and class II mRNA in human monocytes (C) and monocytes after phagocytosis of serum-opsonized HE (H) with (+) or without (−) IFN-γ stimulation. After the start of HE phagocytosis, monocytes were incubated for 24 h before addition of IFN-γ (200 U/ml). After a further 24 h of incubation, total RNA was extracted, RT-PCR was performed in duplicate with 15 ng of total RNA, and amplified MHC class I and class II gene products of 118 and 81 bp, respectively, were analyzed as indicated in the legend to Fig. 2. DNA size standards are indicated on the right. This was one of five similar experiments. (Lower panel) steady-state levels of MHC class I and class II mRNA in human monocytes (C) and monocytes after phagocytosis of opsonized latex beads (L) or opsonized erythrocytes (R) with (+) or without (−) IFN-γ stimulation. After the start of phagocytosis of the latex beads or erythrocytes, monocytes were incubated for 24 h before addition of IFN-γ (200 U/ml). After a further 24 h of incubation, total RNA was extracted, RT-PCR was performed with 15 ng of total RNA, and amplified MHC class I and class II gene products of 118 and 81 bp, respectively, were analyzed as indicated in the legend to Fig. 2. DNA size standards are indicated on the right. This was one of five similar experiments.
Effect of HE phagocytosis on expression of membrane antigens.
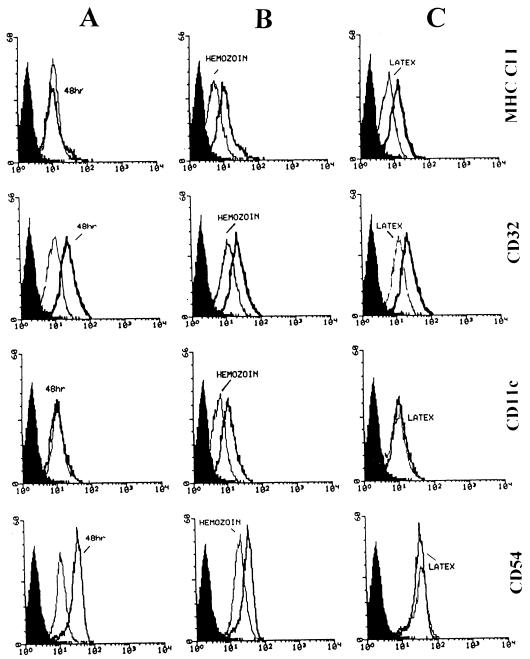
Flow cytometry analysis was performed on the following cell surface antigens constitutively expressed on monocytes: MHC class I, CD32 (low-affinity Fc receptor for aggregated IgG), CD11c (p150,95 integrin), and CD54 (ICAM-1). These cell surface antigens were studied 48 h after phagocytosis of HE and latex beads. As shown in Fig. 4, after 48 h of culturing, expression of MHC class I and CD11c did not change, while expression of CD32 and CD54 was stimulated in unfed control monocytes. Phagocytosis of either HE or latex beads slightly reduced class I expression and abrogated the increase in CD32, while only HE phagocytosis downregulated expression of CD11c and reduced upregulation of CD54. Thus, HE phagocytosis seems to interfere specifically with expression of CD11c and CD54.
FIG. 4.
Expression of surface antigens in unstimulated human monocytes after phagocytosis of serum-opsonized HE or latex beads. Suspended monocytes were allowed to phagocytose HE or latex beads in Teflon-bottom dishes kept in a humidified incubator at 37°C. Forty-eight hours after the start of phagocytosis, monocytes were harvested by aspiration. After being washed, monocytes were immunostained with purified MAb and bound MAb was revealed by FITC-conjugated goat anti-mouse Ig. Flow cytometry analysis of the surface antigens MHC class I, CD32 (low-affinity receptor for aggregated Ig), CD11c (p150,95 integrin), and CD54 (ICAM-1) was performed on a FACScan (Becton Dickinson) flow cytometer. (A) unfed monocytes at time zero (——) and after 48 h in culture (); (B) HE-fed (——) and unfed () monocytes after 48 h in culture; (C) latex-fed (——) and unfed () monocytes after 48 h in culture. Black profiles on the left represent the fluorescence background of monocytes incubated with irrelevant mouse Ig. The y axis represents cell number and the x axis represents fluorescence intensity. This was one of four similar experiments.
The study also included expression of the following cell surface antigens: CD16 (low-affinity Fc receptor for aggregated IgG), CD64 (high-affinity receptor for IgG,, CD11b (receptor for complement component iC3b [CR3]), CD35 (receptor for complement components C3b and C4b [CR1]), and CD36 (non-class-A scavenger receptor [28]). These results are not shown, either because changes (for example, drops in CD16 and CD64) elicited by phagocytosis of latex beads and HE were similar or because HE-specific effects were small (for example, a minor drop in CD11b and CD35 in HE-fed versus latex-fed cells) and not significant.
DISCUSSION
The reduction or lack of immune responsiveness toward a wide range of antigens has been noted in both human and experimental malaria. Altered immune reactivity involves both lower production of specific antibodies and reduced lymphocyte proliferation in culture in the presence of a given antigen. In human malaria, immunosuppression appears late in the acute phase of the disease and can last a long time after the clearance of parasites from the circulation (see references 23 and 46 for reviews). Nigerian children with acute P. falciparum malaria showed diminished antibody responses to the O antigen of Salmonella typhi and to tetanus toxoid (21). Similar results were observed after the vaccination of Nigerian children with meningococcal vaccine (51). There was a significant correlation between the degrees of immunosuppression and parasitemia (5, 51). The mechanism of altered immune reactivity is not clear, although the functional defect of splenic macrophages as antigen-presenting cells has been implicated (49).
The aim of the present work was to determine functional alterations in the monocyte/macrophage system that may be causally connected with altered T-cell-dependent immune reactivity and possibly with defective antigen presentation in the organs of P. falciparum-harboring patients.
Isolated circulating monocytes can be functionally similar to resident macrophages and professional dendritic leukocytes that express MHC class II and adhesive protein, and they can act as antigen-presenting and T-cell-immunostimulating cells in the microenvironment of blood-filtering organs, such as the spleen, the liver, and the bone marrow (24). Macrophages residing in blood-filtering organs and young monocytes under constitutive renewal in bone marrow may take up locally released HE and HE-containing late parasite forms. Dendritic leukocytes reside in the marginal zone between the blood-filtering (red pulp) and the lymphoid (white pulp) compartments. These cells may also take up HE and can be impaired in their T-cell-targeted immunostimulating functions (24).
HE loading in resident macrophages and in circulating leukocytes and monocytes is massive in malaria (8, 16, 27); of note, the percentages of heavily HE-laden leukocytes and macrophages seem to roughly correlate with the severity of the disease (27). In vitro, monocytes also ingest large amounts of HE (38, 40). HE is persistently present in monocytes, where it can be observed to be apparently unmodified after several days in vitro (40) and after several months in vivo after clearance of parasites (8, 16). Under these conditions, HE-laden phagocytes are alive and metabolically active although impaired in important functions (15, 36–40).
The main result of the present work was the defective induction of MHC class II in response to IFN-γ stimulation in HE-laden monocytes. Class II is upregulated in human macrophages by IFN-γ (the major physiological inducer), tumor necrosis factor, IL-4, and granulocyte-macrophage colony-stimulating factor and downregulated by prostaglandin E2, IFN-α/β, colony-stimulating factor 1, α-macroglobulin, α-fetoprotein, and corticosteroids (see reference 18 for a review). The abrogation of MHC class II expression observed here was present at the protein expression level, where no upregulation of class II antigens could be elicited even after 36 h of stimulation with IFN-γ. The effect was also evident at the mRNA level. Low induction of expression of specific MHC class II mRNA was seen under similar conditions of IFN-γ stimulation. Phagocytosis of serum-opsonized latex beads and IgG-opsonized, nonparasitized erythrocytes substantially increased the expression of class II mRNA and class II antigens but did not modify the IFN-γ-induced increase of MHC class II. Thus, the HE-elicited effect seems to be specific and not due to the phagocytic process per se. The effect of HE was seen rather quickly, since occupancy of monocytes by HE for 5 h did not modify the basal level of class II expression but was sufficient to fully abrogate its upregulation.
In bacterial or parasitic diseases such as malaria, upregulation of MHC class II for antigen presentation is important to ensure adequate helper-T-cell development and to determine the outcomes of disease and secondary infections. Consequently, the lack of expression at the cell surface of MHC class II following a specific stimulus such as IFN-γ may also explain defects in T-cell activation observed in malaria and be consistent with manifestations of altered T-cell-dependent immune reactivity mentioned above.
The HE-induced inability of monocytes to upregulate expression of MHC class II after IFN-γ stimulation described in the present study adds to a series of analogous yet very heterogenous observations in other bacterial and parasitic diseases for which impaired class II responses have been described (see reference 34 for a review). For example, it has been observed that after ingestion of Mycobacterium, macrophages showed reduced expression of class II antigens (17); that Leishmania donovani, an obligate intracellular protozoan, suppressed macrophage expression of class II (and class I) in response to IFN-γ stimulation (34, 35); and that supernatants from monocytes inoculated with L. donovani contained a soluble factor which prevented class II upregulation by IFN-γ in naive monocytes (12). An impaired class II response was accompanied by defective antigen presentation and suppression of the T-cell response in mycobacterial (17) and Leishmania (32) infections.
The present data suggest a link between the HE loading of phagocytes, the suppression of IFN-γ responsiveness, the failure of MHC class II upregulation, and disturbances in antigen presentation and immunodepression in malaria. Mechanistically, the abrogation of the IFN-γ signal is unexplained. PKC-mediated phosphorylations have been involved in IFN-γ-mediated enhancement of MHC class II (14, 43). Previous work by our group has shown that HE inhibited PKC translocation and PKC activity (39). Recent data (37) indicate that PKC inhibition may be the consequence of markedly increased levels of 4-hydroxynonenal (HNE) in HE-loaded monocytes. HNE, a highly potent toxic aldehyde originating from lipoperoxidation of unsaturated fatty acids (see reference 13 for a review), accumulates in membranes and may inactivate other protein kinase-dependent processes. Preliminary experiments (35a) showing that low micromolar concentrations of HNE inhibited IFN-γ-mediated MHC class II expression and mimicked HE action seem to substantiate the involvement of PKC.
Quite recently, it has been shown that HE-fed, but not erythrocyte-fed, human monocytes produce high levels of IL-10 (27a). IL-10 seems to be important for the downregulation of the immune response, because it decreases expression of class II and leads to reduced proliferation of human T cells (11). However, other IL-10 effects (increased expression of CD14, CD64, and class I and an increase in phagocytic activity [44]) were not observed after HE phagocytosis. Thus, IL-10 may be only partially responsible for the HE effects reported here.
CD54 (ICAM-1), an adhesion molecule present on the surfaces of monocytes and other antigen-presenting cells, contributes considerably to the capacity of these cells to adhere and stimulate T-cell proliferation by reinforcing the signal from the T-cell receptor (4, 10). CD54 is very weakly expressed in nonstimulated monocytes and is upregulated upon maturation, as confirmed by present data. Our observation that HE-laden but not latex-bead-laden monocytes have reduced spontaneous upregulation of CD54 may help to explain the defective T-cell response in malaria.
CD11c, which belongs to the β2 family of integrins, is expressed mostly on monocytes and granulocytes but also on activated T and B cells (3). By using a CD8+ T-cell line which expressed a high level of CD11c, CD11c has been recently identified as the ligand for ICAM-1 in the rabbit (3). If these data can be confirmed for humans, the lack of spontaneous upregulation of CD11c in HE-laden monocytes may add to the defective response of ICAM-1 as a negative factor for the T-cell response in malaria.
In conclusion only expression of molecules that play essential roles in antigen presentation and the T-cell response, namely, MHC class II, CD54, and CD11c, appeared to be specifically hindered or blocked after HE phagocytosis. Thus, despite their phenomenological character, the present data may offer a way to identify a defective process (IFN-γ-responsive upregulation of critical molecules) and toxic mediators (HE and HNE) as possible causes of alterations of the immune response in human malaria.
ACKNOWLEDGMENTS
This work was supported by grants from WHO/UNDP/World Bank (TDR-Pathogenesis Programme, grant no. 940445), the Italian Ministry of University (MURST), and Compagnia di San Paolo, Torino, Italy.
REFERENCES
- 1.Aikawa M, Suzuki M, Gutierrez Y. Pathology of malaria. In: Kreier J P, editor. Malaria, pathology, vector studies, and culture. Vol. 2. New York, N.Y: Academic Press; 1980. pp. 47–102. [Google Scholar]
- 2.Alessio M, Ghigo D, Garbarino G, Geuna M, Malavasi F. Analysis of the human CD36 leucocyte differentiation antigen by means of the monoclonal antibody NL07. Cell Immuno. 1991;137:487–500. doi: 10.1016/0008-8749(91)90096-t. [DOI] [PubMed] [Google Scholar]
- 3.Blackford J, Reid H W, Pappin D J C, Bowers F S, Wilkinson J M. A monoclonal antibody 3/22 to rabbit CD11c which induces homotypic T cell aggregation. Evidence that ICAM-1 is a ligand for CD11c/CD18. Eur J Immunol. 1996;26:525–531. doi: 10.1002/eji.1830260304. [DOI] [PubMed] [Google Scholar]
- 4.Boyd A W, Wawryk S O, Burns G F, Fecondo J V. Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci USA. 1988;85:3095–3099. doi: 10.1073/pnas.85.9.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brasseur P, Agrapart M, Ballet J J, Druilhe P, Warrell M J, Tharavanij S. Impaired cell-mediated immunity in Plasmodium falciparum-infected patients with high parasitemia and cerebral malaria. Clin Immunol Immunopathol. 1983;27:38–50. doi: 10.1016/0090-1229(83)90054-5. [DOI] [PubMed] [Google Scholar]
- 6.Brémard C, Girerd J J, Kowalewski P, Merlin J C, Moreau S. Spectroscopic investigations of malaria pigment. Appl Spectrosc. 1993;47:1837–1842. [Google Scholar]
- 7.Bussolino F, Turrini F, Arese P. Measurement of phagocytosis utilizing 14C-cyanate-labelled human red cells and monocytes. Br J Haematol. 1987;66:271–274. doi: 10.1111/j.1365-2141.1987.tb01311.x. [DOI] [PubMed] [Google Scholar]
- 8.Clark H C, Tomlinson W J. The pathologic anatomy of malaria. In: Boyd M F, editor. Malariology. Vol. 2. Philadelphia, Pa: W. B. Saunders; 1949. pp. 874–903. [Google Scholar]
- 9.Corte G, Calabi F, Damiani G, Bargellesi A, Tosi R, Sorrentino R. Human Ia molecules carrying DC1 determinants differ in both alpha- and beta-subunits from Ia molecules carrying DR determinants. Nature. 1981;292:357–360. doi: 10.1038/292357a0. [DOI] [PubMed] [Google Scholar]
- 10.Dang L H, Michalek M T, Takei F, Benaceraff B, Rock K L. Role of ICAM-1 in antigen presentation demonstrated by ICAM-1 defective mutants. J Immunol. 1990;144:4082–4091. [PubMed] [Google Scholar]
- 11.De Waal Malefyt R, Haanen J, Spits H, Roncarolo M-G, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries J E. Interleukin 10 (Il-10) and viral Il-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II MHC expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelhorn S, Bruckner A, Remold H G. A soluble factor produced by inoculation of human monocytes with Leishmania donovani promastigotes suppresses IFN-γ-dependent monocyte activation. J Immunol. 1990;145:2662–2668. [PubMed] [Google Scholar]
- 13.Esterbauer H, Schaur R J, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 14.Fan X D, Goldberg M, Bloom B R. Interferon-γ-induced transcriptional activation is mediated by protein kinase C. Proc Natl Acad Sci USA. 1988;85:5122–5125. doi: 10.1073/pnas.85.14.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiori P L, Rappelli P, Mirkarimi S N, Ginsburg H, Cappuccinelli P, Turrini F. Reduced microbicidal and anti-tumor activities of human monocytes after ingestion of Plasmodium falciparum-infected red blood cells. Parasite Immunol. 1993;15:647–655. doi: 10.1111/j.1365-3024.1993.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 16.Garnham P C C. Plasmodium falciparum and Plasmodium Reichenowi. Plasmodium (Laverania) falciparum (Welch, 1897) In: Garnham P C C, editor. Malaria parasites and other haemosporidia. Oxford, England: Blackwell Scientific; 1966. pp. 357–429. [Google Scholar]
- 17.Gercken J, Pryjma J, Ernst M, Flad H-D. Defective antigen presentation by Mycobacterium tuberculosis-infected monocytes. Infect Immun. 1994;62:3472–3478. doi: 10.1128/iai.62.8.3472-3478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glimcher L H, Kara C J. Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg D E. Hemoglobin degradation in Plasmodium-infected red blood cells. Semin Cell Biol. 1993;4:355–361. doi: 10.1006/scel.1993.1042. [DOI] [PubMed] [Google Scholar]
- 20.Goldie P, Roth E F, Jr, Oppenheim J, Vanderberg J P. Biochemical characterization of Plasmodium falciparum hemozoin. Am J Trop Med Hyg. 1990;43:584–596. doi: 10.4269/ajtmh.1990.43.584. [DOI] [PubMed] [Google Scholar]
- 21.Greenwood B M, Bradley-Moore A M, Palit A, Bryceson A D M. Immunosuppression in children with malaria. Lancet. 1972;i:169–172. doi: 10.1016/s0140-6736(72)90569-7. [DOI] [PubMed] [Google Scholar]
- 22.Greenwood B M, Playfair J H L, Torrigiani G. Immunosuppression in murine malaria. I. General characteristics. Clin Exp Immunol. 1971;8:467–478. [PMC free article] [PubMed] [Google Scholar]
- 23.Ho M, Webster H K. Immunology of human malaria. A cellular perspective. Parasite Immunol. 1989;11:105–116. doi: 10.1111/j.1365-3024.1989.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 24.Janeway C A, Jr, Travers P. Immunobiology: the immune system in health and disease. 2nd ed. London, England: Current Biology Ltd.; 1996. [Google Scholar]
- 25.Loose L D, Cook J A, Di Luzio N R. Malarial immunosuppression—a macrophage mediated defect. Proc Helminthol Soc Wash. 1971;39:484–491. [Google Scholar]
- 26.Malavasi F, Funaro A, Bellone G, Caligaris-Cappio F, Berti E, Tetta C, Dellabona P, DeMaria S, Campogrande S, Cappa A P M. Functional and molecular characterization by the CB04 monoclonal antibody of a cell surface structure exerting C3-complement receptor activity. J Clin Immunol. 1985;5:412–420. doi: 10.1007/BF00915339. [DOI] [PubMed] [Google Scholar]
- 27.Metzger W G, Mordmüller B G, Kremsner P G. Malaria pigment in leucocytes. Trans R Soc Trop Med Hyg. 1995;89:637–638. doi: 10.1016/0035-9203(95)90423-9. [DOI] [PubMed] [Google Scholar]
- 27a.Mordmüller, B., F. Turrini, H. Long, P. G. Kremsner, and P. Arese. Submitted for publication. [PubMed]
- 28.Pearson A M. Scavenger receptors in innate immunity. Curr Opin Immunol. 1996;8:20–28. doi: 10.1016/s0952-7915(96)80100-2. [DOI] [PubMed] [Google Scholar]
- 29.Pichyangkul S, Saengkrai P, Webster H K. Plasmodium falciparum pigment induces monocytes to release high levels of tumor necrosis factor-α and interleukin-1β. Am J Trop Med Hyg. 1994;51:430–435. [PubMed] [Google Scholar]
- 30.Prada J, Malinowski J, Müller S, Bienzle U, Kremsner P G. Effects of Plasmodium vinckei hemozoin on the production of oxygen radicals and nitrogen oxides in murine macrophages. Am J Trop Med Hyg. 1996;54:620–624. doi: 10.4269/ajtmh.1996.54.620. [DOI] [PubMed] [Google Scholar]
- 31.Prada J, Malinowski J, Müller S, Bienzle U, Kremsner P G. Hemozoin differentially modulates the production of interleukin 6 and tumor necrosis factor in murine malaria. Eur Cytokine Netw. 1995;6:109–112. [PubMed] [Google Scholar]
- 32.Prina E, Jouanne C, de Souza Lão S, Szabo A, Guillet J-G, Antoine J-C. Antigen presentation capacity of murine macrophages infected with Leishmania amazonensis amastigotes. J Immunol. 1993;151:2050–2061. [PubMed] [Google Scholar]
- 33.Recalde H R. A simple method of obtaining monocytes in suspension. J Immunol Methods. 1984;69:71–77. doi: 10.1016/0022-1759(84)90278-3. [DOI] [PubMed] [Google Scholar]
- 34.Reiner N E. Altered cell signaling and mononuclear phagocyte deactivation during intracellular infection. Immunol Today. 1994;15:374–381. doi: 10.1016/0167-5699(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 35.Reiner N E, Ng W, McMaster W R. Parasite-accessory cell interactions in murine leishmaniasis. II. Leishmania donovani suppresses macrophage expression of class I and class II major histocompatibility complex gene products. J Immunol. 1987;138:1926–1932. [PubMed] [Google Scholar]
- 35a.Schwarzer, E. Unpublished observation.
- 36.Schwarzer E, Arese P. Phagocytosis of malarial pigment hemozoin inhibits NADPH-oxidase activity in human monocyte-derived macrophages. Biochim Biophys Acta. 1996;1316:169–175. doi: 10.1016/0925-4439(96)00021-x. [DOI] [PubMed] [Google Scholar]
- 37.Schwarzer E, Müller O, Arese P, Siems W G, Grune T. Increased levels of 4-hydroxynonenal in human monocytes fed with malarial pigment hemozoin. A possible clue for hemozoin toxicity. FEBS Lett. 1996;388:119–122. doi: 10.1016/0014-5793(96)00523-6. [DOI] [PubMed] [Google Scholar]
- 38.Schwarzer E, Turrini F, Arese P. A luminescence method for the quantitative determination of phagocytosis of erythrocytes, of malaria-parasitized erythrocytes and of malarial pigment. Br J Haematol. 1994;88:740–745. doi: 10.1111/j.1365-2141.1994.tb05112.x. [DOI] [PubMed] [Google Scholar]
- 39.Schwarzer E, Turrini F, Giribaldi G, Arese P. Phagocytosis of P. falciparum malarial pigment hemozoin by human monocytes inactivates monocyte protein kinase C. Biochim Biophys Acta. 1993;1181:51–54. doi: 10.1016/0925-4439(93)90089-j. [DOI] [PubMed] [Google Scholar]
- 40.Schwarzer E, Turrini F, Ulliers D, Giribaldi G, Ginsburg H, Arese P. Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J Exp Med. 1992;176:1033–1041. doi: 10.1084/jem.176.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherry B A, Alava G, Tracey K J, Martiney J, Cerami A, Slater A F G. Malaria-specific metabolite hemozoin mediates the release of several potent endogenous pyrogens (TNF, MIP-1α, and MIP-1β) in vitro, and altered thermoregulation in vivo. J Inflamm. 1995;45:85–96. [PubMed] [Google Scholar]
- 42.Slater A F G, Swiggard W J, Orton B R, Flitter W D, Goldberg D E, Cerami A, Henderson G B. An iron-carboxylate bond links the heme units of malaria pigment. Proc Natl Acad Sci USA. 1991;88:325–329. doi: 10.1073/pnas.88.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith M R, Ramsburg E A, Kung H-F, Durum S K. Components of the protein kinase C pathway induce Ia expression after injection into macrophages. J Immunol. 1992;149:1304–1310. [PubMed] [Google Scholar]
- 44.Spittler A, Schiller C, Willheim M, Tempfer C, Winkler S, Bolt-Nitulescu G. IL-10 augments CD23 expression on U937 cells and down-regulates IL-4-driven CD23 expression on cultured human blood monocytes: effects of IL-10 and other cytokines on cell phenotype and phagocytosis. Immunology. 1995;85:311–317. [PMC free article] [PubMed] [Google Scholar]
- 45.Taramelli D, Basilico N, Pagani E, Grande R, Monti D, Ghione M, Olliaro P. The heme moiety of malaria pigment (β-hematin) mediates the inhibition of nitric oxide and tumor necrosis factor-α production by lipopolysaccharide-stimulated macrophages. Exp Parasitol. 1995;81:501–511. doi: 10.1006/expr.1995.1143. [DOI] [PubMed] [Google Scholar]
- 46.Troye-Blomberg M, Perlmann P. T cell functions in Plasmodium falciparum and other malarias. Prog Allergy. 1988;41:253–287. doi: 10.1159/000415226. [DOI] [PubMed] [Google Scholar]
- 47.Turrini F, Ginsburg H, Bussolino F, Pescarmona G P, Serra M V, Arese P. Phagocytosis of Plasmodium falciparum-infected human red blood cells by human monocytes: involvement of immune and non-immune determinants and dependence on parasite developmental stage. Blood. 1991;80:801–808. [PubMed] [Google Scholar]
- 48.Warhurst D C. Haemozoin and the mode of action of blood schizonticides: more controversy. Parasitol Today. 1995;11:204–205. doi: 10.1016/0169-4758(95)80076-x. [DOI] [PubMed] [Google Scholar]
- 49.Warren H S, Weidanz W P. Malarial immunodepression in vitro: adherent spleen cells are functionally defective as accessory cells in the response to horse erythrocytes. Eur J Immunol. 1976;6:816–819. doi: 10.1002/eji.1830061112. [DOI] [PubMed] [Google Scholar]
- 50.Weidanz W P. Malaria and alterations in immune reactivity. Br Med Bull. 1982;38:167–172. doi: 10.1093/oxfordjournals.bmb.a071754. [DOI] [PubMed] [Google Scholar]
- 51.Williamson W A, Greenwood B M. Impairment of the immune response after acute malaria. Lancet. 1978;i:1328–1329. doi: 10.1016/s0140-6736(78)92403-0. [DOI] [PubMed] [Google Scholar]