Abstract
Three new species of CortinariussectionDelibuti, namely C.fibrillososalor, C.pseudosalor, and C.subtropicus are described as new to science based on morphological and phylogenetic evidences. Cortinariuspseudosalor is extremely morphologically similar to C.salor, but it differs from the latter by smaller coarsely verrucose basidiospores. Cortinariusfibrillososalor can be easily differentiated by its fibrillose pileus. The pileus of C.subtropicus becomes brown without lilac tint at maturity comparing with other members of section Delibuti. A combined dataset of ITS and LSU sequences was used for phylogenetic analysis. The phylogenetic reconstruction of section Delibuti revealed that these three new species clustered and formed independent lineages with full support respectively. A key to the three new species and related species of section Delibuti is provided in this work.
Key words: Morphology, new taxa, phylogeny, taxonomy
Introduction
The genus Cortinarius (Pers.) Gray (Cortinariaceae, Agaricales), which is known for its high species diversity, comprises more than 3000 taxa and exhibits a global distribution (Garnica et al. 2005; Willis 2018). However, the taxonomy of this genus faces an extremely complex challenge due to the overlapping morphological variation within species (Seidl 2000; Dima et al. 2021). Different classification systems of Cortinarius have been proposed by many taxonomists based on the comparison of the morphological characteristics, geographical distribution, ecological traits, chemical features, DNA barcode markers, or diverse combinations of the above through introducing the infrageneric concepts such as subgenus, section, or clade (Moser 1969; Moser and Horak 1975; Singer 1986; Bidaud et al. 1994; Brandrud 1998; Peintner et al. 2002; Garnica et al. 2003; Peintner et al. 2004; Garnica et al. 2005; Stefani et al. 2014; Garnica et al. 2016; Niskanen et al. 2016; Soop et al. 2019). Recently, according to the data of shallow whole genome sequencing and a five-locus analysis of 245 species, the genus Cortinarius was elevated to the Cortinariaceae rank, encompassing 10 genera, namely Aureonarius Niskanen & Liimat., Austrocortinarius Niskanen & Liimat., Calonarius Niskanen & Liimat., Cortinarius, Cystinarius Niskanen & Liimat., Hygronarius Niskanen & Liimat., Mystinarius Niskanen & Liimat., Phlegmacium (Fr.) Wünsche, Thaxterogaster Singer and Volvanarius Niskanen & Liimat. (Liimatainen et al. 2022).
Cortinariussect.Delibuti (Fr.) Sacc., typified by C.delibutus Fr., is widely distributed (Høiland and Holst-Jensen 2000; Peintner et al. 2004). section Delibuti species possess a viscid to glutinous pileus and glutinous cylindrical to clavate stipe, a duplex pileipellis with a gelatinous layer, subglobose and moderately warty basidiospores, basidiome in shades of bluish, yellow, brown, or green, lilac-blue lamellae while brown in the mature stage and a ring zone usually on the upper part of the stipe (Peintner et al. 2004; Garnica et al. 2005; Soop et al. 2019). As a comparatively old lineage, sect. Delibuti used to be placed in the myxacioid group or subgenus Myxacium (Fr.) Trog (Singer 1986; Brandrud et al. 1990, 1992; Seidl 2000; Garnica et al. 2005). In other views, sect. Delibuti was also placed in phlegmacioid group or subg. Phlegmacium, including subsections Delibuti and Anomali (Antonio and Aguirre 2004; Peintner et al. 2004). Based on four- locus (nrITS, nrLSU, rpb1, and rpb2) phylogenetic analysis, sect. Delibuti was placed within larger entity-Anomaloid sections, including sections Anomali, Bolares, Delibuti, Spilomei and Subtorti (Soop et al. 2019). More recently, sect. Delibuti was placed in Cortinariussubgen.Camphorati Liimat., Niskanen & Ammirati, encompassing sections Anomali, Bolares, Lilacinocinerei and Subtorti by Liimatainen et al. (2022).
The research on Cortinarius has mainly been conducted in Europe and North America, while it is still lacking in East Asia (Peintner et al. 2002; Garnica et al. 2003; Peintner et al. 2004; Garnica et al. 2005; Liimatainen et al. 2014; Stefani et al. 2014; Garnica et al. 2016; Niskanen et al. 2016; Soop et al. 2019, Liimatainen et al. 2022). To date, fewer than 30 species were originally reported from China, and only two new species in sect. Delibuti were originally found in China (Yang 1998; Wei and Yao 2013; Xie et al. 2019, 2020, 2021a, 2021b, 2022; Luo and Bau 2021, Zhang et al. 2023; Zhou et al. 2023). With the combination of morphological observations and phylogenetic analysis, we describe three species belonging to sect. Delibuti as new to science in this study.
Materials and methods
Specimens
The specimens were collected from central and southwestern China during 2012–2022. The vouchers are all deposited in the Mycological Herbarium of Hunan Normal University (MHHNU) and Cryptogamic Herbarium of Kunming Institute of Botany, Chinese Academy of Sciences (KUN-HKAS). Detailed information is listed in Table 1.
Table 1.
List of sequences of Cortinarius used for phylogenetic analyses. The sequences newly generated in this study are in bold, and all type specimens are highlighted with an asterisk.
| Species | Voucher | Locality | GenBank Accession No. | Reference | |
|---|---|---|---|---|---|
| ITS | LSU | ||||
| Cortinariusanomalus | TUB011883 | Europe, Germany | AY669645 | AY669645 | Garnica et al. (2005) |
| C.anomalus* | CFP1154 (S) | Europe, Ångermanland | KX302224 | – | Dima et al. (2016) |
| C.barlowensis* | JFA13140 | North America | FJ717554 | – | Harrower et al. (2011) |
| C.bolaris | T40 | Europe, Norway | KC842426 | KC842496 | Stensrud et al. (2014) |
| C.bolaris* | CFP1008 | Europe | KX302233 | – | Dima et al. (2016) |
| C.bolaris | TUB0118524 | Europe, Germany | AY669596 | AY669596 | Garnica et al. (2005) |
| C.calaisopus* | PDD 94050 | New Zealand, Dunedin | NR157880 | MH108373 | Genbank |
| C.calaisopus | PDD103678/CO2106 | New Zealand | KF727395 | KF727338 | Soop et al. (2019) |
| C.camphoratus | SMI193 | North America, Canada | FJ039626 | – | Harrower et al. (2011) |
| C.delibutus | F17048 | North America, Canada | FJ717515 | FJ717515 | Harrower et al. (2011) |
| C.delibutus | SAT01-301-12 | North America, USA | FJ717513 | – | Harrower et al. (2011) |
| C.dysodes | PDD70499/CO1038 HT | New Zealand | GU233340 | GU233394 | Soop et al. (2019) |
| C.ferrusinus* | JB8106 13 | Europe | KY657254 | – | Genbank |
| C.fibrillososalor * | MHHNU 32494 | East Asia, China, Hunan | OR647481 | OR647506 | This study |
| C.fibrillososalor | MHHNU 33520 | East Asia, China: Hunan | OR647485 | OR647507 | This study |
| C.fibrillososalor | MHHNU 33509 | East Asia, China, Hunan | OR647483 | – | This study |
| C.fibrillososalor | MHHNU 8657 | East Asia, China, Hunan | OR647355 | OR647497 | This study |
| C.fibrillososalor | MHHNU 32070 | East Asia, China, Hunan | OR660685 | OR647503 | This study |
| C.illibatus | HMJAU48760 | East Asia, China, Heilongjiang | MW911735 | – | Xie et al. (2021a) |
| C.illibatus | OS574 | Europe | KC842441 | KC842511 | Stensrud et al. (2014) |
| C.pseudocamphoratus* | HMJAU48694 | East Asia, China, Xizang | NR_176776 | – | Xie et al. (2022) |
| C.putorius | TN07411 HT | North America, USA | KR011124 | – | Ariyawansa et al. (2015) |
| C.rotundisporus | PDD96298/ JAC12057 | New Zealand | MH101550 | MH108389 | Soop et al. (2019) |
| C.rotundisporus | PERTH 05255074 | Australia | AY669612 | AY669612 | Garnica et al. (2005) |
| C.salor | TUB011838 | Europe, Germany | AY669592 | AY669592 | Garnica et al. (2005) |
| C.spilomeus* | S: CFP1137 | Europe | KX302267 | – | Dima et al. (2016) |
| C.spilomeus | TUB011523 | Europe | AY669654 | AY669654 | Garnica et al. (2005) |
| C.pseudosalor | MHHNU 8349 | East Asia, China, Hunan | OR647352 | – | This study |
| C.pseudosalor * | MHHNU 32082 | East Asia, China, Hubiei | OR660686 | OR647504 | This study |
| C.pseudosalor | MHHNU 32148 | East Asia, China, Hubiei | OR660688 | OR647505 | This study |
| C.subsalor | HMJAU48758 | East Asia, China, Zhejiang | MW911733 | – | Xie et al. (2021a) |
| C.subsalor* | HMJAU48759 | East Asia, China, Zhejiang | MW911734 | – | Xie et al. (2021a) |
| C.subtortus | F16111 | North America | FJ157044 | FJ157044 | Harrower et al. (2011) |
| C.subtortus | TUB011382 | Europe | AY174857 | AY174857 | Garnica et al. (2003) |
| C.subtropicus | MHHNU 31954 | East Asia, China, Hunan | OR647356 | OR647498 | This study |
| C.subtropicus | KUN-HKAS 75760 | East Asia, China, Guangxi | OR647491 | OR647509 | This study |
| C.subtropicus | MHHNU 31964 | East Asia, China, Hunan | OR660684 | OR647501 | This study |
| C.subtropicus | MHHNU 31981 | East Asia, China, Hunan | OR660687 | OR647502 | This study |
| C.subtropicus * | MHHNU 33533 | East Asia, China, Hunan | OR647488 | OR647508 | This study |
| C.tasmacamphoratus | HO A20606A0 | Australia, Tasmania | AY669633 | AY669633 | Garnica et al. (2005) |
| C.tessiae | PDD107517/CO1450 | New Zealand | MG019356 | MG019356 | Soop et al. (2019) |
| C.tessiae | PDD72611 | New Zealand | HM060317 | HM060316 | Genbank |
| C.tetonensis* | JFA10350 | North America | MZ580436 | – | Dima et al. (2016) |
| C.tibeticisalor* | HMJAU48764 | East Asia, China, Xizang | MW911730 | – | Xie et al. (2021a) |
| C.tibeticisalor | HMJAU48762 | East Asia, China: Xizang | MW911731 | – | Xie et al. (2021a) |
| C.tibeticisalor | HMJAU48763 | East Asia, China, Xizang | MW911732 | – | Xie et al. (2021a) |
| C.viridipileatus | OTA61977 | New Zealand | MK546592 | MK546595 | Nilsen et al. (2021) |
| C.viridipileatus | OTA64087 | New Zealand | MK546593 | MK546596 | Nilsen et al. (2021) |
Morphological observation
The descriptions of macromorphological characters were based on field records and photographs. Color codes were used following Kornerup and Wanscher (1978). The size of basidiomes, as determined by pileus width, was described as small (< 5.0 cm), medium-sized (5.0–9.0 cm) or large (> 9.0 cm). Microscopic features were observed from dried specimens that were mounted with 5% aqueous KOH and stained with 1% Congo red solution under a light microscope (Motic Ltd., China). Melzer’s reagent was used as an indicator of the amyloidity of basidiospores. In the description of basidiospores, the abbreviation [n/m/p] represents that the measurements were made on n basidiospores from m basidiomes of p collections. At least twenty matured basidiospores and basidia from each of the basidiomes were measured. The range (a)b–c(d) stands for the dimensions of basidiospores in which b–c contains a minimum of 90% of the measured values, while a and d indicate the extreme values. In addition, a Q value shows the ratio of length to width of basidiospores, and a Qm value shows the average Q ± standard deviation. A JSM-6380LV scanning electron microscope (JEOL Ltd., Tokyo, Japan) was used for the observation of ornamentations of basidiospores.
DNA extraction, PCR amplification and sequencing
Total genomic DNA was extracted by a Fungal DNA Mini Kit (Omega, USA). ITS 4 and ITS 5 (White et al. 1990), LROR and LR5/LR7 (Vilgalys and Hester 1990), were used for amplification of internal transcribed spacer (ITS), nuclear ribosomal large subunit (nrLSU), respectively. Each PCR mixture contained 1× PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.4 μM of each primer, 1.25 U of Taq polymerase, and 1–2 μl DNA template in a total volume of 25 μl. PCRs were performed with an Eppendorf Mastercycler thermal cycler (Eppendorf Inc., Germany) as follows: initial denaturation at 94 °C for 4 min (ITS; nrLSU; tef1-α); followed by 30–35 cycles of 94 °C for 30 s (ITS; nrLSU), 54 °C for 30 s (ITS) or 55 °C for 1 min (nrLSU), and 72 °C for 30 s (ITS), 1 min (nrLSU); and a final extension at 72 °C for 7–10 min. Amplified PCR products were detected by gel electrophoresis on 2% agarose gels and then sent to Tsingke Biological Technology (China) for sequencing.
Phylogenetic analyses
The sequences newly generated in this study and downloaded from GenBank were used for phylogenetic analysis (Table 1). Alignment was performed by MAFFT v7.149b (Katoh and Standley 2013) and adjusted manually by MEGA5 (Tamura et al. 2011). SequenceMatrix 1.7.8 (Vaidya et al. 2011) was applied to generate multigene matrixes. GTR+I+G was selected as the best-fit model for combined matrix based on the Akaike Information Criterion (AIC) by MrModeltest 2.3 (Nylander 2004). Maximum likelihood (ML) analysis was performed using the W-IQ-TREE web service (http://iqtree.cibiv.univie.ac.at/) with 1000 ultrafast bootstrap replicates (Trifinopoulos et al. 2016). Bayesian inference (BI) was performed in MrBayes v3.2 (Ronquist et al. 2012). Four Metropolis-coupled Monte Carlo Markov chains were run for 5000000 generations, sampling every 1000th generation. Subsequently, the sampled trees were summarized after omitting the first 25% of trees as burn-in.
Results
Phylogenetic analyses
In the concatenated dataset (ITS+LSU), a total of 78 sequences (48 ITS, 30 LSU) from 48 samples were used for phylogenetic analyses among sect. Delibuti, sect. Subtorti, sect. Camphorati, sect. Bolares, sect. Spilomei, and sect. Anomali, of which 24 sequences (13 ITS, 11 LSU) were newly yielded in this study (Table 1). The estimates of tree topology inferred from ML and Bayesian analyses were extremely similar. The ML phylogenetic tree is shown with both bootstrap values (BP) and posterior probabilities (PP) annotated near the nodes (Fig. 1).
Figure 1.
Phylogenetic tree of Cortinariussect.Delibuti inferred from a combined matrix of ITS and LSU through maximum likelihood and Bayesian inference. Bayesian posterior probabilities (PP) > 0.90 and bootstrap values (BP) >85% are reported at the nodes (PP/BP); “–” indicates that the support value was less than the respective threshold. The three newly described species are highlighted in bold. Aus: Australia; EA: East Asia; Eur: Europe; NAm: North America; NZ: New Zealand.
The phylogenetic relationship of sections within the genus Cortinarius in the present study was unclear and weakly supported. In the multi-locus tree, the monophyly of sect. Delibuti was supported with well-supported values (BP = 99%, PP = 1.00), including 12 species. section Camphorati was also monophyletic with fully supported values (BP = 100%, PP = 1.00), emcompassing 5 species. In sect. Delibuti, C.delibutus, C.illibatus, C.salor, C.subsalor, C.tibeticisalor, and three novel species, namely C.fibrillososalor, C.pseudosalor, and C.subtropicus, formed a monophyletic lineage (BP = 95%, PP = 1.00). Cortinariusfibrillososalor, C.subtropicus, and C.tibeticisalor formed a clade only be found in East Asia (BP = 99%, PP = 1.00), while C.tibeticisalor has a special olive-green tint, was only distributed in Tibetan Plateau (Xie et al. 2021a). However, C.pseudosalor, C.salor and C.subsalor clustered with low support values, leaving the position not determined. In addition, 13 specimens from C.pseudosalor, C.fibrillososalor and C.subtropicus collected in this study were fully supported (BP = 100%, PP = 1.00), and the phylogenetic relationships of C.fibrillososalor, C.tibeticisalor and C.subtropicus were clarified (BP = 100%, PP = 1.00).
Taxonomy
. Cortinarius fibrillososalor
P. Long & Z.H. Chen sp. nov.
BB04344F-3F7F-5092-BE9B-7E02A8DADC6E
850393
Figure 2.
Basidiomes of Cortinariusfibrillososalor (a, bMHHNU 32494 c, dMHHNU 8657).
Figure 3.
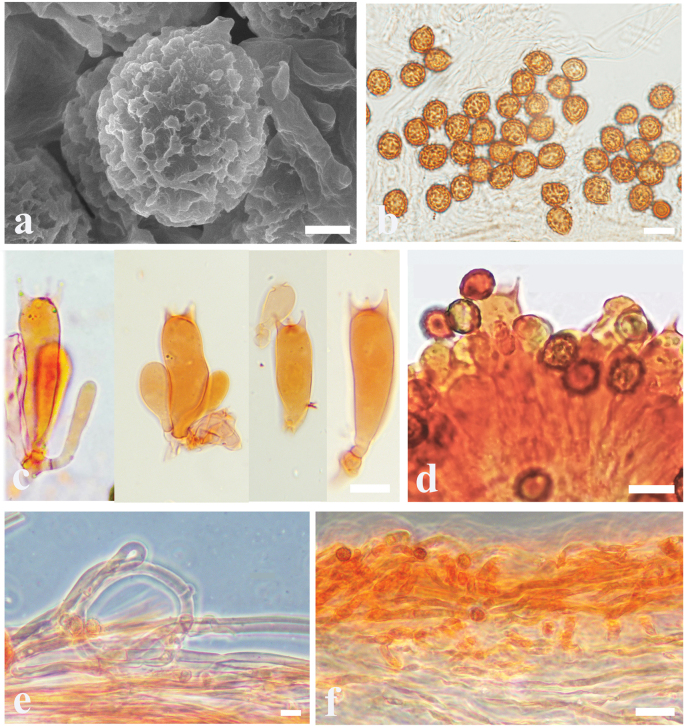
Microscopic features of Cortinariusfibrillososalor (MHHNU 32494) a scanning electron micrograph of basidiospore b basidiospores c lamellae edge d basidia with probasidium e stipitipellis f pileipellis; Scale bars: 1 μm (a); 10 μm (b–e); 20 μm (f).
Etymology.
Fibrillososalor (Latin) refers to the species morphologically similar to Cortinariussalor, but with fibrils on the pileus.
Holotype.
China, Hunan Province: Sangzhi County, Badagongshan National Nature Reserve, at 29.782541°N, 110.084472°E, alt. 1424 m, 8 September 2020, Z.H. Chen, P. Long and S.N. Li, (MHHNU 32494).
Diagnosis.
Differs from the other species of sect. Delibuti from its fibrillose pileus.
Description.
Basidiomes small to medium-sized, telamonia-like, development type stipiocarpic. Pileus 2.9–5.2 cm, at first broadly convex, then lower convex to plane, broadly umbonate at the centre, margin incurved or decurved to upturned; at first violaceous (17B6–17B8), tinged brown (5B4–5C6) at the centre then becoming whitish mauve (16A1–16A2), finely fibrillose, with brown (5A5–5C7) universal veil remains at margin; surface silky when dry or glutinous when wet. Context thin, creamy white, soft, beige (3A1–A2) when bruised. Lamellae adnate to adnexed, lilac (17A2–17B2) to brownish (6C5–6D7), moderately distant, sometimes margin wavy. Stipe cylindrical to clavate, bend, gradually slender to the apex, 3.4–5.9 cm long, 0.4–0.8 cm wide, violaceous (17A4–17B5) when young then fading to whitish mauve (16A2–16A3) tint, leaving an ochraceous (5B6–5D8) ringon the upper stem, hollow. Odour indistinct.
Basidiospores [100/5/5] (6.5–) 7.0–8.8 (–9.2) × (5.0–) 5.9–7.2 (–8.1) μm, av. 8.1 × 6.5 μm, Q = 1.14 (1.16) – 1.31 (1.45), Qm = 1.24 ± 0.02, broadly globose to long ellipsoid, rarely subglobose, yellowish brown, moderately verrucose, without amyloid and dextrinoid reaction. Basidia (27–) 28–35 × (8–) 9–11 μm, 4-spored, sterigmata up to 2.4–3.7 μm, clavate to subcylindrical, colourless or with amber yellow oily inclusions or granules. Pileipellis duplex, hyphae 4–8 μm wide, epicutis strongly gelatinous, 68–128 μm thick, composed of colourless or amber yellow, irregularly arranged and strongly interwoven hyphae, hypocuits 25–38 μm thick, composed of colourless or amber yellow, nearly parallel cylindrical hyphae. Lamellar edges fertile. Cystidia absent. Lamellar trama regular, 40–80 μm thick, composed of parallel arranged hyphae, hyphae 3–6 μm wide. Stipitipellis gelatinous, stipe hyphae 3–6 μm wide, thin-walled, cylindrical, interwoven. Clamp connections present in all tissues.
Habitat, ecology and distribution.
Solitary to gregarious on soil in evergreen broad-leaved forest, known from Hunan, China; July to September.
Additional specimens examined.
China, Hunan Province: Sangzhi County, Badagongshan National Nature Reserve, at 29.769154°N, 110.086577°E, alt. 1405 m, 31 July 2020, Z.H. Chen, P. Long and S.N. Li, (MHHNU 32070). China, Hunan Province: Sangzhi County, Badagongshan National Nature Reserve, at 29.769154°N, 110.477086°E, alt.1482 m, 28 July 2022, Z.H. Chen, J. Wen and Z.J. Jiang (MHHNU 33509, MHHNU 33520). China, Hunan Province: Sangzhi County, Badagongshan National Nature Reserve, at 29.404913°N, 109.491158°E, alt. 1500 m, 10 September 2015, P. Zhang, (MHHNU 8657).
Notes.
Cortinariusfibrillososalor can be differentiated from other species of section Delibuti for its fibrillose pileus, usually under evergreen broad-leaved forest at 1405–1500m. In addition, basidiospores broadly globose to long ellipsoid, rarely subglobose while other members in this section usually subglobose to broadly ellipsoid.
. Cortinarius pseudosalor
P. Long & Z.H. Chen sp. nov.
946C3CE9-E812-5383-85AD-BF278962EE20
850392
Figure 4.
Basidiomes of Cortinariuspseudosalor (a, bMHHNU 32082 c, dMHHNU 8349).
Figure 5.
Microscopic features of Cortinariuspseudosalor (MHHNU 32082) a scanning electron micrograph of basidiospore b basidiospores c basidia with probasidium d lamellae edge e stipitipellis f pileipellis. Scale bars: 1 μm (a); 10 μm (b–e); 20 μm (f).
Etymology.
Pseudosalor (Latin) refers to the species morphologically similar to Cortinariussalor.
Holotype.
China, Hubei Province: Hefeng County, Mulinzi National Nature Reserve, at 30.058935°N, 110.209541°E, alt.1413 m, 1 August 2020, Z.H. Chen, P. Long and S.N. Li, (MHHNU 32082).
Diagnosis.
This species differs from other species in sect. Delibuti for its high morphological similarity with C.salor, but having smaller coarsely verrucose basidiospores.
Description.
Basidiomes small to medium-sized, development type stipiocarpic. Pileus 2.8–6.5 cm, at first broadly convex, then lower convex to plane, margin incurved when young, decurved to upturned at maturity; bluish violaceous (18A3–18C5) when young, tinge of white at the centre when chapped, later fading to ochraceous grey (5B6–5C7) when old with brown (5B8–5C8) universal veil remains at margin; dry, viscid. Context dirty white, soft. Lamellae adnexed, pale yellow (1A2) with lilac tint (16A1–16A2) then brownish (5B6–5D7), moderately distant, sometimes margin wavy. Stipe clavate, gradually slender to the apex, 4–8.4 cm long, 0.4–1.0 cm wide, violaceous (16A2–16A4) when young then fading to upper dirty white, whitish mauve (16A2) at base, leaving an ochraceous ring (5B8–5C8) on the upper stem, hollow in centre. Odour indistinct.
Basidiospores [60/3/3] (7.3–) 7.4–8.4 × (5.7–) 6.0–7.4 (–7.5) μm, av. 7.9 × 6.7 μm, Q = (1.11) 1.12– (1.26) 1.27, Qm = 1.18 ± 0.11, subglobose to broadly ellipsoid, yellowish brown, coarsely verrucose, without amyloid and dextrinoid reaction. Basidia (29–) 30–38 × (8–) 9–12 μm, 4-spored, sterigmata up to 3.7–5.0 μm, clavate to subcylindrical, colourless or with amber yellow granules. Pileipellis duplex obviously, hyphae 2–6 μm wide, epicutis gelatinous, 50–75 μm thick, composed of colourless or amber yellow, moderately interwoven hyphae, hypocuits 50–75 μm thick, composed of colourless or amber yellow, hyphae nearly parallel cylindrical. Lamellar edges fertile. Cystidia absent. Lamellar trama regular, 45–55 μm thick, composed of hyphae and inflated cells, hyphae 2–5 μm wide, inflated cells 14–24 × 5–9 μm. Stipitipellis gelatinous, stipe hyphae 2–7 μm wide, thin-walled, cylindrical, weakly interwoven. Clamp connections present in all tissues.
Habitat, ecology and distribution.
Solitary to gregarious on soil in coniferous and broad-leaved mixed forest or evergreen broad-leaved forest, known from Hunan and Hubei, China; August.
Additional specimens examined.
China, Hunan Province: Yongshun County, Xiaoxi National Nature Reserve, at 28.4215–28.5355°N, 110.650–110.2135°E, alt. 1000–1300 m, 30 August 2014, P. Zhang, (MHHNU 8349); Hubei Province: Hefeng County, Xiaping Town, at 30.046382°N, 110.136712°E, alt. 1223 m, 2 August 2020, Z.H. Chen, P. Long and S.N. Li, (MHHNU 32148).
Notes.
Cortinariuspseudosalor is easily misidentified as C.salor for their high morphological similarity, except the former has smaller coarsely verrucose basidiospores. Besides, C.pseudosalor distributed in Central China under coniferous and broad-leaved mixed forest or evergreen broad-leaved forest at alt. 1000–1413 m.
. Cortinarius subtropicus
P. Long & Z.H. Chen sp. nov.
D523DD7B-2882-534F-A4D3-5E6ACA35006B
850394
Figure 6.
Basidiomes of Cortinariussubtropicus (a, bMHHNU 33533 c, dMHHNU 31964).
Figure 7.
Microscopic features of Cortinariussubtropicus (MHHNU 33533) a scanning electron micrograph of basidiospores b basidiospores c basidia with probasidium d lamellae edge e stipitipellis f pileipellis. Scale bars: 1 μm (a); 10 μm (b–e); 20 μm (f).
Etymology.
Subtropicus (Latin) refers to subtropical distribution range of the species.
Holotype.
China, Hunan Province: Sangzhi County, Badagongshan National Nature Reserve, at 29.050057°N, 110.477119°E, alt. 1642 m, 29 July 2022, Z.H. Chen, J. Wen and Z.J. Jiang, (MHHNU 33533).
Diagnosis.
Differs from the other species of sect. Delibuti species in having an epicutis pileipellis that can be easily separated from the context of the pileus.
Description.
Basidiomes small, development type stipiocarpic. Pileus 2.1–4.6 cm, at first broadly convex, then lower convex to plane, broadly umbonate at the centre, margin incurved; at first violaceous (15A4–15B7), tinged brown (6A5–6C7) at the centre then becoming orange brown (5B2–5B6), brown (5A4–5B6) universal veil remains at margin; surface smooth when dry or glutinous when wet, pileipellis is easy to separate. Context thin, creamy white, soft, beige (3A1–A2) when bruised. Lamellae adnate, bluish violet (18A2–18B2) with pale greyish (18B1) to brownish (5A4–5B7), rust brown (5C7) when dry, moderately distant. Stipe cylindrical to weakly clavate, bend, gradually slender to the apex, 6.4–7.2 cm long, 0.5–1.0 cm wide, lilac (15A2–15B2) when young, dirty white at maturity, leaving an ochraceous (5D7) to orange (5B6) ring on the upper stem, hollow in centre, crumbly. Odour indistinct.
Basidiospores [120/6/6] (6.6–) 7.0–9.1 (–10.3) × (5.9–)6.1–7.9 (– 10.3) μm, av. 7.8 × 6.4 μm, Q = 1.10–1.38 (1.41), Qm = 1.24 ± 0.01, subglobose to ellipsoid, yellowish brown, moderately verrucose, without amyloid and dextrinoid reaction. Basidia 32–48 × 9–12 μm, 4-spored, sterigmata 2.8–4.9 μm, clavate to subcylindrical, colourless or with amber yellow oily inclusions. Pileipellis duplex, hyphae 4–8 μm wide, epicutis gelatinous, 30–40 μm thick, composed of colourless or amber yellow, irregularly arranged and moderately interwoven hyphae, hypocuits 130–200 μm thick, composed of colourless or amber yellow, nearly parallel cylindrical hyphae. Lamellar edges fertile. Cystidia absent. Lamellar trama regular, 40–80 μm thick, composed of parallel arranged hyphae, hyphae 3–6 μm wide. Stipitipellis gelatinous, stipe hyphae 3–6 μm wide, thin-walled, cylindrical, subparallel arranged. Clamp connections present in all tissues of the basidiome.
Habitat, ecology and distribution.
Solitary to gregarious on soil in under evergreen broad-leaved forest, on the ground, known from Hunan, China; July.
Additional specimens examined.
China, Hunan Province: Sangzhi County, Badagongshan National Nature Reserve, at 29.683144°N, 109.754104°E, alt. 1645 m, 27 July 2020, Z.H. Chen, P. Long and S.N. Li, (MHHNU 31954). China, Hunan Province: Sangzhi County, Badagongshan National Nature Reserve, at 29.6767°N, 109.750696°E, alt. 1625 m, 28 July 2020, Z.H. Chen, P. Long and S.N. Li, (MHHNU 31964). China, Hunan Province: Sangzhi County, Badagongshan National Nature Reserve, at 29.676642°N, 109.750674°E, alt. 1625 m, 28 July 2020, Z.H. Chen, P. Long and S.N. Li, (MHHNU 31981). China, Guangxi Province: Xingan County, Maoershan National Nature Reserve, alt. 1900 m, 24 July 2012, X.B. Liu, (KUN-HKAS 75760).
Notes.
Cortinariussubtropicus has an epicutis pileipellis that can be easily separated from the context of the pileus. Besides, pileus become brown without lilac or dark olive tint at maturity comparing with other members of section Delibuti.
A key to species of Cortinariussect.Delibuti
| 1 | Only distributed in the Northern Hemisphere | 2 |
| – | Only distributed in the Southern Hemisphere or distributed both in Northern and Southern Hemisphere | 12 |
| 2 | Pileus usually ochraceous yellow without bluish hue | 3 |
| – | Pileus usually bluish violet, sometimes yellow brown | 4 |
| 3 | Lamellae usually bluish violet at first, veil yellow to pale brown | C.delibutus |
| – | Lamellae pale ochraceous with tinge of pinkish violet, veil not yellowish | C.illibatus |
| 4 | Distributed in Europe ± North America | 5 |
| – | Distributed in China, East Asia | 8 |
| 5 | Growing under coniferous trees and broadleaved trees; pileus bluish violet | 6 |
| – | Only growing under coniferous trees (Abies and Picea); pileus usually orange | C.largodelibutus |
| 6 | Basidiomes small to medium-sized, pileus deep bluish violet to ochraceous; Veil violet to ochraceous | 7 |
| – | Basidiomes small, pileus yellow to olive-ochre at the centre, greyish blue to violet at margin then fading quickly; veil yellow | C.betulinus |
| 7 | Pileus usually olive brown; growing under coniferous trees (Picea) and broadleaved trees (Betula) | C.transiens |
| – | Pileus usually deep bluish violet; growing under broadleaved trees (Quercus, Fagus, Corylus) | C.salor |
| 8 | Pileus usually dark olivaceous to brown at maturity; distributed in Tibetan Plateau of China | C.tibeticisalor |
| – | Pileus usually ochraceous yellow at maturity; distributed in Central China ± Eastern China | 9 |
| 9 | Pileus with fibrils, basidiospores broadly globose to long ellipsoid, rarely subglobose | C.fibrillososalor |
| – | Pileus without fibrils, basidiospores subglobose to broadly ellipsoid | 10 |
| 10 | Basidiospores average length >8 μm | C.subsalor |
| – | Basidiospores average length <8 μm | 11 |
| 11 | Pileipellis is easy to separate; epicutis of pileipellis less than 40 μm thick, distributed from 1625 m to 1900 m | C.subtropicus |
| – | Epicutis of pileipellis more than 50 μm thick, distributed from 1000 m to 1413 m | C.pseudosalor |
| 12 | Growing under trees of Nothofagus | 13 |
| – | Growing under trees of Myrtaceae | 15 |
| 13 | Pileus glutinous, greyish yellow to greyish orange, stipe violet, then becoming white to pale brownish, basidiospores ellipsoid, distributed in Northern and Southern Hemisphere | C.illitus |
| – | Pileus viscid, with a green hue; basidiospores subglobose, distributed in Southern Hemisphere | 14 |
| 14 | Pileus blue‒green to aerugineous; stipe blue green; distributed in Australasia | C.tessiae |
| – | Pileus dark green; stipe white with a purple hue; distributed in New Zealand | C.viridipileatus |
| 15 | Basidiomes distinctly viscid to glutinous, stipe viscid, mainly greyish blue-green | C.rotundisporus |
| – | Basidiomes weakly viscid, stipe often dry, mainly yellow-green to olive | C.calaisopus |
Discussion
In this study, three species of Cortinariussect.Delibuti, namely C.fibrillososalor, C.pseudosalor, and C.subtropicus, were described as new to science based on phylogenetic analyses and morphological characteristics. The phylogenetic relationships of C.fibrillososalor and C.subtropicus in C.sect.Delibuti were resolved with close phylogenetic relationship with C.tibeticisalor. However, the phylogenetic position of C.pseudosalor is still unclear as no supported sister relationship was revealed in the phylogenetic analysis.
Cortinariusfibrillososalor, C.pseudosalor, C.salor, C.subsalor, C.subtropicus and C.tibeticisalor have morphological homogeneity of the basidiomes. The macromorphological characters of C.pseudosalor, and C.salor are similar to basidiomes, coloured bluish violet, while C.subsalor is coloured purple to purplish red in pileus centre. Besides, C.pseudosalor has smaller coarsely verrucose basidiospores comparing C.salor and C.subsalor (Kibby and Tortelli 2021; Xie et al. 2021a). Meanwhile, C.salor is characterized by its lilaceous lamellae all the time and the narrow distribution in European woodlands, while C.pseudosalor and C.subsalor occurs in Asia (Xie et al. 2021a). Cortinariusfibrillososalor with violaceous to whitish mauve tint differ from other species in this section in the appearance of fibrils on the pileus and its broadly globose to long ellipsoid basidiospores (Kibby and Tortelli 2021; Xie et al. 2021a). Cortinariussubtropicus was found in the subtropical monsoon climate region of the Hunan and Guangxi provinces distributed from 1625 m to 1900 m. Cortinariustibeticisalor was only distributed in Tibetan Plateau and was usually olivaceous to brown at maturity, while olivaceous species in sect. Delibuti mainly occurred in the South Pacific (Soop et al. 2019; Xie et al. 2021a).
Phylogenetic analysis was first applied to the taxonomic study of Cortinarius with ITS (Liu et al. 1997). Later, phylogenetic relationships were inferred mainly based on ITS, LSU sequences, and rpb1, rpb2 were also confirmed to help elucidate the relationships of species in Cortinarius (Frøslev et al. 2005; Soop et al. 2019; Xie et al. 2022). Species delimitation could be justified by the combination of ITS and LSU sequences (Nilsen et al. 2021; Zhou et al. 2023), a two-locus dataset (ITS and LSU) was used for the research of three new species and their similar species in the present study. However, it needs more sequence data and DNA markers for recognising higher taxonomic rank such as subgenus or genus. In section rank, a two-locus dataset (ITS, LSU) and four-locus dataset (ITS, LSU, rpb1 and rpb2) were first employed for phylogenetic analyses, and the latter provided a higher BP value and clearer tree structure, although with limited rpb1 and rpb2 (Soop et al. 2019). Besides, the first phylogenomic study based on shallow whole genome sequencing was conducted for Cortinariaceae revision (Liimatainen et al. 2022).
Supplementary Material
Acknowledgements
We thank Professor Ping Zhang, Master Zi-Juan Jiang and Jing Wen (Hunan Normal University) and Dr. Xiao-Bin Liu (Kunming Institute of Botany, Chinese Academy of Sciences) for specimen collection. We also thank Prof. Zhu-Liang Yang (Kunming Institute of Botany, Chinese Academy of Sciences), Dr. Zheng-Mi He and Yu-Ting Su (Hunan Normal University) for improving the manuscript.
Citation
Long P, Zhou S-Y, Li S-N, Liu F-F, Chen Z-H (2024) Three new species of Cortinarius section Delibuti (Cortinariaceae, Agaricales) from China. MycoKeys 101: 143–162. https://doi.org/10.3897/mycokeys.101.114705
Funding Statement
This study was financially supported by the National Science Foundation of China (Grant No. 32170016) and the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment, China (Grant No. 2019HJ2096001006).
Additional information
Conflict of interest
The authors have declared that no competing interests exist.
Ethical statement
No ethical statement was reported.
Funding
This study was financially supported by the National Science Foundation of China (Grant No. 32170016) and the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment, China (Grant No. 2019HJ2096001006).
Author contributions
Conceptualization: Zuo-Hong Chen and Pan Long; methodology: Fei-Fei Liu and Pan Long; performing the experiment: Pan Long; resources: Zuo-Hong Chen, Pan Long, Sai-Nan Li, and Song-Yan Zhou; writing – original draft preparation: Pan Long; writing – review and editing: Zuo-Hong Chen and Song-Yan Zhou; supervision: Zuo-Hong Chen; project administration: Zuo-Hong Chen; funding acquisition: Zuo-Hong Chen. All authors have read and agreed to the published version of the manuscript.
Data availability
All of the data that support the findings of this study are available in the main text or Supplementary Information. The sequence data generated in this study are deposited in NCBI GenBank.
Supplementary materials
Multiple sequence alignment
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Pan Long, Song-Yan Zhou, Sai-Nan Li, Fei-Fei Liu, Zuo-Hong Chen
Data type
fas
References
- Antonio J, Aguirre C. (2004) Fungi Non Delineati Vol. 29. Edizioni Candusso, Italy, 3.
- Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B, Chethana KWT, Dai DQ, Dai YC, Daranagama DA, Jayawardena RS, Lücking R, Ghobad-Nejhad M, Niskanen T, Thambugala KM, Voigt K, Zhao RL, Li GJ, Doilom M, Boonmee S, Yang ZL, Cai Q, Cui YY, Bahkali AH, Chen J, Cui BK, Chen JJ, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, Hashimoto A, Hongsanan S, Jones EBJ, Larsson E, Li WJ, Li QR, Liu JK, Luo ZL, Maharachchikumbura SSN, Mapook A, McKenzie EHC, Norphanphoun C, Konta S, Pang KL, Perera RH, Phookamsak R, Phukhamsakda C, Pinruan U, Randrianjohany E, Singtripop C, Tanaka K, Tian CM, Tibpromma S, Abdel-Wahab MA, Wanasinghe DN, Wijayawardene NN, Zhang JF, Zhang H, Abdel-Aziz FA, Wedin M, Westberg M, Ammirati JF, Bulgakov TS, Lima DX, Callaghan TM, Callac P, Chang CH, Coca LF, Dal-Forno M, Dollhofer V, Fliegerová K, Greiner K, Griffith GW, Ho HM, Hofstetter V, Jeewon R, Kang JC, Wen TC, Kirk PM, Kytövuori I, Lawrey JD, Xing J, Li H, Liu ZY, Liu XZ, Liimatainen K, Lumbsch HT, Matsumura M, Moncada B, Nuankaew S, Parnmen S, Santiago ALCMA, Sommai S, Song Y, Souza CAF, Souza-Motta CM, Su HY, Suetrong S, Wang Y, Wei SF, Wen TC, Yuan HS, Zhou LW, Réblová M, Fournier J, Camporesi E, Luangsa-ard JJ, Tasanathai K, Khonsanit A, Thanakitpipattana D, Somrithipol S, Diederich P, Millanes AM, Common RS, Stadler M, Yan JY, Li XH, Lee HW, Nguyen TTT, Lee HB, Battistin E, Marsico O, Vizzini A, Vila J, Ercole E, Eberhardt U, Simonini G, Wen HA, Chen XH, Miettinen O, Spirin V. (2015) Fungal diversity notes 111–252-taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 75(1): 27. 10.1007/s13225-015-0346-5 [DOI] [Google Scholar]
- Bidaud A, Moënne-Loccoz P, Reumaux P. (1994) Atlas des Cortinaires, Clé générale des sous-genres, sections, sous-sections et séries. Èditions Fèdération mycologique Dauphiné-Savoie, France, 121–198, 222–263.
- Brandrud TE. (1998) CortinariussubgenusPhlegmaciumsectionPhlegmacioides (= Variecolores) in Europe. Edinburgh Journal of Botany 55(1): 65–156. 10.1017/S0960428600004364 [DOI] [Google Scholar]
- Brandrud TE, Lindstrom H, Marklund H, Melot J, Muskos S. (1990) Cortinarius Flora Photographica I (English version). Cortinarius HB, Matfors, 60 pp. [Google Scholar]
- Brandrud TE, Lindstrom H, Marklund H, Melot J, Muskos S. (1992) Cortinarius Flora Photographica II (English version). Cortinarius HB, Matfors, 60 pp. [Google Scholar]
- Dima B, Lindström H, Liimatainen K, Olson Å, Soop K, Kytövuori I, Dahlberg A, Niskanen T. (2016) Typification of Friesian names in Cortinarius sections Anomali, Spilomei, and Bolares, and description of two new species from northern Europe. Mycological Progress 15(9): 903–919. 10.1007/s11557-016-1217-5 [DOI] [Google Scholar]
- Dima B, Liimatainen K, Niskanen T, Bojantchev D, Harrower E, Papp V, Nagy LG, Kovács GM, Ammirati JF. (2021) Type studies and fourteen new North American species of CortinariussectionAnomali reveal high continental species diversity. Mycological Progress 20(11): 1399–1439. 10.1007/s11557-021-01738-0 [DOI] [Google Scholar]
- Frøslev TG, Matheny PB, Hibbett D. (2005) Lower level relationships in the mushroom genus Cortinarius (Basidiomycota, Agaricales): A comparison of RBP1, RPB2 and ITS phylogenies. Molecular Phylogenetics and Evolution 37(2): 602–618. 10.1016/j.ympev.2005.06.016 [DOI] [PubMed] [Google Scholar]
- Garnica S, Weiss M, Oberwinkler F. (2003) Morphological and molecular phylogenetic studies in South American Cortinarius species. Mycological Research 107(10): 1143–1156. 10.1017/S0953756203008414 [DOI] [PubMed] [Google Scholar]
- Garnica S, Weiß M, Oertel B, Oberwinkler F. (2005) A framework for a phylogenetic classification in the genus Cortinarius (Basidiomycota, Agaricales) derived from morphological and molecular data. Canadian Journal of Botany 83(11): 1457–1477. 10.1139/b05-107 [DOI] [Google Scholar]
- Garnica S, Schön ME, Abarenkov K, Riess K, Liimatainen K, Niskanen T, Dima B, Soop B, Frøslev TG, Jeppesen TS, Peintner U, Kuhnert-Finkernagel R, Brandrud TE, Saar G, Oerter B, Ammirati JF. (2016) Determining threshold values for barcoding fungi: Lessons from Cortinarius (Basidiomycota), a highly diverse and widespread ectomycorrhizal genus. FEMS Microbiology Ecology 92(4): fiw045. 10.1093/femsec/fiw045 [DOI] [PubMed]
- Harrower E, Ammirati JF, Cappuccino AA, Ceska O, Kranabetter JM, Kroeger P, Lim S, Taylor T, Berbee ML. (2011) Cortinarius species diversity in British Columbia and molecular phylogenetic comparison with European specimen sequences. Botany 89(11): 799–810. 10.1139/b11-065 [DOI] [Google Scholar]
- Høiland K, Holst-Jensen A. (2000) Cortinarius phylogeny and possible taxonomic implications of ITS rDNA sequences. Mycologia 92(4): 694–710. 10.1080/00275514.2000.12061210 [DOI] [Google Scholar]
- Katoh K, Standley DM. (2013) MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30(4): 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibby G, Tortelli M. (2021) The genus Cortinarius in Britain. privately published, Great Britain, 20–22.
- Kornerup A, Wanscher JH. (1978) Methuen handbook of colour, 3rd edn. Methuen, London, 252 pp. [Google Scholar]
- Liimatainen K, Niskanen T, Dima B, Kytövuori I, Ammirati JF, Frøslev TG. (2014) The largest type study of Agaricales species to date: Bringing identification and nomenclature of Phlegmacium (Cortinarius) into the DNA era. Persoonia 33(1): 98–140. 10.3767/003158514X684681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liimatainen K, Kim JT, Pokorny L, Kirk PM, Dentinger B, Niskanen T. (2022) Taming the beast: A revised classification of Cortinariaceae based on genomic data. Fungal Diversity 112(1): 89–170. 10.1007/s13225-022-00499-9 [DOI] [Google Scholar]
- Liu Y, Rogers SO, Ammirati JF. (1997) Phylogenetic relationships in Dermocybe and related Cortinarius taxa based on nuclear ribosomal DNA internal transcribed spacers. Canadian Journal of Botany 75(4): 519–532. 10.1139/b97-058 [DOI] [Google Scholar]
- Luo Y, Bau T. (2021) Cortinariusjiaoheensis (Cortinariaceae), a new species of CortinariussubgenusTelamoniasectionFlexipedes, from northeast China. Phytotaxa 494(1): 113–121. 10.11646/phytotaxa.494.1.7 [DOI] [Google Scholar]
- Moser M. (1969) Uber einige kritische oder neue Cortinarien aus der untergattung Myxacium Fr. aus Smaland und Halland. Friesia 9: 142–150. [Google Scholar]
- Moser M, Horak E. (1975) Cortinarius Fr. und nahe verwandte Gattungen in Sudamerika. Beihefte zur Nova Hedwigia 52: 1–628. [Google Scholar]
- Nilsen AR, Macrae OC, Andrew KM, Wang XY, Tana MCT, Soop K, Brown CM, Summerfield TC, Orlovich DA. (2021) Studies of New Zealand Cortinarius: Resolution of taxonomic conflicts in section Subcastanelli (Agaricales), new species and key to rozitoid species. New Zealand Journal of Botany 59(4): 457–475. 10.1080/0028825X.2021.1879877 [DOI] [Google Scholar]
- Niskanen T, Liimatainen K, Kytövuori I, Lindström H, Dentinger B, Ammirati JF. (2016) CortinariussubgenusCallistei in North America and Europe-type studies, diversity, and distribution of species. Mycologia 108(5): 1018–1027. 10.3852/16-033 [DOI] [PubMed] [Google Scholar]
- Nylander JAA. (2004) MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University.
- Peintner U, Horak E, Moser MM, Vilgalys R. (2002) Phylogeny of Rozites, Cuphocybe and Rapacea inferred from ITS and LSU rDNA sequences. Mycologia 94(4): 620–629. 10.1080/15572536.2003.11833190 [DOI] [PubMed] [Google Scholar]
- Peintner U, Moncalvo JM, Vilgalys R. (2004) Toward a better understanding of the infrageneric relationships in Cortinarius (Agaricales, Basidiomycota). Mycologia 96(5): 1042–1058. 10.1080/15572536.2005.11832904 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhn S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl MT. (2000) Phylogenetic relationships within CortinariussubgenusMyxacium, sections Defibulati and Myxacium. Mycologia 92(6): 1091–1102. 10.1080/00275514.2000.12061257 [DOI] [Google Scholar]
- Singer R. (1986) The Agaricales in modern taxonomy 4th ed. Koenigstein, Koeltz Scientific Books, 981 pp. [Google Scholar]
- Soop K, Dima B, Cooper JA, Park D, Oertel B. (2019) A phylogenetic approach to a global supraspecific taxonomy of Cortinarius (Agaricales) with an emphasis on the southern mycota. Persoonia 42(1): 261–290. 10.3767/persoonia.2019.42.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani F, Jones RH, May TW. (2014) Concordance of seven gene genealogies compared to phenotypic data reveals multiple cryptic species in Australian dermocyboid Cortinarius (Agaricales). Molecular Phylogenetics and Evolution 71: 249–260. 10.1016/j.ympev.2013.10.019 [DOI] [PubMed] [Google Scholar]
- Stensrud Ø, Orr RJS, Reier-Røberg K, Schumacher T, Høiland K. (2014) Phylogenetic relationships in Cortinarius with focus on North European species. Karstenia 54(2): 57–71. 10.29203/ka.2014.464 [DOI] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. (2011) MEGA5: Molecular Evolutionary Genetic Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution 28(10): 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ. (2016) W-IQTREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44(W1): 1–4. 10.1093/nar/gkw256 [DOI] [PMC free article] [PubMed]
- Vaidya G, Lohman DJ, Meier R. (2011) Sequence matrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27(2): 171–180. 10.1111/j.1096-0031.2010.00329.x [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172(8): 4238–4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei TZ, Yao YJ. (2013) Cortinariuskorfii, a new species from China. Junwu Xuebao 32(3): 557–562. 10.13346/j.mycosystema.2013.03.017 [DOI] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Academic Press, San Diego, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Willis KJ. (2018) State of the World’s Fungi. Royal Botanic Gardens, Kew, London, 18–23.
- Xie ML, Li D, Wei SL, Ji RQ, Li Y. (2019) Cortinariussubcaesiobrunneus sp. nov., (Cortinariaceae, Agaricales) a new species from northwest China. Phytotaxa 392(3): 217–224. 10.11646/phytotaxa.392.3.4 [DOI] [Google Scholar]
- Xie ML, Wei TZ, Fu YP, Li D, Qi L, Xing PJ, Cheng GH, Ji RQ, Li Y. (2020) Three new species of CortinariussubgenusTelamonia (Cortinariaceae, Agaricales) from China. MycoKeys 69: 91–109. 10.3897/mycokeys.69.49437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie ML, Chen JL, Phukhamsakda C, Dima B, Fu YP, Ji RQ, Wang K, Wei TZ, Li Y. (2021a) Cortinariussubsalor and C.tibeticisalor spp. nov., two new species from the section Delibuti from China. PeerJ 9: e11982. 10.7717/peerj.11982 [DOI] [PMC free article] [PubMed]
- Xie ML, Wei TZ, Dima B, Fu YP, Ji RQ, Li Y. (2021b) Cortinariuskhinganensis (Agaricales), a new species of section Illumini from Northeast China. Phytotaxa 500(1): 1–10. 10.11646/phytotaxa.500.1.1 [DOI] [Google Scholar]
- Xie ML, Phukhamsakda C, Wei TZ, Li JP, Wang K, Wang Y, Ji RQ, Li Y. (2022) Morphological and phylogenetic evidence reveal five new Telamonioid species of Cortinarius (Agaricales) from East Asia. Journal of Fungi (Basel, Switzerland) 8(3): 257. 10.3390/jof8030257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZL. (1998) Revision of the genera Rozites and Descolea from China. Fungal Science 13(3 & 4): 61–74. 10.7099/FS.199812.0061 [DOI]
- Zhang QY, Jin C, Zhou HM, Ma ZY, Zhang YZ, Liang JQ, Si J, Li HJ. (2023) Enlargement of the knowledge of CortinariussectionAnomali (Agaricales, Basidiomycota): Introducing three new species from China. Frontiers in Cellular and Infection Microbiology 13: 1–15. 10.3389/fcimb.2023.1215579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SY, Long P, Yang ZL. (2023) Three new species and a new record of CortinariussectionCamphorati from southwestern China. Mycologia 115(6): 904–917. 10.1080/00275514.2023.2251365 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple sequence alignment
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Pan Long, Song-Yan Zhou, Sai-Nan Li, Fei-Fei Liu, Zuo-Hong Chen
Data type
fas
Data Availability Statement
All of the data that support the findings of this study are available in the main text or Supplementary Information. The sequence data generated in this study are deposited in NCBI GenBank.