Abstract
We have previously identified two mucin-type sialoglycoproteins from porcine intestinal epithelial cells with approximate molecular masses of 210 (intestinal mucin-type glycoprotein IMTGP-1) and 240 kDa (IMTGP-2) as receptors for the K88ab and K88ac fimbrial adhesins of Escherichia coli. These receptors are detected in intestinal brush border membrane preparations from pigs with adhesive phenotypes but not from pigs with nonadhesive phenotypes and are postulated to be important determinants of the susceptibility of pigs to K88ab+ and K88ac+ enterotoxigenic E. coli infections. Using exoglycosidase digestion studies, we have now determined that β-linked galactose is an important component in the recognition of IMTGP-1 and IMTGP-2 by the K88ac adhesin. In addition, we observed a differential distribution of the K88ac adhesin binding activity of IMTGP-1 and IMTGP-2 along the crypt-villus axis, suggesting that receptor activity is dependent on the maturation state of the intestinal epithelial cells. Brush borders from immature intestinal epithelial cells possessed the highest concentrations of IMTGP-1 and IMTGP-2 receptor activity, with a progressive decrease in receptor activity as the cells mature. To characterize the differences in the carbohydrate moieties of IMTGP-1 and IMTGP-2, we developed a procedure for purifying the receptors, using phenol extraction followed by serial lectin affinity chromatography. Carbohydrate compositional analysis of the purified receptors indicated that the carbohydrate moieties of IMTGP-1 and IMTGP-2 consist of both N- and O-glycans containing galactose, glucose, sialic acid, mannose, N-acetylgalactosamine, N-acetylglucosamine, and fucose. The major difference between the two receptors is that IMTGP-2 contains a higher percentage of monosaccharides (mannose and glucose) commonly found in N-glycans.
Enterotoxigenic Escherichia coli cells (ETEC) that express the K88 fimbrial adhesin on their surface are a common cause of diarrhea in newborn and weaned piglets (16, 22). K88 fimbrial adhesins are filamentous surface appendages whose lectin activity allows ETEC to attach to specific glycoconjugates (receptors) on porcine intestinal epithelial cells (16). Attachment is the initial step in the colonization of the small intestine by ETEC and allows bacteria to avoid elimination by intestinal peristalsis (14).
Using microscopic observation of the binding of K88+ ETEC to purified intestinal brush border preparations, Sellwood et al. (30) identified two phenotypes of pigs with respect to adherence of K88+ ETEC. Brush borders of the adhesive phenotype bind K88+ ETEC, while those of the nonadhesive phenotype do not. If the three variants of the K88 adhesin (K88ab, K88ac, and K88ad) are considered, as many as six phenotypes of pigs can be distinguished in relation to the bacterial adhesion (2, 3, 26). These six porcine phenotypes and the K88 adhesin variants that bind to them are as follows: phenotype A (all three variants), phenotype B (K88ab and K88ac), phenotype C (K88ab and K88ad), phenotype D (K88ad), phenotype E (none of the variants), and phenotype F (K88ab). Ability to bind K88+ ETEC was found to be inherited in a simple Mendelian fashion, with fimbrial adhesin binding ability (receptor presence) dominant over inability to bind fimbrial adhesin (receptor absence). Inheritance of the receptor as determined by the brush border adherence assay was found to correlate with susceptibility of pigs to K88+ ETEC infections (11, 27).
We have previously identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) two glycoproteins from porcine intestinal epithelial cells with apparent molecular masses of 210 and 240 kDa that bind the K88ab and K88ac adhesin only in pigs with phenotypes A and B (4, 8, 9). Because these glycoproteins are detected only in pigs that have been characterized as having brush borders that are adhesive for K88ab and K88ac, they have the potential to be important receptors in the phenotype-specific adherence of K88+ ETEC to intestinal epithelial cells. These receptors have been characterized as mucin-type sialoglycoproteins that appear as wide bands on SDS-PAGE separations due to the microheterogeneity within their carbohydrate moieties (8). Previous references to these receptors by their molecular masses (210 and 240 kDa) are misleading, since they do not migrate at single well-defined molecular masses. For the purpose of clarity, we have designated the lower-molecular-mass receptor, which migrates in the range of 210 to 230 kDa, intestinal mucin-type glycoprotein 1 (IMTGP-1) and the higher-molecular-mass receptor, which migrates in the range of 240 to 300 kDa, IMTGP-2. These two K88 adhesin receptors have similar amino acid compositions, reactivities with lectins, elution characteristics by gel filtration and hydroxyapatite chromatography, and susceptibilities to neuraminidase treatment (8).
Mucin-type sialoglycoproteins are a diverse group of cell surface glycoproteins that structurally resemble mucins found in epithelial secretions except that they are attached to the membrane and are not cross-linked to other mucins via disulfide bonds (6). Mucin-type sialoglycoproteins may be optimal targets for microbial attachment in the intestine because they contain a diverse array of oligosaccharide structures which may function as binding sites for the microbial organism. Also, the rigidity of the mucin-type sialoglycoproteins may function to move the binding site recognized by the adhesin away from the surface of the cell and out of range of interference by the cell’s crowded glycocalyx region (15).
The results presented here provide further characterization of the structures of the carbohydrate moieties of IMTGP-1 and IMTGP-2 and their localization along the crypt-villus axis. We present evidence indicating that (i) galactose residues are important in the recognition of these receptors by K88ac adhesin, (ii) these receptor activities are differentially expressed along the crypt-villus axis, and (iii) IMTGP-2 contains a higher percentage of N-glycans than IMTGP-1.
MATERIALS AND METHODS
Phenol extraction of brush border glycoproteins.
Intestinal brush border vesicles were prepared from frozen adult porcine small intestine as described by Erickson et al. (9). Glycoproteins were phenol extracted from brush border vesicles by the method described by Howe et al. (13). Briefly, brush border vesicles suspended in phosphate-buffered saline (PBS; 15 mM KH2PO4, 8 mM Na2HPO4, 137 mM NaCl, 2.6 mM KCl [pH 7.4]) were mixed with 20 volumes of phenol-saline solution (liquefied phenol diluted with an equal volume of 1% NaCl). The mixture was stirred for 30 min at room temperature (RT) and then centrifuged (1,000 × g for 2 h at RT). The phenol layer was removed and extracted twice more with an equal volume of 0.45% NaCl. The aqueous layers from each extraction were pooled, dialyzed extensively against water, and lyophilized.
Protein and neutral sugar determination.
Protein concentrations were determined by the modified Lowry assay described by Peterson (24), with bovine serum albumin as the standard. Neutral sugar concentrations were determined by the phenol-sulfuric method, with a mixture of glucose and galactose as the standard (25).
SDS-PAGE.
SDS-PAGE was performed as described by Laemmli (18). Prior to electrophoresis, the samples were denatured by heating at 100°C for 3 min in sample buffer containing 62.5 mM Tris-HCl (pH 6.8)–0.6 mM β-mercaptoethanol–2% (wt/vol) SDS–10% (vol/vol) glycerol. Separated molecules were transferred to polyvinylidene difluoride (PVDF) membranes according to the method of Towbin et al. (33). Periodic acid-Schiff (PAS) staining of the glycoproteins immobilized to PVDF membranes was performed as described by Stromqvist and Gruffman (31).
K88ac adhesin binding assay.
Detection of K88ac receptor activity was achieved with the biotinylated K88ac adhesin overlay assay (BAOA) described by Erickson et al. (9). This overlay assay can be performed with receptors immobilized on polystyrene plates or on PVDF membranes. One unit of receptor activity binds 1 nmol of biotinylated K88ac adhesin.
Exoglycosidase treatments.
Brush border vesicles (400 μg of protein) were solubilized in 50 mM sodium citrate (pH 5.0 for neuraminidase and pH 4.0 for β-galactosidase) containing 0.1% taurodeoxycholate. Dissolved brush border proteins were then treated separately or sequentially with Arthrobacter ureafaciens neuraminidase (200 mU; Boehringer Mannheim) or E. coli β-galactosidase (4 U; Sigma) for 48 h at 37°C, with stirring at 120 rpm/min. After treatment, exoglycosidase activity was stopped by heating the sample at 100°C for 5 min. The treated samples were dialyzed against 0.1 M NH4HCO3 (pH 8.5) for 16 h. These samples were then tested for ability to bind biotinylated K88ac adhesin by the BAOA.
Fractionation of intestinal epithelial cells along the crypt-villus axis.
Intestinal cells were fractionated by the method of Weiser (34). One meter of adult porcine jejunum was washed three times at 4°C with 20 ml of 0.9% NaCl containing 1 mM dithiothreitol and then for 15 min at 37°C with a solution of 500 ml of 27 mM sodium citrate, 8 mM KH2PO4, 5.6 mM Na2HPO4, 96 mM NaCl, 1.5 mM KCl (pH 7.3). Intestinal epithelial cells were then sequentially eluted by filling the intestine with 500 ml of elution buffer (1.5 mM KH2PO4, 6.5 mM Na2HPO4, 1.5 mM EDTA, 0.5 mM dithiothreitol, 137 mM NaCl, 3 mM KCl [pH 7.3]). After 4 min of incubation at 37°C, the elution buffer was removed from the intestine and 500 ml of fresh elution buffer was added. This process was repeated six more times with incubation intervals of 4, 3, 4, 5, 7, and 15 min. The eluted cells were collected by centrifugation (250 × g for 5 min) and washed (for 5 min at 4°C) twice in PBS and twice in PBS-EDTA (8 mM KH2PO4, 5.6 mM Na2HPO4, 96 mM NaCl, 1.5 mM KCl, 10 mM EDTA [pH 7.4]). The cells were collected by centrifugation (250 × g for 5 min) after each wash. Brush border vesicles were prepared from fractionated epithelial cells by hypotonic lysis followed by differential centrifugation as described by Dean-Nystrom (7). Alkaline phosphatase activity, which is a marker of the differentiated intestinal epithelial cells, was determined for each cell fraction as described by Weiser (34), with p-nitrophenyl phosphate as substrate. One unit of phosphatase activity hydrolyzes 1 nmol of p-nitrophenyl phosphate.
Purification and separation of IMTGP-1 and IMTGP-2.
The lyophilized phenol-extracted brush border proteins were dissolved in 0.1 M Tris-HCl–1 mM CaCl2–1 mM MgCl2–0.5 M NaCl (pH 7.0; buffer A). The resulting solution was clarified by centrifugation (1,500 × g for 10 min) and loaded at RT onto a water-jacketed concanavalin A (ConA)-Sepharose column (1.2 cm in diameter by 30 cm in length) equilibrated with buffer A at a flow rate of 9 ml/h. After loading, the flow through the column was stopped for 16 h to allow the sample to interact with the immobilized ConA. Components of the sample not recognized by ConA were removed by washing the column with 5 column volumes of buffer A. Elution of the glycoproteins bound to the ConA column was accomplished by the addition of 30 ml of 0.3 M methyl-α-d-glucopyranoside dissolved in buffer A at RT followed by the addition of 25 ml of 0.3 M methyl-α-d-mannopyranoside dissolved in buffer A at 60°C. The fractions (1.5 ml) containing K88ac-binding activity were pooled, desalted by extensive dialysis against water, and lyophilized. This chromatography step separated the majority of IMTGP-1 from IMTGP-2. Final purification of IMTGP-1 was achieved by chromatography on a Superose 12 HR 10/30 column (Pharmacia) equilibrated with 0.1 M NH4HCO3 (pH 8.5) at RT at a flow rate of 0.4 ml/min. Five hundred micrograms of protein in a volume of 200 μl of 0.1 M NH4HCO3 (pH 8.5) was loaded for each Superose 12 chromatography run. Purification of IMTGP-2 was achieved by loading the pooled IMTGP-2-containing fractions from the ConA-Sepharose chromatography onto a Jacalin agarose column (0.7 cm in diameter by 15 cm in length) equilibrated with 0.1 M Tris-0.5 M NaCl (pH 7.0; buffer B) at a flow rate of 6 ml/h. After this column was washed with 5 column volumes of buffer B, the bound glycoproteins were eluted with 25 ml of 0.3 M melibiose dissolved in buffer B, followed by 25 ml of 0.3 M methyl-α-d-galactopyranoside dissolved in buffer B. One-milliliter fractions were collected during the elution. Purification of IMTGP-2 was completed by gel filtration chromatography on a Superose 12 column as described above for IMTGP-1.
Monosaccharide analysis.
Carbohydrate compositional analysis was performed on purified IMTGP-1 and IMTGP-2 at the Complex Carbohydrate Research Center at the University of Georgia, Athens, Ga. Trimethylsilylated methyl glycosides were prepared by methanolysis, N-(re)acetylation, and trimethylsilylation as described by Merkle and Poppe (20). These trimethylsilylated methylglycosides were separated and quantified on a Hewlett-Packard 5890 gas chromatograph coupled to a 5970 mass spectrometer, with a 50-m Quadrex methyl silicone capillary column.
RESULTS
Determination of which monosaccharides on IMTGP-1 and IMTGP-2 are recognized by K88ac adhesin.
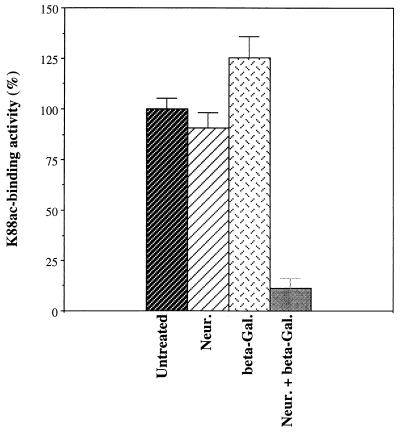
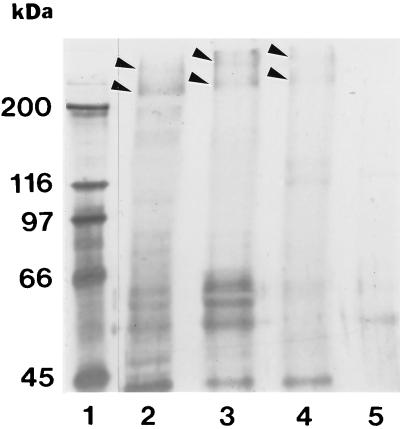
Based on previous studies with lectins, we knew that the oligosaccharides present on IMTGP-1 and IMTGP-2 contain both sialic acid and galactose residues in terminal positions on the reducing end of oligosaccharides (8, 28). To determine the identities of the monosaccharides on IMTGP-1 and IMTGP-2 that are recognized by the K88ac adhesin, we treated the receptors with specific exoglycosidases to sequentially remove terminal monosaccharides. The effect of exoglycosidase treatment on the K88ac adhesin binding activity of the receptors was evaluated by BAOAs performed on brush border proteins immobilized to polystyrene plates (Fig. 1) or separated by SDS-PAGE (Fig. 2). Treatment of the intestinal brush border protein preparations with neuraminidase did not reduce K88ac adhesin binding activity (Fig. 1) but did lead to an upward shift in the mobility of IMTGP-1 and IMTGP-2 (Fig. 2, lane 3). These results indicate that terminal sialic acid residues were removed but that their removal had little effect on the recognition of IMTGP-1 and IMTGP-2 by the K88ac adhesin (Fig. 2, lane 3). Treatment of brush border protein preparations with β-galactosidase increased K88ac-binding activity by 25% over that of the untreated control (Fig. 1) and led to an upward shift in the mobility of IMTGP-1 and IMTGP-2 on SDS-PAGE (Fig. 2, lane 4). Sequential treatment of brush border preparations with neuraminidase followed by β-galactosidase significantly (P < 0.0002) decreased the K88ac adhesin binding activity to 11.3% of that of the untreated control (Fig. 1) and completely abolished recognition of IMTGP-1 and IMTGP-2 by K88ac adhesin (Fig. 2, lane 5). These results indicate that the receptors contain both sialic acid and β-linked galactosyl residues and that the presence of sialic acid residues prevents the removal of many of the galactose residues by β-galactosidase treatment. In addition, galactose residues that are exposed by treatment of the IMTGPs with neuraminidase are essential in recognition of the receptors by the K88ac adhesin.
FIG. 1.
Exoglycosidase treatment of IMTGP-1 and IMTGP-2. Intestinal brush border glycoproteins were subjected to treatment with no addition, neuraminidase from Arthrobacter ureafaciens (Neur.), β-galactosidase from E. coli (beta-Gal.), and sequentially with neuraminidase and β-galactosidase (Neur. + beta-Gal.) as described in Materials and Methods. K88ac adhesin binding activity (expressed as a percentage of the untreated control) was determined by BAOA, with the samples immobilized to polystyrene plates. The results shown are the means ± standard errors (n = 4) for untreated and exoglycosidase-treated intestinal brush border proteins.
FIG. 2.
Exoglycosidase treatment of IMTGP-1 and IMTGP-2. Intestinal brush border glycoproteins were subjected to treatment with no addition (lane 2), neuraminidase from A. ureafaciens (lane 3), β-galactosidase from E. coli (lane 4), and (sequentially) neuraminidase and β-galactosidase (lane 5) as described in Materials and Methods. The treated glycoproteins (25 μg per lane) were separated by SDS-PAGE (7% polyacrylamide) and transferred to a PVDF membrane. K88ac adhesin receptor activity was detected with biotinylated K88ac adhesin. Molecular mass standards (lane 1) are indicated at the left.
Distribution of IMTGP-1 and IMTGP-2 along the crypt-villus axis.
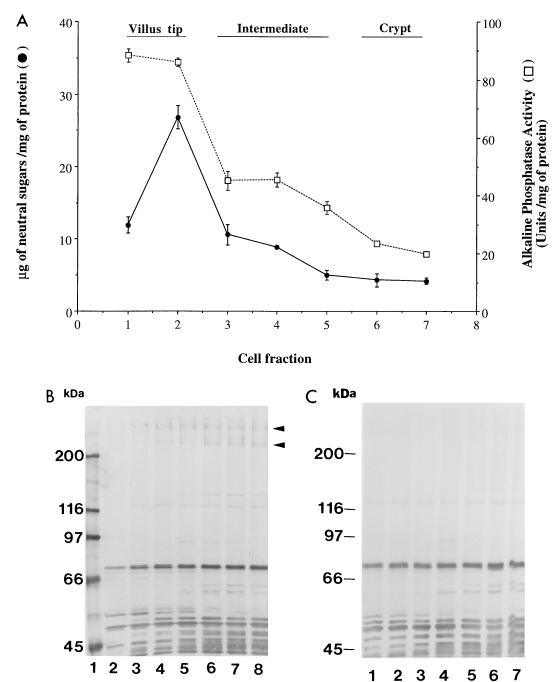
To characterize the localization of IMTGP-1 and IMTGP-2 along the crypt-villus axis, intestinal epithelial cells were sequentially eluted from the villus tip to the crypt by incubating segments of small intestine from pigs with adhesive and nonadhesive phenotypes for various periods of time in a buffered solution containing EDTA (34). The stage of differentiation of the cells in each fraction was determined by assaying the cell fractions for activity of alkaline phosphatase, an enzyme expressed by the mature enterocytes. Based on these results, we grouped the cell fractions as follows: villus tip (fractions 1 and 2), intermediate (fractions 3 to 5), and crypt (fractions 6 and 7) (Fig. 3A). We determined the carbohydrate distribution along the crypt-villus axis by quantitation of the neutral sugar concentration in each cell fraction. Villus tip cells were found to have a high concentration of neutral sugars (≥12 mg of neutral sugar/mg of protein), whereas that of crypt cells is lower (≤4.5 mg of neutral sugar/mg of protein). These results are similar to those previously reported by Weiser (34). To determine the distribution of IMTGP-1 and IMTGP-2 along the crypt-villus axis, we evaluated the brush border portion of the enterocytes from the seven cell fractions for the presence of IMTGP-1 and IMTGP-2 by the BAOA to detect K88ac adhesin binding activity (Fig. 3B). Brush borders from crypt cell fractions possessed the highest concentrations of IMTGP-1 and IMTGP-2 receptor activity, with a progressive decrease in receptor activity as the cells matured (Fig. 3B). A small amount of IMTGP-2 receptor activity, but no IMTGP-1 receptor activity, was detected in the villus tip fractions. These results indicate that IMTGP-1 and IMTGP-2 from immature intestinal epithelial cells possess higher K88ac adhesin binding activity or are present in higher concentrations than IMTGPs in more mature enterocytes. A number of other K88ac adhesin binding molecules, whose molecular masses range from 45 to 120 kDa, show a differential distribution of K88ac adhesin binding activity along the crypt-villus axis (Fig. 3B and C). All these other molecules are detected in brush borders from both adhesive and nonadhesive phenotypes of pigs, whereas IMTGP-1 and IMTGP-2 are detected only in adhesive phenotypes of pigs (Fig. 3B and C). These results indicate that these other molecules possess sites recognized by the K88ac adhesin some time during their maturation, but the importance of these binding activities is not clear, since their presence is not correlated with the phenotype (adhesive or nonadhesive for K88ac adhesin) of the pigs.
FIG. 3.
Distribution of IMTGP-1 and IMTGP-2 along the crypt-villus axis. Intestinal epithelial cells were sequentially eluted as described in Materials and Methods. (A) The eluted cell fractions were assayed for neutral sugar content (•) and alkaline phosphatase activity (□). Glycoproteins (50 μg) from each cell fraction were separated by SDS-PAGE (7% polyacrylamide) and electrotransferred to a PVDF membrane. Fractions 1 to 7 in panel A correspond to lanes 2 to 8 of adhesive phenotype (B) and lanes 1 to 7 of nonadhesive phenotype (C). K88ac adhesin binding activity was detected with biotinylated K88ac adhesin.
Purification and separation of IMTGP-1 and IMTGP-2.
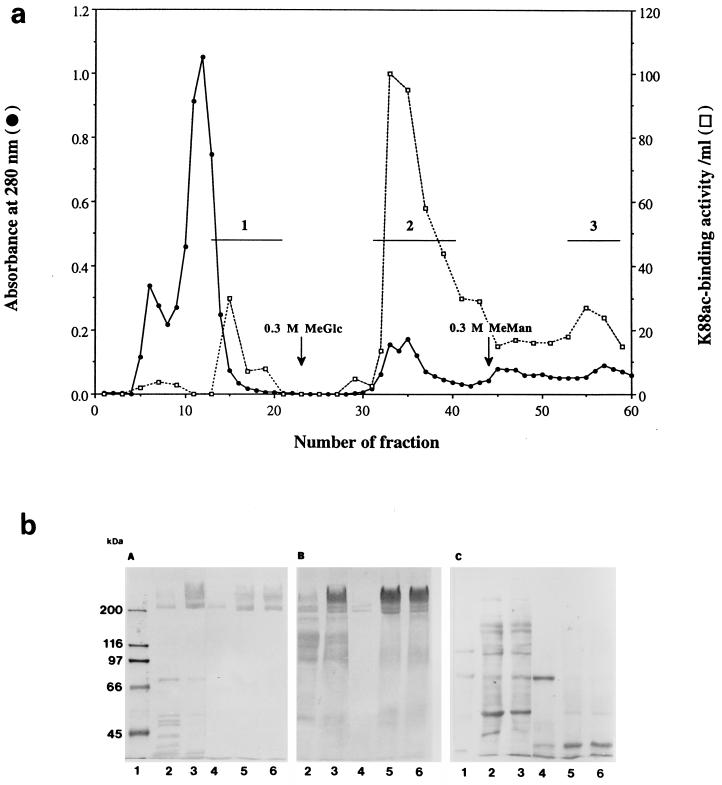
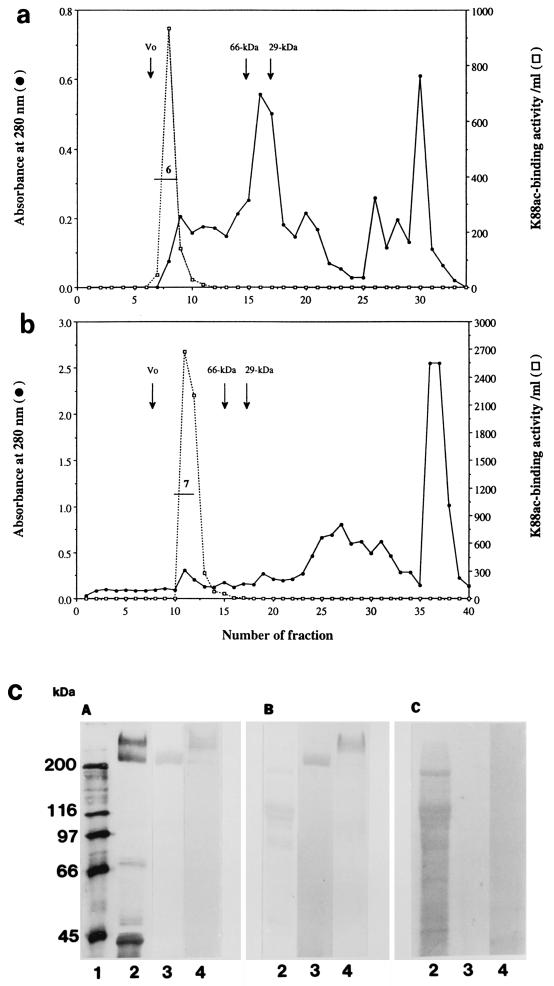
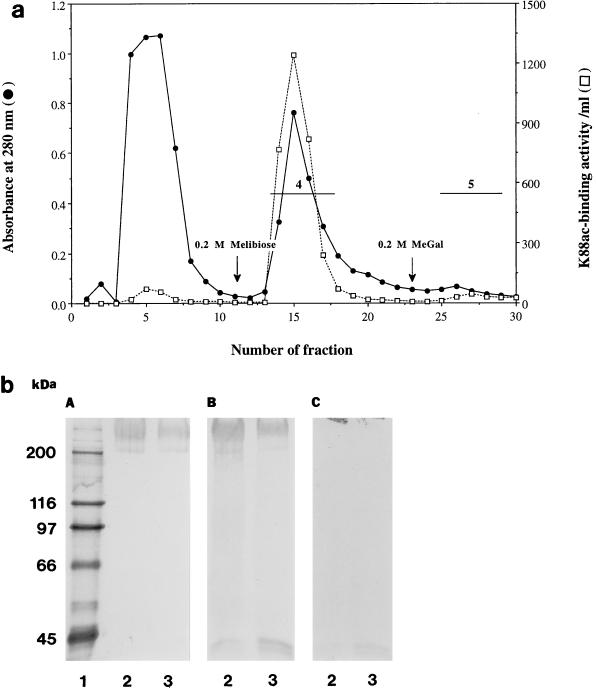
The results of a typical IMTGP-1 and IMTGP-2 purification are summarized in Table 1. Phenol extraction of brush borders resulted in a 17.2-fold increase in the specific activity (K88 receptor activity per unit of protein) of the resulting extract (Table 1 and Fig. 4b, lanes 3). The extracted glycoproteins were then fractionated onto a ConA-Sepharose column (Fig. 4a). Some IMTGP-1 did not bind tightly to the ConA column and eluted just after the proteins that did not interact with the column (Fig. 4a). This retarded fraction contained only IMTGP-1 along with a 66-kDa protein contaminant (Fig. 4b, panel C, lane 4). Final purification of IMTGP-1 was achieved by Superose 12 gel filtration chromatography (Fig. 6a) during which the 66-kDa protein contaminant was completely removed (Fig. 6c, lanes 3). The amount of pure IMTGP-1 obtained after this step was 0.7 mg, with a specific activity of 4.3 U/mg (Table 1). IMTGP-2 along with a small amount of IMTGP-1 bound tightly to the ConA-Sepharose column and required elution of the column with methyl-α-d-glucopyranoside and methyl-α-d-mannopyranoside for removal (Fig. 4a). These results demonstrate that there are differences in the glycanic moieties of IMTGP-1 and IMTGP-2. IMTGP-1 appears to exist in two forms; the first is not recognized by ConA and likely does not contain a large number of N-glycans, and the second is recognized by ConA and likely contains more N-glycans. Based on interaction with ConA-Sepharose, IMTGP-2 appears to exist only in a form which possesses numerous N-glycans. After ConA chromatography, IMTGP-2 was further purified by Jacalin chromatography (Fig. 5a) during which IMTGP-2 bound tightly to the column and was removed by elution with melibiose followed by methyl-α-d-galactopyranoside (Fig. 5b). During this step, approximately 80% of the remaining contaminating proteins were removed (Table 1). To remove the final contaminants from IMTGP-2, we subjected the pooled fractions from Jacalin agarose chromatography to Superose 12 gel filtration chromatography (Fig. 6b). Using this purification scheme, we obtained 9.4 mg of IMTGP-2, with a final specific activity of 4.2 U/mg (Table 1 and Fig. 6c, lane 4).
TABLE 1.
Purification of IMTGP-1 and IMTGP-2 receptors
| Purification step | Protein (mg) | Neutral sugars (μg/mg of protein) | Total units | Sp act (U/mg) | Receptor recovery (%) |
|---|---|---|---|---|---|
| Crude brush borders | 6,700 | 77.8 | 804 | 0.12 | |
| Phenol extract | 410 | 153.0 | 845 | 2.06 | 100 |
| IMTGP-1 | |||||
| ConA-Sepharose | 16.2 | 251.6 | 55 | 3.39 | 6.5 |
| Superose 12 | 0.7 | 640.0 | 3.1 | 4.30 | 0.4 |
| IMTGP-2 | |||||
| ConA-Sepharose | 87.4 | 438.0 | 332.1 | 3.80 | 39.3 |
| Jacalin agarose | 26.8 | 677.1 | 115.2 | 4.30 | 13.6 |
| Superose 12 | 9.4 | 943.0 | 39.5 | 4.20 | 4.7 |
FIG. 4.
Purification of IMTGP-1 and IMTGP-2 by chromatography on ConA-Sepharose. (a) K88ac adhesin binding activity (□) was determined with biotinylated K88ac adhesin as described in Materials and Methods. Protein concentration was monitored by UV absorption at 280 nm (•). Horizontal lines represent the pooled fractions containing K88ac adhesin receptors. Fraction 1 was obtained by eluting the column with buffer A (0.1 M Tris-HCl, 0.5 M NaCl, 1 mM CaCl2, 1 mM MgCl2 [pH 7.0]). Fractions 2 and 3 were obtained after elution with buffer A containing 0.3 M methyl-α-d-glucopyranoside (MeGlc) or 0.3 M methyl-α-d-mannopyranoside (MeMan), respectively. (b) Glycoproteins (25 μg each) were separated by SDS-PAGE (7% polyacrylamide). (A) Biotinylated K88ac adhesin was used to detect receptor activity. (B) PAS was used to detect glycoproteins. (C) Coomassie blue was used to detect protein. Lanes 1, biotinylated molecular mass markers; lanes 2, crude intestinal brush borders; lanes 3, phenol-extracted glycoproteins; lanes 4, 5, and 6, ConA fractions 1, 2, and 3, respectively.
FIG. 6.
Purification of IMTGP-1 and IMTGP-2 by gel filtration chromatography. Gel filtration chromatography of IMTGP-1 (a) and IMTGP-2 (b) was performed on Superose 12 HR 10/30 on a fast-performance liquid chromatography system as described in Materials and Methods. Void volume (Vo) was determined with thyroglobulin (669 kDa), and the elution positions for bovine serum albumin (66 kDa) and carbonic anhydrase (29 kDa) are indicated by arrows. Protein concentration was monitored at 280 nm (•). K88ac adhesin binding activity (□) was determined with biotinylated K88ac adhesin as described in Materials and Methods. Horizontal lines labeled 6 and 7 indicate the pooled fractions containing IMTGP-1 and IMTGP-2, respectively. (c) Glycoproteins were separated by SDS-PAGE (7% polyacrylamide) and transferred to PVDF membranes. (A) K88ac-binding activity was detected with biotinylated K88ac adhesin. (B) Glycoproteins were detected by PAS staining. (C) Proteins were detected by Coomassie blue staining. Lane 1, biotinylated molecular mass markers; lanes 2, crude intestinal brush borders (25 μg); lanes 3 and 4, 15 μg of pooled gel filtration fractions 6 and 7, respectively.
FIG. 5.
Purification of IMTGP-2 by chromatography on Jacalin agarose. (a) K88ac adhesin binding activity (□) was determined with biotinylated K88ac adhesin as described in Materials and Methods. Protein concentration was monitored by UV absorption at 280 nm (•). Horizontal lines represent the pooled fractions containing K88ac adhesin receptors. Fraction 4 was obtained by eluting the column with buffer B containing 0.3 M melibiose. Fraction 5 was obtained after elution with buffer B containing 0.3 M methyl-α-d-galactopyranoside (MeGal). (b) Glycoproteins (25 μg) were separated by SDS-PAGE (7% polyacrylamide) and transferred to PVDF membranes. (A) K88ac-binding activity was detected with biotinylated K88ac adhesin. (B) Glycoproteins were detected by PAS staining. (C) Proteins were detected by Coomassie blue staining. Lane 1, biotinylated molecular mass markers; lanes 2 and 3, Jacalin fractions 4 and 5, respectively.
Monosaccharide composition.
The monosaccharide compositions of purified IMTGP-1 and IMTGP-2 are shown in Table 2. The carbohydrate moieties of these two glycoproteins were found to be composed of galactose, glucose, sialic acid, mannose, N-acetylgalactosamine, N-acetylglucosamine, and fucose. Both glycoproteins contained high percentages of galactosyl residues, 38.5% for IMTGP-1 and 26.1% for IMTGP-2, indicating that they contain a large proportion of O-glycans. IMTGP-2 contained higher percentages of mannosyl and glucosyl than did IMTGP-1 residues, indicating that IMTGP-2 contains a larger proportion of N-glycans than IMTGP-1.
TABLE 2.
Monosaccharide composition of the glycan moieties of IMTGP-1 and IMTGP-2
| Monosaccharide | Composition of glycan moiety (% [wt/wt])
|
|
|---|---|---|
| IMTGP-1 | IMTGP-2 | |
| Fuc | 4.0 | 1.5 |
| Man | 6.5 | 18.6 |
| Gal | 38.5 | 26.1 |
| Glc | 4.0 | 24.1 |
| GalNAc | 18.0 | 6.8 |
| GlcNAc | 9.2 | 9.0 |
| NeuAc | 19.7 | 14.0 |
DISCUSSION
Many research groups have reported the identification of putative K88 fimbrial adhesin receptors in both intestinal brush border (9, 12, 17, 19, 35) and intestinal mucus (5, 19, 21, 37) preparations. In contrast to many of the other K88 receptors identified, IMTGP-1 and IMTGP-2 are detected only in adhesive phenotypes of pigs; consequently, it is very likely that these receptors are important in the in vivo attachment of K88ab and K88ac ETEC to porcine intestine (4, 8, 9, 28). Our objective in the present study was to further characterize IMTGP-1 and IMTGP-2. We found that (i) galactose residues are important in the recognition of IMTGP-1 and IMTGP-2 by K88ac adhesin, (ii) the K88ac adhesin binding activities of IMTGP-1 and IMTGP-2 are differentially expressed along the crypt-villus axis, and (iii) the major difference in carbohydrate composition between IMTGP-1 and IMTGP-2 is that IMTGP-2 has a higher proportion of monosaccharides commonly found in N-glycans.
Knowledge of the carbohydrate specificity of the K88ac adhesin is essential to understanding the molecular mechanism of K88+ ETEC adhesion. Results of previous studies to determine the carbohydrate specificity of the K88 adhesin have not been definitive. Data from monosaccharide-blocking studies indicate that N-acetylglucosamine, N-acetylgalactosamine, N-acetylmannosamine (1), and d-galactosamine (29) may be involved. Results from glycoprotein-blocking studies indicate that terminal N-acetylgalactosamine, N-acetylglucosamine, and galactose may play a role in K88 adhesin-brush border receptor interaction (1, 10). Also, galactose has been reported to be an important residue in the recognition of putative intestinal mucus receptors and glycosphingolipids by the K88ab adhesin (5, 23). In the present study, we determined that β-linked galactose is essential in the recognition of IMTGP-1 and IMTGP-2 by K88ac adhesin. Our study also indicated that terminal sialic acid residues do not contribute significantly to the binding of K88ac adhesin to the receptors. It is interesting that treatment of the IMTGPs with β-galactosidase removed terminal galactose residues, as indicated by the shift in mobility, but did not significantly decrease K88ac adhesin binding to the receptors. Inactivation of receptor activity required the removal of sialic acid residues so that another group of galactose residues could be exposed to hydrolysis by β-galactosidase. While we know that galactose is an essential component of the IMTGP site that is recognized by the K88ac adhesin, we do not know if the carbohydrate that is β-linked to galactose is also essential. Future studies will be directed at determining the entire glycanic structure of the oligosaccharides on IMTGP-1 and IMTGP-2 that are recognized by the K88ac adhesin. Determination of this structure will make possible the synthesis of structural analogs of the receptor recognition sequence which can be used to prevent and treat K88ac+ ETEC-induced disease in pigs.
To gain a better understanding of the differences in the carbohydrate moieties of IMTGP-1 and IMTGP-2, we needed to purify relatively large amounts of the receptors for carbohydrate structural analysis. We have previously published a procedure for purifying IMTGP-1 and IMTGP-2 (8). This purification scheme involves the solubilization of the glycoproteins from intestinal brush border vesicles with deoxycholate and the purification of the receptors by gel filtration chromatography on Sepharose CL-4B, followed by hydroxyapatite chromatography performed in the presence of 0.1% SDS. This procedure consistently yields a highly purified mixture of IMTGP-1 and IMTGP-2 but produces low yields and a final product that contains SDS, which must be removed before carbohydrate characterization experiments can be performed. To overcome these problems, we developed the purification procedure described in this study, which involves phenol extraction of glycoproteins followed by serial lectin affinity and gel filtration chromatographies.
The carbohydrate composition of IMTGP-1 and IMTGP-2 that was determined in the current study is similar to those reported for other mucin-type sialoglycoproteins that possess both N- and O-glycans (32). The high percentage of galactose found in both receptors is an excellent indicator that these glycoproteins contain a large number of O-glycans, which are attached to threonine and serine residues along the polypeptide chain. It has been previously reported that IMTGP-1 and IMTGP-2 contain high percentages of threonine residues, consistent with our conclusion that they contain large numbers of O-glycans (8). Removal of these O-glycans by alkaline β-elimination abolishes recognition of the receptors by the K88ac adhesin (28). O-glycans are usually relatively short and found clustered in certain sections of the glycoprotein. The presence of these clusters of O-glycosylated residues makes that section of the glycoprotein relatively resistant to degradation by proteases (32) and rigid and elongated, helping to facilitate the scaffolding function that is hypothetically attributed to mucin-type domains within glycoproteins (15). In addition, clustering of O-glycans recognized by K88ac+ ETEC into a small area on the receptor may strengthen the avidity of the interaction between K88ac fimbriae and receptors by increasing the number of interactions between the two structures. A similar effect has been observed for rotavirus binding to mucin-type sialoglycoproteins (36).
Our study involving sequential removal of epithelial cells from the intestine showed that brush borders from immature intestinal epithelial cells possessed the highest concentrations of IMTGP-1 and IMTGP-2 receptor activity, with a progressive decrease in receptor activity as the cells matured. Mucin-type sialoglycoproteins are initially synthesized as a single large polypeptide which is N glycosylated cotranslationally in the endoplasmic reticulum. En route to the cell membrane, biosynthesis of O-glycans on mucin-type sialoglycoproteins begins by the attachment of GalNAc to threonine and serine residues and continues by the sequential addition of monosaccharides (primarily GalNAc, GlcNAc, and Gal) by glycosyltransferases present in the Golgi network of the cell (6). The O-glycans which initially reach the cell membrane are not always complete. Many of the mucin-type sialoglycoproteins are internalized from the membrane and recycled into the Golgi network (6). During recycling, more monosaccharides (sialic acid, fucose, GalNAc, and GlcNAc) are added to the O-linked oligosaccharides in the trans-Golgi network (6). One hypothesis that would explain the decrease in the K88ac adhesin receptor activity of the IMTGPs as intestinal epithelial cells mature is the modification of the carbohydrate structures recognized by the K88ac adhesin during the recycling of mucin-type glycoproteins. This modification may mask the site recognized by the K88ac adhesin and render the receptor less active. An alternative hypothesis is that the concentration of IMTGP-1 and IMTGP-2 on the brush border membrane may decrease due to the shedding of these receptors into the lumen of the intestine as the cell matures.
One of our long-term goals is to identify the gene(s) that makes some pigs susceptible to K88+ ETEC infections. The protein coded for by this gene is likely either the polypeptide portion of a glycoprotein receptor for the K88 adhesin or a glycosyltransferase enzyme that is involved in the assembly of the oligosaccharide recognized by the K88 adhesin. Because the K88 adhesin binding activity of IMTGP-1 and IMTGP-2 is expressed only in brush borders of adhesive animals, we believe that further characterization of the carbohydrate and protein moieties of these glycoproteins will provide the information necessary to identify the gene(s) responsible for susceptibility to K88+ ETEC infections. The purification scheme described in the current study is being used to purify amounts of IMTGP-1 and IMTGP-2 that are sufficient for complete analysis of the carbohydrate structure recognized by the K88 adhesin and for amino acid sequencing of the polypeptide, which will facilitate the cloning of the gene that encodes these receptors.
ACKNOWLEDGMENTS
We gratefully acknowledge USDA grant 94-02419, NSF grant OSR-9452894, the South Dakota Future Fund, and the South Dakota Agricultural Experiment Station (paper no. 3040) for providing financial assistance. This research was supported in part by the National Institutes of Health (NIH)-funded Resource Center for Biomedical Complex Carbohydrates (NIH grant no. 2-P41-RR05351-06) to the Complex Carbohydrate Research Center.
We thank David Benfield and Mike Hildreth for their critical review of the manuscript.
REFERENCES
- 1.Anderson M J, Whitehead J S, Kim Y S. Interaction of Escherichia coli K88 antigen with porcine intestinal brush border membranes. Infect Immun. 1980;29:897–901. doi: 10.1128/iai.29.3.897-901.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker D R, Billey L O, Francis D H. Distribution of K88 Escherichia coli-adhesive and nonadhesive phenotypes among pigs of four breeds. Vet Microbiol. 1997;54:123–132. doi: 10.1016/s0378-1135(96)01277-1. [DOI] [PubMed] [Google Scholar]
- 3.Bijlsma I G W, de Nijs A, van der Meer C, Frik J F. Different pig phenotypes affect adherence of Escherichia coli to jejunal brush borders by K88ab, K88ac, or K88ad antigen. Infect Immun. 1982;37:891–894. doi: 10.1128/iai.37.3.891-894.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billey, L. O., A. K. Erickson, and D. H. Francis. Multiple receptors on porcine intestinal epithelial cells for the three variants of Escherichia coli K88 fimbrial adhesin. Vet. Microbiol., in press. [DOI] [PubMed]
- 5.Blomberg L, Krivan H C, Cohen P S, Conway P L. Piglet ileal mucus contains protein and glycolipid (galactosylceramide) receptors specific for Escherichia coli K88 fimbriae. Infect Immun. 1993;61:2526–2531. doi: 10.1128/iai.61.6.2526-2531.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carraway K L, Hull S R. Cell surface mucin-type glycoproteins and mucin-like domains. Glycobiology. 1991;1:131–138. doi: 10.1093/glycob/1.2.131. [DOI] [PubMed] [Google Scholar]
- 7.Dean-Nystrom E. Identification of intestinal receptors for enterotoxigenic Escherichia coli. Methods Enzymol. 1995;253:315–324. doi: 10.1016/s0076-6879(95)53027-4. [DOI] [PubMed] [Google Scholar]
- 8.Erickson A K, Baker D R, Bosworth B T, Casey T A, Benfield D A, Francis D H. Characterization of porcine intestinal receptors for the K88ac fimbrial adhesin of Escherichia coli as mucin-type sialoglycoproteins. Infect Immun. 1994;62:5404–5410. doi: 10.1128/iai.62.12.5404-5410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson A K, Willgohs J A, McFarland S Y, Benfield D A, Francis D H. Identification of two porcine brush border glycoproteins that bind the K88ac adhesin of Escherichia coli and correlation of these glycoproteins with the adhesive phenotype. Infect Immun. 1992;60:983–988. doi: 10.1128/iai.60.3.983-988.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbons R A, Jones G W, Sellwood R. An attempt to identify the intestinal receptor for the K88 adhesin by means of a hemagglutination inhibition test using glycoproteins and fractions from sow colostrum. J Gen Microbiol. 1975;86:228–240. doi: 10.1099/00221287-86-2-228. [DOI] [PubMed] [Google Scholar]
- 11.Gibbons R A, Sellwood R, Burrows M, Hunter P A. Inheritance of resistance to neonatal Escherichia coli diarrhea in the pig: examination of the genetic system. Theor Appl Genet. 1977;81:65–70. doi: 10.1007/BF00299479. [DOI] [PubMed] [Google Scholar]
- 12.Grange P A, Mouricout M A. Transferrin associated with the porcine intestinal mucosa is a receptor specific for K88ab fimbriae of Escherichia coli. Infect Immun. 1996;64:606–610. doi: 10.1128/iai.64.2.606-610.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe C, Lloyd K O, Lee L T. Isolation of glycoproteins from red cell membranes using phenol. Methods Enzymol. 1972;28:236–244. [Google Scholar]
- 14.Isaacson R E. Molecular and genetic basis of adherence for enteric Escherichia coli in animals. In: Roth J A, editor. Virulence mechanisms of bacterial pathogens. Washington, D.C: American Society for Microbiology; 1988. pp. 28–44. [Google Scholar]
- 15.Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990;15:291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- 16.Jones G W, Rutter J M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972;6:918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearns M J, Gibbons R A. The possible nature of the pig intestinal receptor for the K88 antigen of Escherichia coli. FEMS Microbiol Lett. 1979;6:165–168. [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Laux D C, McSweegan E F, Williams T J, Wadolkowski E A, Cohen P S. Identification and characterization of mouse small intestine mucosal receptors for Escherichia coli K-12(K88ab) Infect Immun. 1986;52:18–25. doi: 10.1128/iai.52.1.18-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merkle R K, Poppe I. Carbohydrate composition analysis of glycoconjugates by gas-liquid chromatography/mass spectrometry. Methods Enzymol. 1994;118:3–40. doi: 10.1016/0076-6879(94)30003-8. [DOI] [PubMed] [Google Scholar]
- 21.Metcalfe J W, Krogfelt K A, Krivan H C, Cohen P S, Laux D C. Characterization and identification of a porcine small intestine mucus receptor for the K88ab fimbrial adhesin. Infect Immun. 1991;59:91–96. doi: 10.1128/iai.59.1.91-96.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris J, Sojka W. Escherichia coli as a pathogen in animals. Spec Publ Soc Gen Microbiol. 1985;13:47–77. [Google Scholar]
- 23.Payne D, O’Reilly M, Williamson D. The K88 fimbrial adhesin of enterotoxigenic Escherichia coli binds to β-1-linked galactosyl residues in glycosphingolipids. Infect Immun. 1993;61:3673–3677. doi: 10.1128/iai.61.9.3673-3677.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson G L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- 25.Rao P, Pattabiraman T. Reevaluation of the phenol-sulfuric acid reaction for the estimation of hexoses and pentoses. Anal Biochem. 1989;181:18–22. doi: 10.1016/0003-2697(89)90387-4. [DOI] [PubMed] [Google Scholar]
- 26.Rapacz J, Hasler-Rapacz J. Polymorphism and inheritance of swine small intestinal receptors mediating adhesion of three serological variants of Escherichia coli-producing K88 pilus antigen. Anim Genet. 1986;17:305–321. doi: 10.1111/j.1365-2052.1986.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 27.Rutter J M, Burrows M R, Sellwood R, Gibbons R A. A genetic basis for resistance to enteric disease caused by Escherichia coli. Nature (London) 1975;257:135–136. doi: 10.1038/257135a0. [DOI] [PubMed] [Google Scholar]
- 28.Seignole D, Grange P, Duval-Iflah Y, Mouricout M. Characterization of O-glycan moieties of the 210 and 240 kDa pig intestinal receptors for Escherichia coli K88ac fimbriae. Microbiology. 1994;140:2467–2473. doi: 10.1099/13500872-140-9-2467. [DOI] [PubMed] [Google Scholar]
- 29.Sellwood R. The interaction of the K88 antigen with porcine intestinal epithelial cell brush borders. Biochim Biophys Acta. 1980;632:326–335. doi: 10.1016/0304-4165(80)90090-2. [DOI] [PubMed] [Google Scholar]
- 30.Sellwood R, Gibbons G, Jones W, Rutter J. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975;8:405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- 31.Stromqvist M, Gruffman H. Periodic acid/Schiff staining of glycoproteins immobilized to a blotting matrix. BioTechniques. 1992;13:744–749. [PubMed] [Google Scholar]
- 32.Strous G J, Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27:57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- 33.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiser M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973;248:2536–2541. [PubMed] [Google Scholar]
- 35.Willemsen P T J, de Graaf F K. Age and serotype dependent binding of K88 fimbriae to porcine intestinal receptors. Microb Pathog. 1992;12:367–375. doi: 10.1016/0882-4010(92)90099-a. [DOI] [PubMed] [Google Scholar]
- 36.Willoughby R E. Rotaviruses preferentially bind O-linked sialylglycoconjugates and sialomucins. Glycobiology. 1993;3:437–444. doi: 10.1093/glycob/3.5.437. [DOI] [PubMed] [Google Scholar]
- 37.Wilson I B, Staley T E, Bush L J, Gilliland S E. Recovery of intestinal binding sites for K88 E. coli from pig mucosal organ cultures. Mol Cell Biochem. 1984;62:57–65. doi: 10.1007/BF00230078. [DOI] [PubMed] [Google Scholar]