Abstract
Background
Sect. Tuberculata belongs to Camellia, and its members are characterized by a wrinkled pericarp and united filaments. All the plants in this group, which are endemic to China, are highly valuable for exploring the evolution of Camellia and have great potential for use as an oil source. However, due to the complex and diverse phenotypes of these species and the difficulty of investigating them in the field, their complex evolutionary history and interspecific definitions have remained largely unelucidated.
Results
Therefore, we newly sequenced and annotated 12 chloroplast (cp) genomes and retrieved the published cp genome of Camellia anlungensis Chang in sect. Tuberculata. In this study, comparative analysis of the cp genomes of the thirteen sect. Tuberculata species revealed a typical quadripartite structure characterized by a total sequence length ranging from 156,587 bp to 157,068 bp. The cp.genome arrangement is highly conserved and moderately differentiated. A total of 130 to 136 genes specific to the three types were identified by annotation, including protein-coding genes (coding sequences (CDSs)) (87–91), tRNA genes (35–37), and rRNA genes (8). The total observed frequency ranged from 23,045 (C. lipingensis) to 26,557 (C. anlungensis). IR region boundaries were analyzed to show that the ycf1 gene of C. anlungensis is located in the IRb region, while the remaining species are present only in the IRa region. Sequence variation in the SSC region is greater than that in the IR region, and most protein-coding genes have high codon preferences. Comparative analyses revealed six hotspot regions (tRNA-Thr(GGT)-psbD, psbE-petL, ycf15-tRNA-Leu(CAA), ndhF-rpl32, ndhD, and trnL(CAA)-ycf15) in the cp genomes that could serve as potential molecular markers. In addition, the results of phylogenetic tree construction based on the cp genomes showed that the thirteen sect. Tuberculata species formed a monophyletic group and were divided into two evolutionarily independent clades, confirming the independence of the section.
Conclusions
In summary, we obtained the cp genomes of thirteen sect. Tuberculata plants and performed the first comparative analysis of this group. These results will help us better characterize the plants in this section, deepen our understanding of their genetic characteristics and phylogenetic relationships, and lay the theoretical foundation for their accurate classification, elucidation of their evolutionary changes, and rational development and utilization of this section in the future.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-024-09982-w.
Keywords: Sect. Tuberculata, Cp genomes, Phylogenetic relationship, Features, Comparative analysis
Background
Camellia is the largest genus of Theaceae; it consists primarily of evergreen shrubs or trees and contains more than 200 species in 18 sections according to the taxonomy of Chang et al. [1, 2]. Compared with other genera of Theaceae, Camellia is a relatively primitive lineage with many flowers and a basal phylogenetic position. Camellia species are distributed on both sides of the Tropic of Cancer in East Asia, with approximately 238 species present in China. Most of the species are found in Yunnan, Guangxi, Guangdong and Sichuan, China, and the rest are distributed in the Indo-China Peninsula and Japan, eastern India and the Philippines. Camellia species are typical representatives of the Chinese flora, and many Camellia species are endemic to China [1, 3]. These species have a high utilization value and are important sources of materials for tea and oil production [4]. For example, the leaves of Camellia species are used to make tea, which is one of the three most famous nonalcoholic drinks worldwide and is a vital commodity in global trade. In addition, some species of Camellia in China are essential woody oil plants. These fruits are often used to produce safe oil products with high economic value and abundant health benefits.
In sect. Tuberculata Chang belongs to Camellia and is named for the “tuberculiform protuberance on the surface of the ovary and fruit”; it is thus considered a specialized taxon within sect. Tuberculata that has retained its primitive shape [5]. In 1939, the famous botanist Chongshu Qian first discovered a Camellia plant with a raised capsule peel in Sichuan Province, China, and named it Camellia tuberculata Chien, after which it was merged into sect. Pseudocamellia Sealy Rev. [6]. It was not until 1981 that sect. Tuberculata Chang was first identified by Chang and is now considered to include six species [1]. Twelve new species were reported over the next decade [7]. The species were divided into two subgroups (subsect. Tuberculata Chang and subsect. Nudicarpa Chang) according to whether the ovary had a wrinkled pericarp [7]. Currently, the division of sects. Tuberculata into groups has been recognized by many Camellia taxonomists. However, there is notable taxonomic conflict regarding the interspecific taxonomic relationships of these plants. Min [5] eliminated the classification levels of “subgroup” and “lineage” in this group and merged the 18 species into 6 species and 4 varieties. Notably, these classifications are based only on morphological data and lack a basis on data from molecular biology and other disciplines. This problematic systematic classification of sect. Tuberculata has aroused great interest among researchers, who have started to search for various methods, including classical classification methods, to address this problem. At present, the widely accepted classification system for sects. Tuberculata [1, 3] is based on the morphological characteristics of the plants. However, traditional morphological identification of species can be affected by environmental and human interference, resulting in unreliable results. Therefore, the main reason for the divergence in the classification of sect. Tuberculata is the use of purely morphological traits [8], which greatly hinders the conservation and utilization of plant germplasm resources.
The chloroplast (cp) is an important energy converter that functions in higher plants and some algae to promote life activities and is rich in genetic information; furthermore, cp has evolved slowly in most angiosperms [9]. Because of their conserved overall structure and high base substitution rates, they are useful for resolving the kinship of plant species and clarifying the evolution of developmental systems [10, 11]. Although cp genes evolve slowly and are conserved in sequence and structure, narrowing and widening of their edges in IR regions are common and important factors contributing to cp genome length differences and structural variation. In contrast to the plant genome, the cp genome has a simple structure, a small molecular weight, multiple copies, conserved sequences, and slow evolution, as well as additional repetitive sequences and rich genetic information; these characteristics are useful for constructing a molecular phylogenetic tree [12, 13]. Furthermore, phylogenies constructed based on complete cp genome sequences are highly convincing and credible [14]. As next-generation DNA sequencing (NGS) technology has matured and sequencing prices have decreased, obtaining complete plasmid sequences in the laboratory has become common [15]. By searching the literature and the National Center for Biotechnology Information (NCBI) database, we found that only four complete cp.genomes in sect. Tuberculata have been reported [16–19]. This lack of data limits further exploration of this species.
In this study, we performed high-throughput sequencing on 12 samples of 12 species taken from the field. The cp genomes of sect. Tuberculata plants were analyzed in combination with information in the NCBI database. This study aimed to address the following: (1) perform a comparative evaluation of the genomes to discover structural aspects and variations in the sequences, (2) analyze codon preferences and cp genome diversity among species and identify hotspots with high codon usage preferences and high nucleotide diversity among species, and (3) construct a phylogenetic tree based on the cp genomes of the 13 sect. Tuberculata plants to preliminarily explore their phylogeny and relationships. In this way, we aimed to enrich the cp genome database of sect. Tuberculata and provide a basis for further taxonomic identification and utilization of sect. Tuberculata species.
Results
Chloroplast genome structure and features
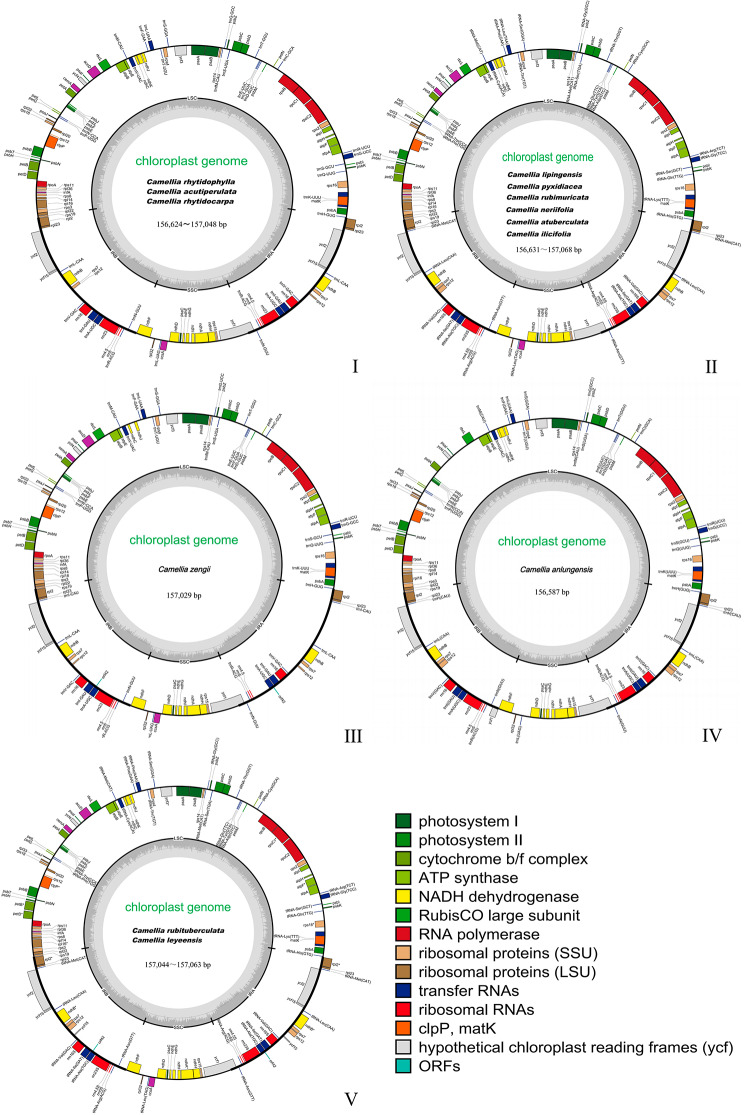
Comparative analysis of the cp genomes of the thirteen sect. Tuberculata species revealed a typical quadripartite structure characterized by a total sequence length of 156,587 bp (C. anlungensis) to 157,068 bp (C. atuberculata), including the LSC region (86,212−86,732 bp), the SSC region (18,276−18,339 bp), and two identical inverted repeat (IR) regions (51,986−52,130 bp) (Fig. 1; Table 1). Moreover, the thirteen sect. Tuberculata species were classified according to the number of genes, and five cp genome types were obtained (Fig. 1; Table 1). A total of 130 to 136 genes specific to the three types were obtained by annotation, including protein-coding genes (coding sequences (CDSs)), tRNA genes, and rRNA genes (87–91, 35–37, and 8, respectively). The GC content ranged from 37.30 to 37.34%. The SSC region (30.59–30.63%), LSC region (35.31–35.37%), and IR region (42.94–42.98%) are in order from small to large. The cumulative length of the CDSs ranged from 79,099 (C. neriifolia) to 80,175 bp (C. leyeensis), and the GC contents of the CDSs ranged from 37.52 to 37.65%. In addition, the first codon position, the second codon position, and the third codon position had the following order of GC content from low to high in the CDSs: third position (29.40-29.53%), second position (37.94-38.04%), and first position (45.19-45.42%) (Table 1).
Fig. 1.
Maps of the thirteen sect. Tuberculata cp genomes
Table 1.
Structure of the complete cp genomes of sect. Tuberculata plants
|
C. rhytidophylla |
C. acutiperulata |
C. rhytidocarpa |
C. lipingensis |
C. pyxidiacea |
C. rubimuricata |
C. neriifolia |
C. atuberculata |
C. ilicifolia |
C. zengii |
C. anlungensis |
C. rubituberculata |
C. leyeensis |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome size (bp) | 156,625 | 156,624 | 157,048 | 157,011 | 156,677 | 156,631 | 157,067 | 157,068 | 157,067 | 157,029 | 156,587 | 157,044 | 157,063 | |
| GC (%) | 37.34 | 37.33 | 37.31 | 37.30 | 37.33 | 37.33 | 37.30 | 37.31 | 37.31 | 37.31 | 37.33 | 37.31 | 37.30 | |
| LSC size (bp) | 86,227 | 86,212 | 86,648 | 86,732 | 86,261 | 86,237 | 86,675 | 86,675 | 86,674 | 86,630 | 86,262 | 86,689 | 86,661 | |
| SSC size (bp) | 18,282 | 18,282 | 18,282 | 18,281 | 18,286 | 18,276 | 18,282 | 18,283 | 18,283 | 18,281 | 18,339 | 18,279 | 18,284 | |
| IR size (bp) | 52,116 | 52,130 | 52,118 | 51,998 | 52,130 | 52,118 | 52,110 | 52,110 | 52,110 | 52,118 | 51,986 | 52,076 | 52,118 | |
| GC of LSC (%) | 35.37 | 35.36 | 35.33 | 35.32 | 35.35 | 35.37 | 35.31 | 35.31 | 35.31 | 35.33 | 35.31 | 35.31 | 35.31 | |
| GC of SSC (%) | 30.60 | 30.59 | 30.62 | 30.63 | 30.62 | 30.59 | 30.61 | 30.61 | 30.61 | 30.62 | 30.60 | 30.62 | 30.60 | |
| GC of IR (%) | 42.95 | 42.95 | 42.95 | 42.96 | 42.95 | 42.94 | 42.96 | 42.97 | 42.97 | 42.95 | 42.96 | 42.98 | 42.96 | |
| GC of CDSs (%) | 37.55 | 37.61 | 37.54 | 37.53 | 37.53 | 37.52 | 37.61 | 37.61 | 37.61 | 37.61 | 37.54 | 37.65 | 37.55 | |
| 1st position GC (%) | 45.26 | 45.37 | 45.23 | 45.23 | 45.19 | 45.34 | 45.37 | 45.37 | 45.37 | 45.34 | 45.24 | 45.42 | 45.26 | |
| 2nd position GC (%) | 37.97 | 38.04 | 37.98 | 37.94 | 37.94 | 37.94 | 38.04 | 38.03 | 38.04 | 38.04 | 37.97 | 38.00 | 37.97 | |
| 3rd position GC (%) | 29.40 | 29.43 | 29.40 | 29.42 | 29.43 | 29.43 | 29.46 | 29.43 | 29.43 | 29.46 | 29.40 | 29.53 | 29.40 | |
| Length of CDSs | 79,487 | 79,500 | 79,659 | 79,753 | 79,767 | 79,925 | 79,099 | 79,161 | 79,160 | 79,395 | 79,671 | 80,155 | 80,175 | |
| Number of genes | 130 | 130 | 130 | 132 | 132 | 132 | 132 | 132 | 132 | 133 | 134 | 136 | 136 | |
| Number of CDSs | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 88 | 89 | 91 | 91 | |
| Number of tRNAs | 35 | 35 | 35 | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 | |
| Number of rRNAs | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | |
| Genome type | I | I | I | II | II | II | II | II | II | III | IV | In | In | |
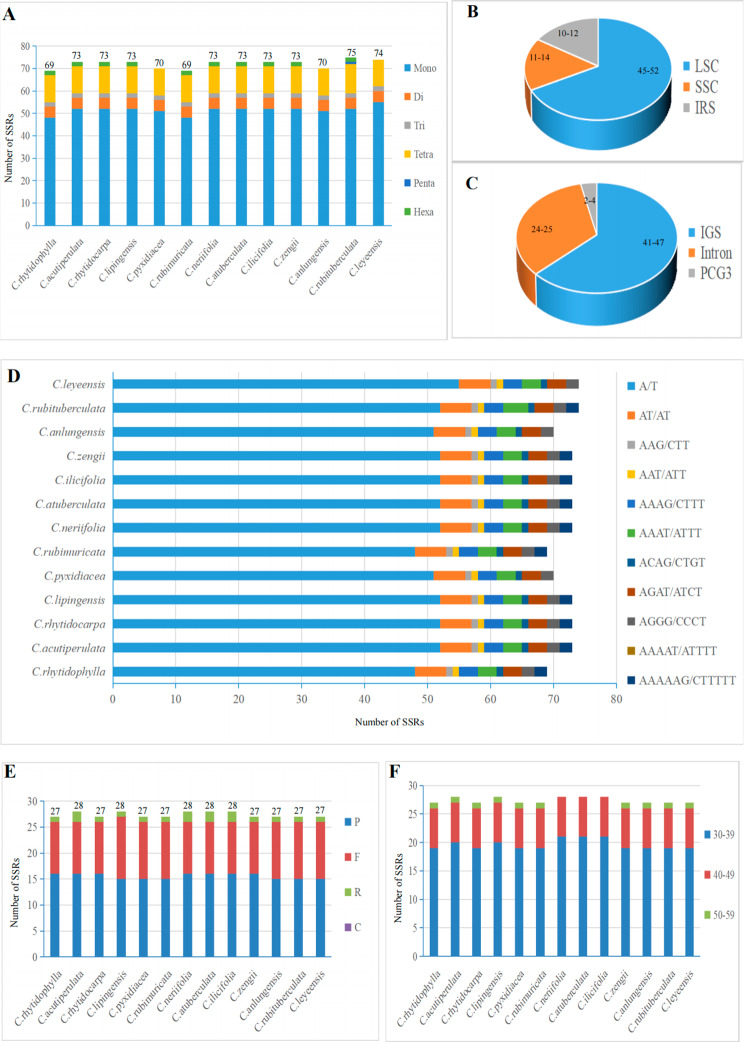
Repeat structure and simple sequence repeat analyses
In this study, a total of 69 (C. rhytidophylla and C. rubimuricata) to 75 (C. rubituberculata) SSRs of six types were detected, including 48–55 mononucleotides (mono-), 5 dinucleotides (di-), 2 trinucleotides (tri-), 12 tetranucleotides (tetra-), 0–1 pentanucleotide (penta-), and 0–2 hexanucleotides (hex-) (Fig. 2A). Among them, one pentanucleotide distributed in the IR region, AAAAT/ATTTT, was found only in C. rubituberculata (Fig. 2D). A statistical evaluation of all the identified SSRs revealed 45–52, 11–14, and 10–12 SSRs in the LSC, SSC, and IR regions, respectively (Fig. 2B). In addition, we found large differences in the distribution of these SSRs, with the largest number of SSRs occurring in intergenic sequences (IGSs) (41–47), followed by CDSs (24–25) and introns (2–4) (Fig. 2C). Among the six SSR repeat types, A/T was the only single-nucleotide SSR type, and the remaining five types of repeat units consisted mainly of A or T (Fig. 2D). These long repeat sequences ranged from 30 to 50 bp in length (Fig. 2F). Analysis of the scattered repeats with REPuter identified 27–28 scattered repeats, and no complementary (C) repeats were found. (Fig. 2E).
Fig. 2.
Comparative analysis of cp genome repeat sequences of thirteen species from sect. Tuberculata. (A): The numbers of the six SSR types; (B): The numbers of SSRs distributed in different copy regions; (C): The numbers of SSRs distributed in different gene regions; (D): The numbers of different SSR repeat unit types; (E): The numbers of the four long repeat types. (F): The length of the four long repeat types
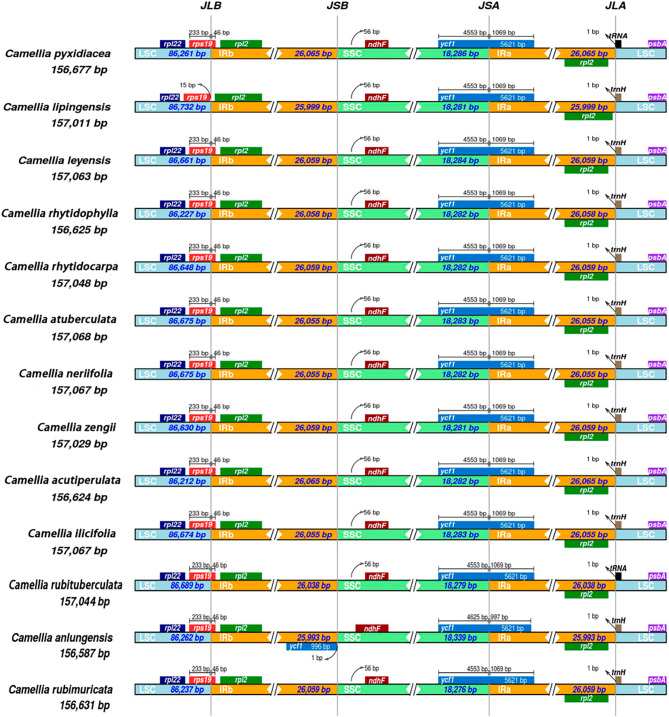
Contraction and expansion of IR boundaries
Narrowing and widening of the edge of the IR region are very common; this phenomenon has become a popular topic of research and is the major driver of size differences between cp genomes [20, 21]. To clarify the spreading status of the IR region boundaries, the boundaries of the cp genomes of the thirteen sect. Tuberculata species were compared in this study. The results showed that the cp genome was conserved. However, there were still some structural variations in the various boundary zones (Fig. 3). In the sect. Tuberculata plants, the LSC/IRb boundary was located within the rps19 gene, 46 bp of which extended into the IRb region. However, the rps19 gene of C. lipingensis did not extend into the IRb region, and this region was separated from the LSC/IRb border by 15 bp.
Fig. 3.
Analysis of the cp genome IR region boundaries of the thirteen species of sect. Tuberculata
The SSC/IRa boundary of C. rubituberculata, C. rubimuricata, C. anlungensis, C. pyxidiacea, C. lipingensis, C. neriifolia, C. rhytidophylla, C. rhytidocarpa, C. atuberculata, C. acutiperulata, C. ilicifolia, C. zengii and C. leyeensis was located in ycf1. In C. anlungensis, 997 bp of ycf1 extended into the IRa region, while in the other species, the extension length was 1,069 bp. Interestingly, in C. anlungensis, 996 bp of the ycf1 gene was located in the IRb region 1 bp from the SSC region. All the rps19 genes were located at the LSC/IRb boundary, with rps19 from C. lipingensis differing by 15 bp from the IRb region and all the other species extending 46 bp toward the IRb region. The ndhF, tRNA and trnH genes located in the SSC and LSC regions in the thirteen cp genomes showed no expansion to the IR regions. The IR regions of C. rubituberculata and C. pyxidiacea were located in the tRNA gene coding area, and those of C. rubimuricata, C. lipingensis, C. leyeensis, C. anlungensis, C. neriifolia, C. rhytidophylla, C. rhytidocarpa, C. atuberculata, C. acutiperulata, C. ilicifolia and C. zengii were located in the trnH gene (Fig. 3).
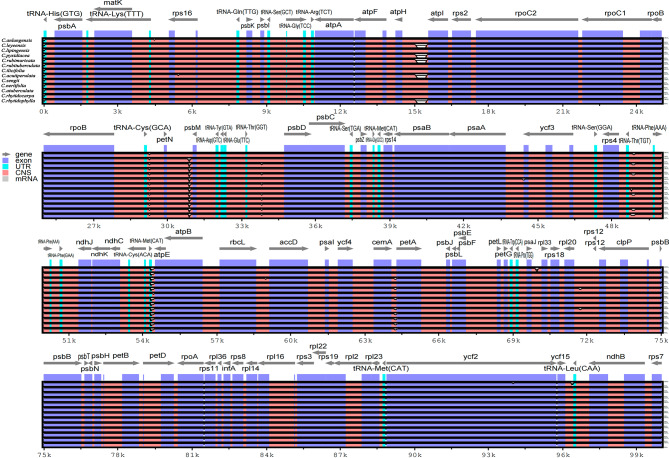
Comparative cp Genome analysis
In this study, the cp genomes of the thirteen sect. Tuberculata species were visually compared with those of mVISTA, and the C. szechuanensis was selected as a reference. The results showed that the cp genomes of the thirteen sect. Tuberculata species were conserved. Moreover, there was a difference in the IR region compared with the LSC and SSC regions, and the same was true for the noncoding and coding regions (Fig. 4). The regions with greater variation were located mainly in tRNA-His(GTG)-psbA, atpH-atpl, petN-psbM, tRNA-Thr(TGT)-tRNA-Phe(AAA), and tRNA-Met(CAT)-atpE.
Fig. 4.
Sequence identity plot of the cp genomes of the thirteen sect. Tuberculata species. The C. szechuanensis sequence was used as a reference
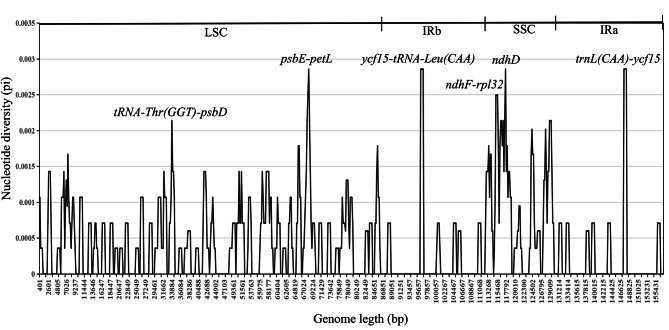
DNA molecular markers are usually highly variable regions of sequences that can be used for the differentiation of relationships between species. Therefore, to further understand the DNA polymorphisms (Pi), mutation hotspot regions in the cp genomes of the thirteen sect. Tuberculata plants were screened using DnaSP (Fig. 5). Pi analysis revealed that the pi values ranged from 0 to 0.00286, and the cp genome was relatively structurally conserved, small, and highly variable among the species. A total of six mutation hotspot regions (Pi > 0.0022) were detected, and they can be used as potential molecular markers. Among these, tRNA-Thr(GGT)-psbD and psbE-petL were located in the LSC region; ndhF-rpl32 and ndhD were located in the SSC region; and trnL (CAA)-ycf15 and ycf15-tRNA-Leu (CAA) were located in the IR region (Fig. 5).
Fig. 5.
Comparison of nucleotide diversity (Pi) values among the thirteen sect. Tuberculata species in each section (window length: 800 bp; step size: 200 bp)
Codon usage
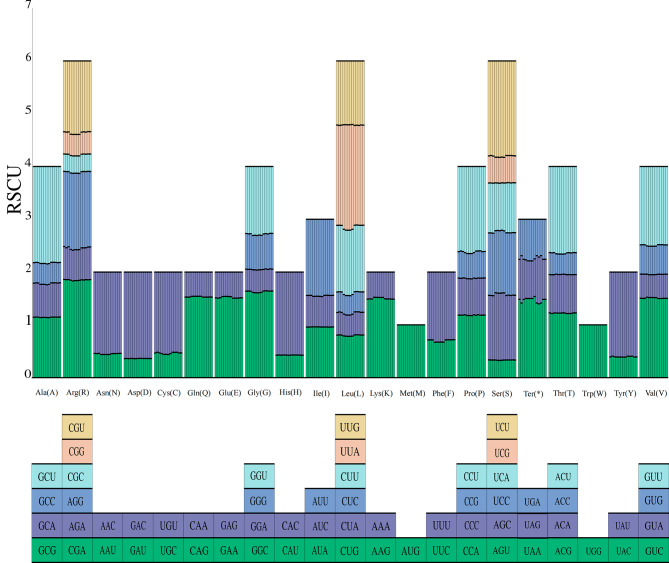
Codon usage preferences are developed by organisms during long-term evolution and reflect the combined effects of genetics, evolution, and mutation on genes and species [22, 23]. In this study, the Thirteen sect. Tuberculata cp genomes were statistically analyzed and visually mapped based on relative synonymous codon usage (RSCU) (Fig. 6). A total of 64 different RSCU values were obtained for each species. For each of the thirteen sect. Tuberculata species, 64 codons were detected, and the remaining 61 codons edited 20 amino acids, except for the stop codons UAA, UAG, and UGA. The total observed frequency ranged from 23,045 (C. lipingensis) to 26,557 (C. anlungensis) (Table S2). Notably, both methionine and tryptophan have only one synonymous codon, while leucine, serine, and arginine have six synonymous codons.
Fig. 6.
Codon content for 20 amino acids and stop codons in all the protein-coding genes of the cp genomes of the thirteen sect. Tuberculata species
In sect. Tuberculata, the most common encoded amino acid is leucine, the least common encoded amino acid is cysteine, the most common codon is UUU, and the least common codon is GCG. A codon with an RSCU value greater than 1.00 was the preferred codon. In addition, many preferred codons end in A or T, while nonpreferred codons end in C or G, which suggests a reduction in GC content in the coding region, which is a widespread phenomenon [24, 25].
Phylogenetic analysis
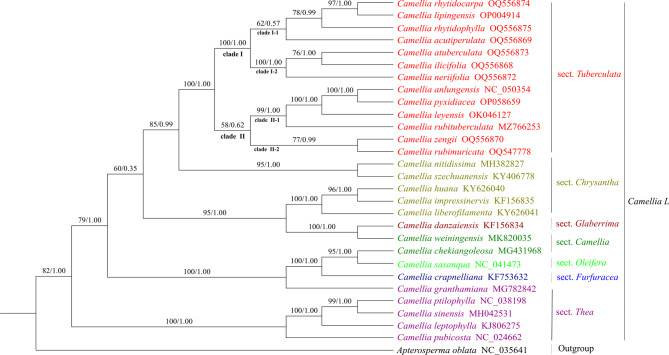
A phylogenetic tree was constructed using 29 cp genomes, and the phylogenetic trees constructed by the ML method and the BI method were merged, illustrating the genetic relationships of the species (Fig. 7). The thirteen species from sect. Tuberculata used in this study, another 15 species and 1 outgroup are shown. In the phylogenetic trees, all plants in section Tuberculata were clustered on one large branch, and the plants in section Tuberculata were divided into groups different from those in other groups of Camellia L., confirming the independence of this section. In addition, the thirteen species of plants belonging to sect. Tuberculata were divided among two typical subbranches and four minor branches. Sect. Tuberculata was divided into four small branches: (clade I-1) C. rhytidophylla, C. rhytidocarpa, C. atuberculata and C. lipingensis on a branch (BS, PP = 62%, 0.57, respectively); (clade I-2) C. atuberculata, C. ilicifolia and C. neriifolia on a branch (BS, PP = 100%, 1.00, respectively); (clade II-1) C. rubituberculata, C. anlungensis, C. pyxidiacea and C. leyeensis on a branch (BS, PP = 99%, 1.00, respectively); and (clade II-2) C. rubimuricata and C. zengii on a branch (BS, PP = 77%, 0.99, respectively).
Fig. 7.
Phylogenetic tree obtained using the maximum likelihood (ML) and Bayesian inference (BI) methods for the sect. Tuberculata species based on complete cp genomes
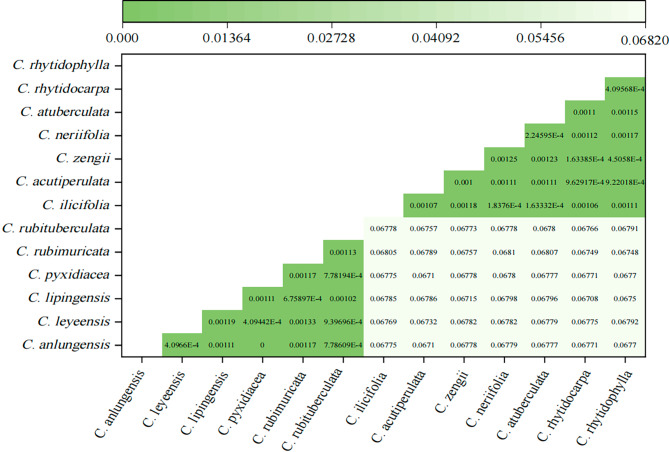
Among these species, C. anlungensis and C. pyxidiacea formed a subgroup distinct from C. leyeensis, which further supports the idea that they come from the same origin and are evolutionarily related. Additionally, C. leyeensis and C. rubituberculata converged on the same small branch. C. rhytidocarpa first matched C. lipingensis and then gradually joined C. rhytidophylla and C. atuberculata. The reliability of the phylogenetic tree was further validated by the genetic distance of the sect. Tuberculata cp genomes, which ranged from 0.00000 (C. anlungensis and C. pyxidiacea) to 0.06805 (C. rubimuricata and C. ilicifolia) (Fig. 8).
Fig. 8.
Genetic distances of the cp genomes of sect. Tuberculata
Discussion
Previous studies have shown that the length of land plant cp genomes ranges from 120 to 170 kb [26]. In this study, comparative analysis of the cp genomes of the thirteen sect. Tuberculata species revealed a typical quadripartite structure characterized by a total sequence length of 156,587 bp (C. anlungensis) to 157,068 bp (C. atuberculata), including the LSC region (86,212−86,732 bp), the SSC region (18,276−18,339 bp), and two identical inverted repeat (IR) regions (51,986−52,130 bp). The GC content of the thirteen sect. Tuberculata plants ranged from 37.30 to 37.34%. In particular, in agreement with the results of many studies on angiosperms, the GC content was highest in the IR regions [27]. The differences in the cp genome among the species were obvious and attributed to changes in base composition. The conversion between sequences and higher GC content may be important factors leading to greater conservation of IR regions [28, 29].
For cp genomes, long repeat sequences facilitate the integration of transferred plasmid sequences into the host genome [30]. In this study, we found 69 (C. rhytidophylla and C. rubimuricata) to 75 (C. rubituberculata) SSR loci, and the LSC regions accounted for approximately 68% of the loci. The mononucleotide A/T was the most common, with a frequency of 71.64–74.32% in the cp genomes of sect. Tuberculata, consistent with values in other genera [31, 32]. Additionally, the cp SSRs in sect. Tuberculata also exhibited a high A/T content, which was positively correlated with the variability in cp genome sequences [33]. In conclusion, the statistical analysis of SSRs and interspersed repeats in the 13-sect. Tuberculata cp genomes laid the foundation for identification of similar molecular markers, genetic modifications, and species in sect. Tuberculata.
In angiosperms, the IR region is relatively conserved in sequence and structure, and the narrowing and widening of its edges are not only important factors for length variation but also the main cause of the emergence of pseudogenes [34, 35]. Although cp genes evolve slowly and are relatively conserved in terms of sequence and structure, boundary contraction and expansion in the IR regions are common phenomena. In this study, the LSC/IRb boundaries were located within the rps19 gene, 46 bp of which extended into the IRb region. However, the rps19 gene did not extend into the IRb region in C. lipingensis, and the region was separated from the LSC/IRb boundary by 15 bp. Another interesting finding was that ycf1 was distributed at different boundaries. Therefore, the ycf1 gene influences the expansion or contraction of the IR boundary in sect. Tuberculata.
For all the studied species in sect. Tuberculata, the 64 PCGs encoded 223,045 to 26,557 codons. These results were comparable to those in Trapa, in which 85 genes were found to encode 26,160−26,590 codons [36]. All thirteen species exhibited a highly conserved cp genome in sect. Tuberculata, as in Trapa [36], where the RSCU value of a single amino acid increased with the number of codons encoding that amino acid. Furthermore, the preference for half of all codons ending in A/T may be related to the high A/T content in the cp genome [37].
Molecular markers have many distinct advantages over conventionally applied genetic markers. By selecting highly polymorphic loci for DNA molecular markers, effective support for species kinship identification and gene pool construction can be provided [38, 39]. A good DNA barcode must be a short fragment of DNA that is representative of the species and is highly variable and amenable to amplification [40]. In our study, both the sequence and structure of the sect. Tuberculata cp genomes were highly conserved. mVISTA revealed that most of the species in sect. Tuberculata were structurally similar, except for C. leyeensis, C. pyxidiacea, C. rubimuricata, C. acutiperulata, and C. rhytidophylla. We observed that most of the variation in nucleotide sequences occurred in noncoding regions, which is the same as the findings of previous reports, from which we can infer that this variation may be a basic feature of angiosperms [41–43]. In addition, based on nucleotide diversity (Pi) analysis, six regions with large hotspot areas were identified as key loci for genetic studies, including five intergenic regions (tRNA-Thr(GGT)-psbD, psbE-petL, ycf15-tRNA-Leu(CAA), ndhF-rpl32, and trnL(CAA)-ycf15) and one genic region (ndhD). In conclusion, these mutation hotspot regions will play an important role in the identification and characterization of plant species in sect. Tuberculata.
The cp genome is the main object of molecular biology research and has become a current research hotspot for species genealogy identification. In particular, phylogenetic analysis via whole-genome sequencing has become an important tool due to the improvements in sequencing technology and low cost. Phylogenetic trees constructed based on a single or a few gene sequences can have inconsistent or even conflicting topologies due to differences in evolutionary rates and horizontal shifts between genes, making it difficult to fully determine the correct evolutionary relationships of species [44, 45]. In this study, a phylogenetic tree was constructed using the BI method and the ML method. The cp genomes of the thirteen sect. Tuberculata species converged into a branch with high support. C. rhytidocarpa was similar to C. lipingensis and may be the same species. C. ilicifolia was classified on the same branch as C. atuberculata and C. neriifolia (BS and PP = 100% and 1.00, respectively), indicating that the three are similar and supporting the merger of C. ilicifolia into sect. Tuberculata [3]. The classification of C. atuberculata, C. neriifolia and C. ilicifolia as different species was supported [1]. C. acutiperulata, C. anlungensis, C. pyxidiacea, C. leyeensis, C. rubimuricata, C. zengii and C. rubituberculata formed a monophyletic group, which suggested that C. pyxidiacea and C. rubituberculata are separate species and that C. anlungensis and C. leyeensis are separate species [3]. On the basis of the phylogenetic tree and genetic distance results, we speculate that C. anlungensis, C. pyxidiacea, and C. leyeensis evolved from C. rubituberculata. Together, C. zengii and C. rubimuricata form a small branch, but the support rate is low, with support for only the independent taxonomic statuses of the two species [1]. As shown in our study, high-resolution cp genome sequences provide resources for broad research on the genetic information and species identification of sect. Tuberculata.
Conclusion
In this study, for the first time, the whole cp genomes of thirteen species of plants in sect. Tuberculata were sequenced and compared. These cp genomes all had relatively conserved quadripartite structures. The repeat sequences, codon usage, and mutation hotspot regions of sect. Tuberculata were compared and analyzed, and six mutation hotspot regions were found to be potential molecular markers in this section. Phylogenetic trees and genetic distances were initially used to explore the affinities within sect. Tuberculata. The independent taxonomic status of the section was highly supported, with the thirteen species divided into two typical subclades and four minor clades. In summary, these results not only fill the data gap regarding the cp genome of sect. Tuberculata but also provide an important basis for the comprehensive exploration of cp phylogenetic relationships and the resolution of taxonomic and identification problems.
Materials and methods
Plant materials and DNA extraction
In this study, thirteen sect. Tuberculata samples were used. The cp genome sequence of one of the samples (C. anlungensis) was downloaded from the NCBI database, and the other samples were collected from Guizhou and Guangxi provinces in China. Fresh, young, or old leaves in good growth condition and not contaminated by other organisms were collected from 12 plants, placed in self-sealing bags with color-changing silica gel, and stored at -80 °C until use. The specimens were arranged and preserved in the tree herbarium of the School of Forestry, Guizhou University (GZAC) (Fig. 9, Table S1). Total DNA from the 12 samples was extracted in the laboratory using the CTAB method. The purity of the DNA was determined by an Ultra-Micro spectrophotometer, and the quality of the extracted DNA was examined via 1% agarose gel electrophoresis.
Fig. 9.
Morphological characteristics of thirteen species in sect. Tuberculata. (1: Fruit; 2: Flower; A: C. rhytidophylla; B: C. acutiperulata; C: C. rubituberculata; D: C. lipingensis; E: C. pyxidiacea; F: C. atuberculata; G: C. neriifolia; H: C. atuberculata; I: C. ilicifolia; J: C. zengii; K: C. anlungensis; L: C. leyeensis; M: C. rhamnoea.)
DNA sequencing, assembly and annotation
Sequencing libraries were constructed by DNA fragment end repair, ligation splicing, PCR amplification, and magnetic bead sorting to purify the ligated products. The gene library concentrations were determined using a Thermo Qubit 4.0 fluorescence quantification instrument. Libraries were sequenced using the Illumina high-throughput sequencing platform. A total of 3.7–8.37 GB of raw data were obtained (Table 1). Low-quality sequences based on quality statistics were trimmed with Trimmomatic software [46]. The clean screened cp reads were compared with published sequences from sect. Tuberculata in the NCBI database. A circular cp gene map was obtained by de novo splicing of the filtered data using SOAPdenovo 2 and NOVOPlasty [47, 48]. Finally, the complete cp genomes were obtained by online annotation, BLAST comparison, and manual correction using C. rubituberculata Chang & Yu (MZ766253) as the reference sequence. Cp genome mapping was performed using the online tool OGDRAW v1.3.1 (http://ogdraw.mpimp-golm.mp-g.de/) [49]. All annotated genomes were uploaded to NCBI, and GenBank accession numbers were obtained (Table 2).
Table 2.
Sampling information for sect. Tuberculata
| Number | Species | Location | Specimen number | GenBank accession number |
|---|---|---|---|---|
| 1 | Camellia rhytidophylla Y. K. Li &; M.Z.Yang | Kaiyang County, Guizhou, China | GZAC, LZ20220802 | OQ556875 |
| 2 | Camellia acutiperulata Chang & Ye | Longlin County, Guangxi, China | GZAC, LZ20221103 | OQ556869 |
| 3 | Camellia rhytidocarpa Chang & Liang | Longsheng County, Guangxi, China | GZAC, LZ20221106 | OQ556874 |
| 4 | Camellia lipingensis Chang | Liping County, Guizhou, China | GZAC, LZ20210830 | OP004914 |
| 5 | Camellia pyxidiacea Xu F.P. Chen & C.Y.Deng | Xingyi City, Guizhou, China | GZAC, LZ20211204 | OP058659 |
| 6 | Camellia rubimuricata Chang & Z.R.Xu | Libo County, Guizhou, China | GZAC, LZ20211213 | OQ547778 |
| 7 | Camellia neriifolia Hung T.Chang | Chishui City, Guizhou, China | GZAC, LZ20220821 | OQ556872 |
| 8 | Camellia atuberculata Chang | Chishui City, Guizhou, China | GZAC, LZ20220820 | OQ556873 |
| 9 | Camellia ilicifolia Y.K.Li | Chishui City, Guizhou, China | GZAC, LZ20221108 | OQ556868 |
| 10 | Camellia zengii Chang | Liping County, Guizhou, China | GZAC, LZ20210829 | OQ556870 |
| 11 | Camellia anlungensis Chang | Wangmo County, Guizhou, China | - | NC_050354 |
| 12 | Camellia rubituberculata Chang & Yu | Xingren County, Guizhou, China | GZAC, LZ20210411 | MZ766253 |
| 13 | Camellia leyeensis Chang & Y.C. Zhong | Leye County, Guangxi, China | GZAC, LZ20210413 | OK046127 |
Repeat sequence analysis
MISA v2.1 [50] software was used to find SSRs in the organelle genomes. The minimum repeat units and number of repeats were set as follows: at least 10 for mononucleotide (mono-) repeats, at least 5 for dinucleotide (di-) repeats, at least 4 for trinucleotide (tri-) repeats, at least 3 for tetranucleotide (tetra-) repeats, at least 3 for pentanucleotide (penta-) repeats, and at least 3 for hexanucleotide (hexa-) repeats. The REPuter (https://bibiserv.cebitec.uni-bielefeld.de/reputer) [51] online tool was used to search for larger repeat sequences with a Hamming distance of 3 and a minimum repeat size of 30 bp. The four types were forward (F), reverse (R), complement (C), and palindromic (P).
Genome structure, genome comparison and divergence hotspot identification
The boundary regions of the cp genomes of the 13 sect. Tuberculata plants were visualized and compared by using IRscope [52] to obtain a macroscopic view of the cp genome structure of the section. The cp genomes of 13 sect. Tuberculata were sequenced using MAFFT v7 [53] and imported into the mVISTA online program (https://genome.lbl.gov/vista/mvista/submit.shtml) [54] for cp genome visualization and comparison using the model Shufe-Lagan (C. szechuanensis C. W. Chi was used as the reference). Molecular evolutionary genetics analysis (MEGA) v11 [55] was used to analyze the codon usage distribution and GC content and for phylogenomic inference. Nucleotide diversity (Pi) analysis of the cp genome after sequence alignment was performed using DnaSP v6 [56].
Codon usage and phylogenetic analysis
Statistical analysis of the preferential relatively synonymous codon usage (RSCU) of the cp genome codons of the 13 sect. Tuberculata plants was performed using CodonW 1.4.2 [57]. Phylogenetic analysis was performed using 28 cp genomes of Camellia species, with Apterosperma oblata (NC_035641) set as the outgroup. The substitution saturation indices (Iss) of the cp genomes were evaluated using DAMBE v5.3.19 [58], and the results were as follows: Iss (0.0809) < Iss.c (0.8428), P = 0.0000. Because the 29 cp genome sequences did not reach saturation, a phylogenetic tree was reconstructed using the maximum likelihood (ML) method in IQ-TREE v1.6.12 [59]. The optimal model (GTR + I + G) was identified using MrModeltest v2.3, and a Bayesian inference (BI) phylogenetic tree was subsequently reconstructed using MrBayes v3.2.7 [60]. The genetic distances of the thirteen sect. Tuberculata cp genomes after alignment were calculated by MEGA11 with 1000 bootstrap replicates.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
ZL conceived the study. ZL and XX collected the samples. CY and MTA performed the experiments and data analysis. ZHR and ZL wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Guizhou Provincial Basic Research Program (Natural Science) 2022 (072), the Characteristic Forestry Research Project of the Guizhou Forestry Bureau (Special Forestry Research 2020-06) and the Survey and Evaluation of New National Key Protected Wild Plants Resources in Guizhou Province (Phase I) (MCHC-ZC20222009).
Data availability
The data provided in this study were uploaded to the NCBI repository (https://www.ncbi.nlm.nih.gov/) under accession numbers NC_050354, MZ766253, OK046127, OP058659, OP004914, OQ547778, OQ556872-OQ556875, OQ556868, and OQ556869-OQ556870.
Declarations
Ethics approval and consent to participate
For the sample collection in this study, no special permission was needed. The research in this paper followed the guidelines and regulations of the Ethics Committee. National and international ethical principles were followed during the sampling, experiments, and investigations of the plants involved in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chang HT. Systematic study of the genus Camellia. In Journal of Sun Yatsen University (Natural Science Edition) Forum. 1981;pp.108–125.
- 2.Wu Q, Tong W, Zhao H, Ge RH, Li RP, Huang J, et al. Comparative transcriptomic analysis unveils the deep phylogeny and secondary metabolite evolution of 116 Camellia plants. Plant J. 2022;111(2):406–21. doi: 10.1111/tpj.15799. [DOI] [PubMed] [Google Scholar]
- 3.Min TL. Studies on the genus Camellia in the world. Kunming: Yunnan Science and Technology Press; 2000. pp. 3–20. [Google Scholar]
- 4.Xia EH, Tong W, Wu Q, Wei S, Zhao J, Zhang ZZ, et al. Tea plant genomics: achievements, challenges and perspectives. Hortic Res. 2020;7. 10.1038/s41438-019-0225-4. [DOI] [PMC free article] [PubMed]
- 5.Min TL, Zhong YC. Revision of the plants of the genus Camellia in the group Verrucosa. Yunnan Plant Research. 1993; (02):123–30.
- 6.Sealy JR. A revision of the genus Camellia. London: the Royal Horticulture Society; 1958. [Google Scholar]
- 7.Chang HT, Ren SX. A classification on the section Tuberculata of Camellia. Act Sci Nat Univ Sunyats. 1991;30(4):86–91. [Google Scholar]
- 8.Luo CQ, Tan XF, Chi LL. A review of the taxonomy of Camellia sinensis. J Cent South Forestry Coll. 1999;19(3):78–81. [Google Scholar]
- 9.Birky CW. Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proc Natl Acad Sci U S A. 1995;92(25):11331. doi: 10.1073/pnas.92.25.11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore MJ, Soltis PS, Bell CD, Burleigh JG, Soltis DE. Phylogenetic analysis of 83 plastid genes further resolves the early diversifification of eudicots. Proc Natl Acad Sci U S A. 2010;107:4623–8. doi: 10.1073/pnas.0907801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaut BS, Morton BR, Mccaig BC. Substitution rate comparisons between grasses and palms: synonymous rate differences at the nuclear gene Adh parallel rate differences at the plastid gene rbcL. Proc Natl Acad Sci U. S. A. 1996;93:10274–10279. 10.1073/pnas.93.19.10274. [DOI] [PMC free article] [PubMed]
- 12.Zheng XM, Wang JR, Feng L, Liu S, Pang HG, Qi L, et al. Inferring the evolutionary mechanism of the chloroplast genome size by comparing whole-chloroplast genome sequences in seed plants. Sci Rep. 2017;7(1):1555. doi: 10.1038/s41598-017-01518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravi V, Khurana JP, Tyagi AK, Khurana P. An update on chloroplast genomes. Plant Syst Evol. 2008; 271(1–2):101–22. 10.1007/s00606-007-0608-0.
- 14.Parks M, Cronn R, Liston A. Increasing phylogenetic resolution at low taxonomic levels using massively parallel sequencing of chloroplast genomes. BMC Biol. 2009;7:84. doi: 10.1186/1741-7007-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi L, Chen H, Jiang M, Wang L, Wu X, Huang L, et al. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res. 2019;47(W1):W65–W73. doi: 10.1093/nar/gkz345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu YY, Xu J, Wang G, Yuan CJ, Luo Y, Dai XY. Characterization of the complete chloroplast genome of Camellia anlungensis. Mitochondrial DNA Part B. 2020;5:873–4. doi: 10.1080/23802359.2020.1716639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao X, Lu JG, Yang GY, Li Z. The complete chloroplast genome of Camellia leyeensis (theaceae). 2022; 7:735–7. 10.1080/23802359.2022.2068980. [DOI] [PMC free article] [PubMed]
- 18.Li YY, Liu HY, Zou J. Complete chloroplast genome of Camellia rubituberculata: a species endemic to Guizhou, China. Mitochondrial DNA Part B. 2021;6:2596–8. doi: 10.1080/23802359.2021.1961625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XF, Sun YB, Huang LL, Xu YC, Zhao CY, Yu B. Complete chloroplast genome sequence of Camellia rhytidophylla, comparative and phylogenetic analysis. Mitochondrial DNA Part B. 2021;6:161–3. doi: 10.1080/23802359.2020.1856010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zong D, Zhou AP, Zhang Y, Zou XL, Li D, Duan A, et al. Characterization of the complete chloroplast genomes of five Populus species from the western Sichuan plateau, Southwest China: comparative and phylogenetic analyses. Peer J. 2019;7:e6386. doi: 10.7717/peerj.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang RJ, Cheng CL, Chang CC, Wu CL, Su TM, Chaw SM. Dynamics and evolution of the inverted repeat-large single copy junctions in the chloroplast genomes of monocots. BMC Evol Biol. 2008;8(1):1–14. doi: 10.1186/1471-2148-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazumdar P, Othman R, Mebus K, Ramakrishnan N, Harikrishna J. Codon usage and codon pair patterns in non-grass monocot genomes. Ann Bot. 2017;00:1–17. doi: 10.1093/aob/mcx112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sloan D, Taylor D. Testing for selection on Synonymous sites in Plant mitochondrial DNA: the role of Codon Bias and RNA editing. J Mol Evol. 2010;70:479–91. doi: 10.1007/s00239-010-9346-y. [DOI] [PubMed] [Google Scholar]
- 24.LaBella A, Opulente D, Steenwyk J, Hittinger C, Rokas A. Variation and selection on codon usage bias across an entire subphylum. PLoS Genet. 2019;15:e1008304. doi: 10.1371/journal.pgen.1008304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li GL, Pan ZL, Gao SC, He YY, Xia QY, Jin Y, et al. Analysis of synonymous codon usage of chloroplast genome in Porphyra umbilicalis. Genes Genomics. 2019;41(10):1173–81. doi: 10.1007/s13258-019-00847-1. [DOI] [PubMed] [Google Scholar]
- 26.Ruhlman TA, Jansen RK. The plastid genomes of fowering plants. Methods Mol Biol. 2014;1132:3–38. doi: 10.1007/978-1-62703-995-6_1. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Nie L, Xu Z, Li P, Wang Y, He C, et al. Comparative and phylogenetic analysis of the complete chloroplast genomes of three paeonia section moutan species (Paeoniaceae). Front Genet. 2020;11. 10.3389/fgene.2020.00980. [DOI] [PMC free article] [PubMed]
- 28.Khakhlova O, Bock R. Elimination of deleterious mutations in plastid genomes by gene conversion. Plant J Cell Mol Biol. 2006;46:85–94. doi: 10.1111/j.1365-313X.2006.02673. [DOI] [PubMed] [Google Scholar]
- 29.Fan WB, Wu Y, Yang J, Shahzad K, Li ZH. Comparative chloroplast genomics of dipsacales species: insights into sequence variation, adaptive evolution, and phylogenetic relationships. Front Plant Sci. 2018;9:689. doi: 10.3389/fpls.2018.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng G, Wei L, Ma L, Wu Z, Chen K. Comparative analyses of chloroplast genomes from 13 Lagerstroemia (Lythraceae) species: identifcation of highly divergent regions and inference of phylogenetic relationships. Plant Mol Biol. 2020;102:659–76. doi: 10.1007/s11103-020-00972-6. [DOI] [PubMed] [Google Scholar]
- 31.Asaf S, Khan AL, Khan AR, Waqas M, Kang SM, Khan MA, et al. Complete chloroplast genome of Nicotiana otophora and its comparison with related species. Front Plant Sci. 2016;7:843. doi: 10.3389/fpls.2016.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuang DY, Wu H, Wang YL, Gao LM, Lu L. Complete chloroplast genome sequence of Magnolia kwangsiensis (Magnoliaceae): implication for DNA barcoding and population genetics. Genome. 2011;54(8):663–73. doi: 10.1139/g11-026. [DOI] [PubMed] [Google Scholar]
- 33.Hong Z, Wu Z, Zhao K, Yang Z, Zhang N, Guo J, et al. Comparative analyses of fve complete chloroplast genomes from the genus Pterocarpus (Fabacaeae) Int J Mol Sci. 2020;21(11):3758. doi: 10.3390/ijms21113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim KJ, Lee HL. Complete chloroplast genome sequences from Korean ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA res. 2004;11(4):247–61. doi: 10.1093/dnares/11.4.247. [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Fan X, Li X, Chen Y, Zucc, Lythraceae The complete chloroplast genome sequence of Trapa Incisa Sieb. Mitochondrial DNA Part B. 2021;6(6):1732–3. doi: 10.1080/23802359.2021.1930601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan XG, Wang WC, Wagutu GF, Li W, LI XL, Chen YY. Fifteen complete chloroplast genomes of Trapa species (Trapaceae): insight into genome structure, comparative analysis and phylogenetic relationships. BMC Plant Biol. 2022;22:230. doi: 10.1186/s12870-022-03608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eguiluz M, Rodrigues NF, Guzman F, Yuyama P, Margis R. The chloroplast genome sequence from Eugenia Unifora, a Myrtaceae from Neotropics. Plant Syst Evol. 2017;303:1199–212. doi: 10.1007/s00606-017-1431-x. [DOI] [Google Scholar]
- 38.Singh RB, Mahenderakar MD, Jugran AK, Singh RK, Srivastava RK. Assessing genetic diversity and population structure of sugarcane cultivars, progenitor species and genera using microsatellite (SSRs) markers. Gene. 2020;753:144800. doi: 10.1016/j.gene.2020.144800. [DOI] [PubMed] [Google Scholar]
- 39.Menezes AP, Resende LC, Buzatti RS, Nazareno AG, Carlsen M, Evanguedes FP, et al. Chloroplast genomes of Byrsonima species (Malpighiaceae): comparative analysis and screening of high divergence sequences. Sci Rep. 2018;8(1):2210. doi: 10.1038/s41598-018-20189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song Y, Wang SJ, Ding YM, Xu J, Li MF, Zhu SF, et al. Chloroplast genomic resource of Paris for species discrimination. Sci Rep. 2017;7:3427. doi: 10.1038/s41598-017-02083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng H, Li JF, Zhang H, Cai BH, Gao ZH, Qiao YS, et al. The complete chloroplast genome sequence of strawberry (Fragaria × ananassa Duch.) And comparison with related species of Rosaceae. PeerJ. 2017;5:e3919. doi: 10.7717/peerj.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clegg MT, Gaut BS, Learn GH, Morton BR. Rates and patterns of chloroplast DNA evolution. Proc Nati Acad Sci USA. 1994;91(15):6795–801. doi: 10.1073/pnas.91.15.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyagi S, Jung JA, Kim JS, Won SY. Comparative analysis of the complete chloroplast genome of mainland Aster Spathulifolius and other Aster species. Plants. 2020;9:568. doi: 10.3390/plants90505680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Firetti F, Zuntini AR, Gaiarsa JW, Oliveira RS, Lohmann LG, VanSluys MA. Complete chloroplast genome sequences contribute to plant species delimitation: a case study of the Anemopaegma species complex. Am J Bot. 2017;104(10):1493–509. doi: 10.3732/ajb.1700302. [DOI] [PubMed] [Google Scholar]
- 45.Yu XQ, Drew BT, Yang JB, Gao LM, Li DZ. Comparative chloroplast genomes of eleven Schima (Theaceae) species: insights into DNA barcoding and phylogeny. PLoS One. 2017;12(6):e0178026. 10.1371/journal.pone.0178026. [DOI] [PMC free article] [PubMed]
- 46.Bolger AM, Lohse M. Trimmomatic: a flflexible trimmer for illumina sequence data. Bioinf Oxf Engl. 2014;30(15):2114–20. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo RB, Liu BH, Xie YL, Li ZY, Huang WH, Yuan JY, et al. SOAPdenovo 2: an empirically improved memory-effificient short-read de novo assembler. Gigascience. 2012;1:1–6. doi: 10.1186/2047-217x-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dierckxsens N, Mardulyn P, Smits G. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017;45(4):e18. doi: 10.1093/nar/gkw955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lohse M, Drechsel O, Kahlau S, Bock R. OrganellarGenomeDRAW-a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013;41:W575–81. 10.1093/nar/gkt289. [DOI] [PMC free article] [PubMed]
- 50.Beier S, Thiel T, Munch T, Scholz U, Mascher M. MISA-web: a web server for microsatellite prediction, Bioinformatics. 2017;33(16):2583–2585. 10.1093/bioinformatics/btx198. [DOI] [PMC free article] [PubMed]
- 51.Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001;29(22):4633–42. doi: 10.1093/nar/29.22.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amiryousef A, Hyvönen J, Poczai P, IRscope An online program to visualize the junction sites of chloroplast genomes. Bioinformatics. 2018;34(17):3030–1. doi: 10.1093/bioinformatics/bty220. [DOI] [PubMed] [Google Scholar]
- 53.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004;32:W273–9. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamura K, Stecher G, Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol. 2021;38(7):3022–3027. 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed]
- 56.Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34(12):3299–3302. 10.1093/molbev/msx248. [DOI] [PubMed]
- 57.Shield DC, Sharp PM. Synonymous codon usage in Bacillus subtilis reflects both translational selection and mutational biases. Nucleic Acids Res. 1987;15(19):8023–40. doi: 10.1093/nar/15.19.8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xia XH. DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Mol Biol Evol. 2013;30(7):1720–8. doi: 10.1093/molbev/mst064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen LT, Schmidt HA, Haeseler A, Minh BQ. IQTREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–74. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huelsenbeck JP, Ronquist FMRBAYES. Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17(8):754–5. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data provided in this study were uploaded to the NCBI repository (https://www.ncbi.nlm.nih.gov/) under accession numbers NC_050354, MZ766253, OK046127, OP058659, OP004914, OQ547778, OQ556872-OQ556875, OQ556868, and OQ556869-OQ556870.