Abstract
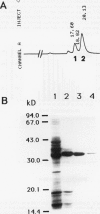
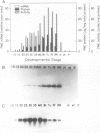
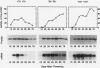
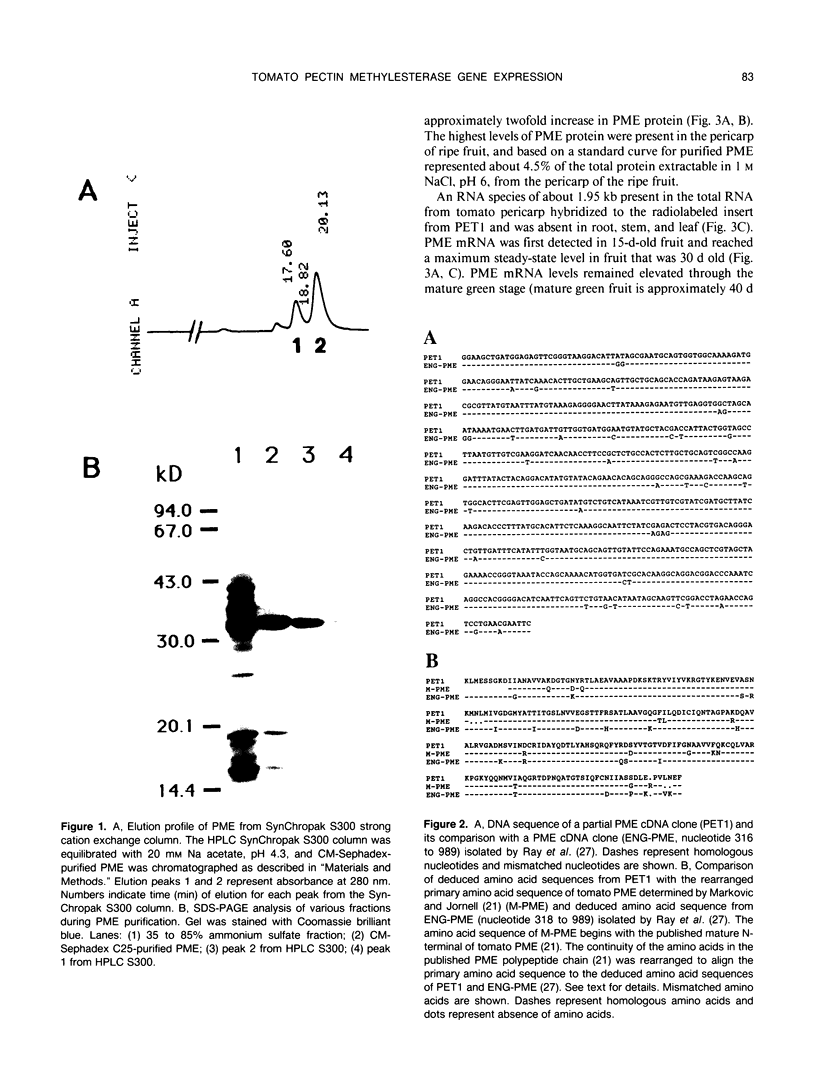
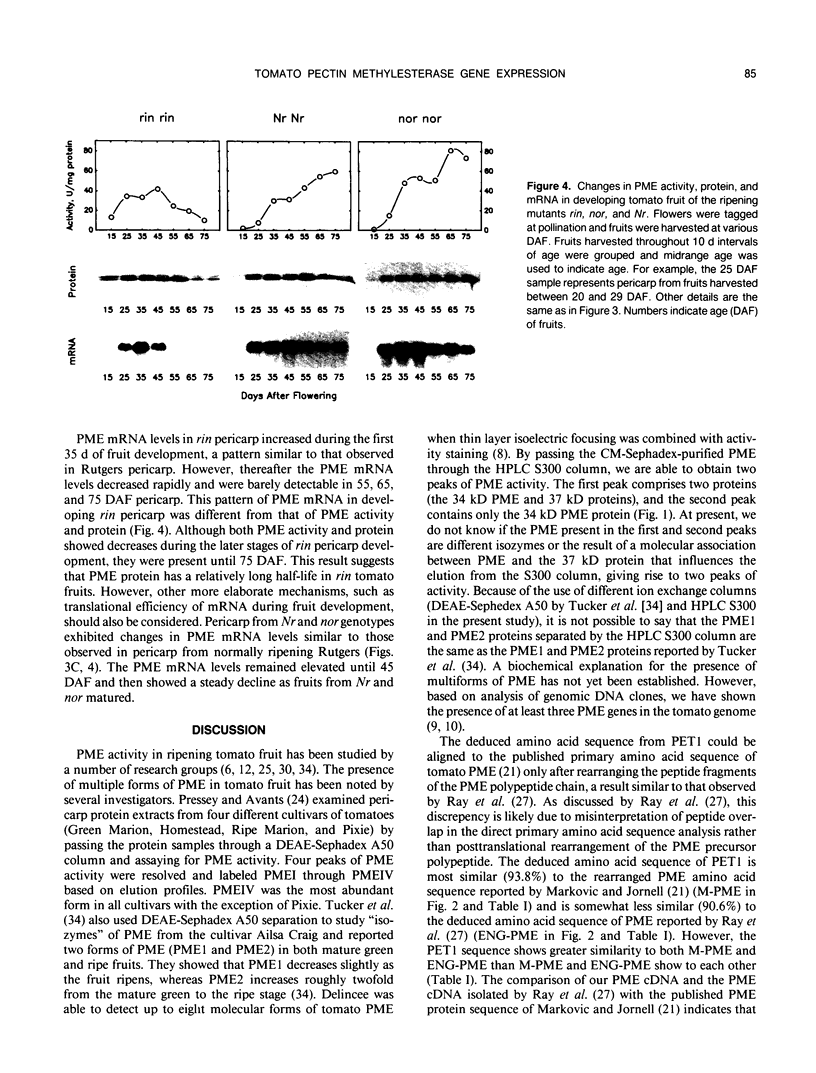
We have purified pectin methylesterase (PME; EC 3.1.11) from mature green (MG) tomato (Lycopersicon esculentum Mill. cv Rutgers) pericarp to an apparent homogeneity, raised antibodies to the purified protein, and isolated a PME cDNA clone from a λgtll expression library constructed from MG pericarp poly(A)+ RNA. Based on DNA sequencing, the PME cDNA clone isolated in the present study is different from that cloned earlier from cv Ailsa Craig (J Ray et al. [1989] Eur J Biochem 174:119-124). PME antibodies and the cDNA clone are used to determine changes in PME gene expression in developing fruits from normally ripening cv Rutgers and ripening-impaired mutants ripening inhibitor (rin), nonripening (nor), and never ripe (Nr). In Rutgers, PME mRNA is first detected in 15-day-old fruit, reaches a steady-state maximum between 30-day-old fruit and MG stage, and declines thereafter. PME activity is first detectable at day 10 and gradually increases until the turning stage. The increase in PME activity parallels an increase in PME protein; however, the levels of PME protein continue to increase beyond the turning stage while PME activity begins to decline. Patterns of PME gene expression in nor and Nr fruits are similar to the normally ripening cv Rutgers. However, the rin mutation has a considerable effect on PME gene expression in tomato fruits. PME RNA is not detectable in rin fruits older than 45 days and PME activity and protein begin showing a decline at the same time. Even though PME activity levels comparable to 25-day-old fruit were found in root tissue of normal plants, PME protein and mRNA are not detected in vegetative tissues using PME antibodies and cDNA as probes. Our data suggest that PME expression in tomato pericarp is highly regulated during fruit development and that mRNA synthesis and stability, protein stability, and delayed protein synthesis influence the level of PME activity in developing fruits.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awad M., Young R. E. Postharvest Variation in Cellulase, Polygalacturonase, and Pectinmethylesterase in Avocado (Persea americana Mill, cv. Fuerte) Fruits in Relation to Respiration and Ethylene Production. Plant Physiol. 1979 Aug;64(2):306–308. doi: 10.1104/pp.64.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs M. S., Handa A. K. Temporal regulation of polygalacturonase gene expression in fruits of normal, mutant, and heterozygous tomato genotypes. Plant Physiol. 1989 Jan;89(1):117–125. doi: 10.1104/pp.89.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs M. S., Harriman R. W., Handa A. K. Changes in Gene Expression during Tomato Fruit Ripening. Plant Physiol. 1986 Jun;81(2):395–403. doi: 10.1104/pp.81.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBSON G. E. Pectinesterase in normal and abnormal tomato fruit. Biochem J. 1963 Feb;86:358–365. doi: 10.1042/bj0860358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Koch J. L., Nevins D. J. Tomato fruit cell wall : I. Use of purified tomato polygalacturonase and pectinmethylesterase to identify developmental changes in pectins. Plant Physiol. 1989 Nov;91(3):816–822. doi: 10.1104/pp.91.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic O., Jörnvall H. Pectinesterase. The primary structure of the tomato enzyme. Eur J Biochem. 1986 Aug 1;158(3):455–462. doi: 10.1111/j.1432-1033.1986.tb09775.x. [DOI] [PubMed] [Google Scholar]
- Marlow S. J., Handa A. K. Immuno slot-blot assay using a membrane which covalently binds protein. J Immunol Methods. 1987 Jul 16;101(1):133–139. doi: 10.1016/0022-1759(87)90226-2. [DOI] [PubMed] [Google Scholar]
- Paull R. E., Chen N. J. Postharvest Variation in Cell Wall-Degrading Enzymes of Papaya (Carica papaya L.) during Fruit Ripening. Plant Physiol. 1983 Jun;72(2):382–385. doi: 10.1104/pp.72.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J., Knapp J., Grierson D., Bird C., Schuch W. Identification and sequence determination of a cDNA clone for tomato pectin esterase. Eur J Biochem. 1988 May 16;174(1):119–124. doi: 10.1111/j.1432-1033.1988.tb14070.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C. S., Yu J. H., Bai D. H., Hester P. Y., Kim K. H. Antibodies to the alpha-subunit of insulin receptor from eggs of immunized hens. J Immunol. 1985 Nov;135(5):3354–3359. [PubMed] [Google Scholar]