Abstract
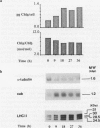
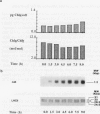
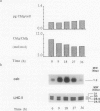
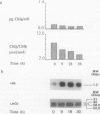
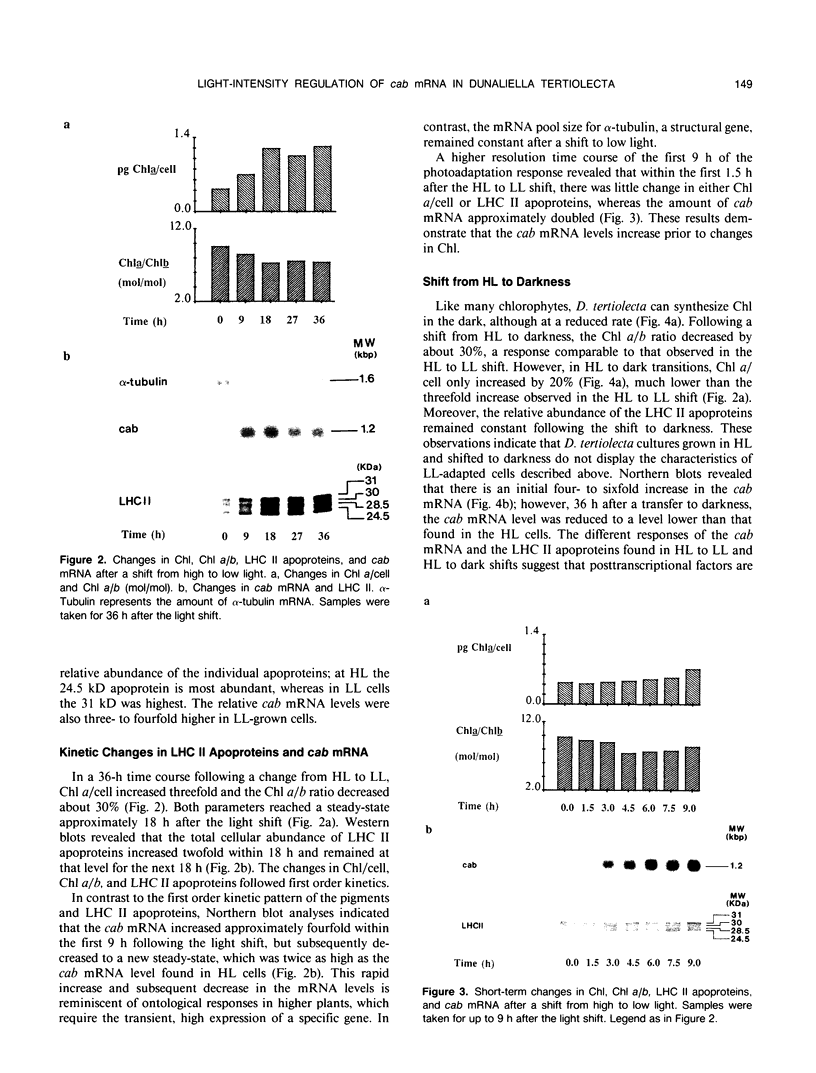
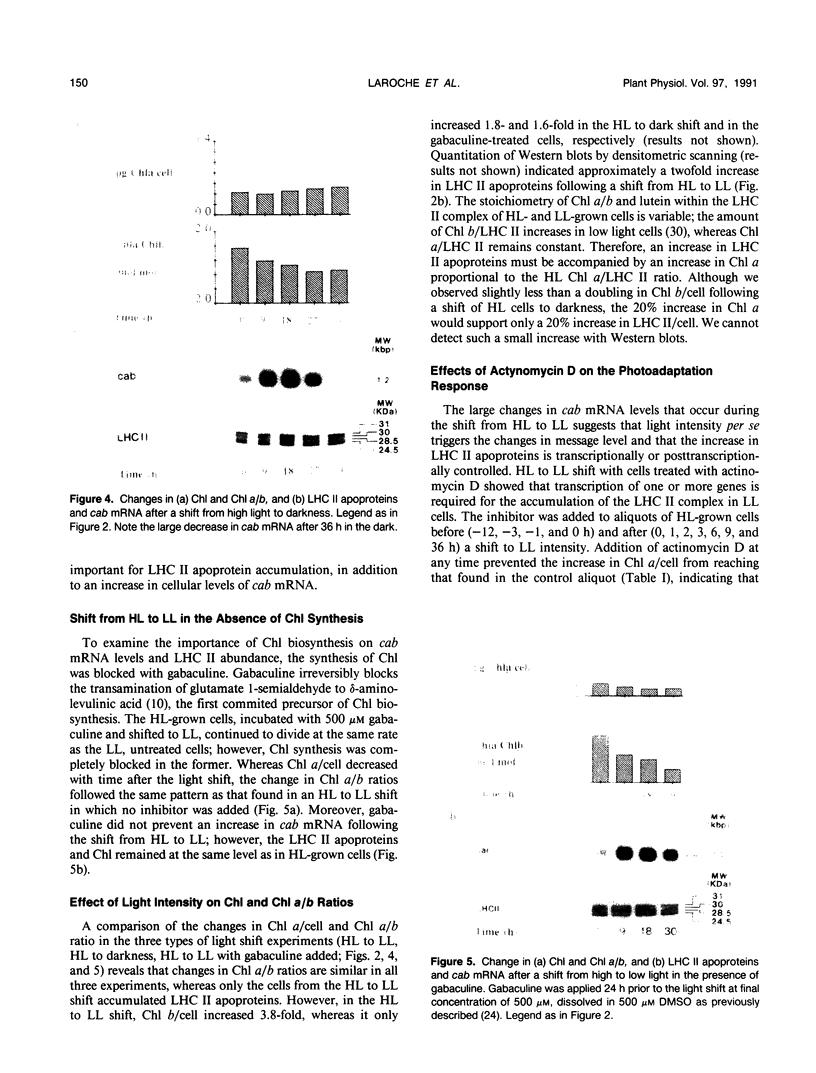
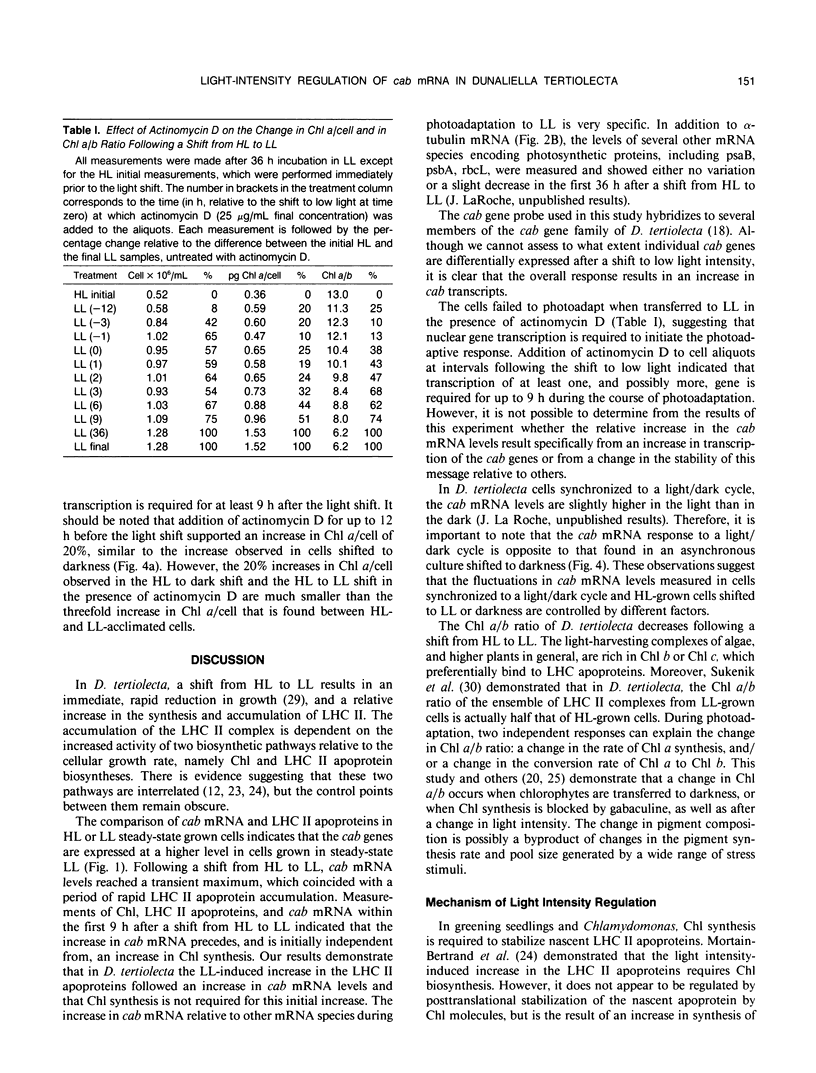
During a transition from high growth irradiance (700 micromoles quanta per square meter per second) to low growth irradiance (70 micromoles quanta per square meter per second), the unicellular marine chlorophyte Dunaliella tertiolecta Butcher increases the cellular pool size of the light-harvesting complex of photosystem II (LHC II). We showed that the increase in LHC II apoproteins and in chlorophyll content per cell is preceded by an approximately fourfold increase in cab mRNA. The increase in cab mRNA is detectable within 1.5 hours following a shift from high to low light intensity. An increase in the relative abundance of cab mRNA was also found following a shift from high light to darkness and from high light to low light in the presence of gabaculine, a chlorophyll synthesis inhibitor. However, the LHC II apoproteins did not accumulate in the latter experiments, suggesting that LHC II apoprotein synthesis is coupled to chlorophyll synthesis at or beyond translation. We propose that changes in energy balance brought about by a change in light intensity may control a regulatory factor acting to repress cab mRNA expression in high light.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I., Appleman D. Chlorophyll synthesis in chlorella: regulation by degree of light limitation of growth. Plant Physiol. 1971 Feb;47(2):230–235. doi: 10.1104/pp.47.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskins K., Westhoff P., Beremand P. D. Light Quality and Irradiance Level Interaction in the Control of Expression of Light-Harvesting Complex of Photosystem II: Pigments, Pigment-Proteins, and mRNA Accumulation. Plant Physiol. 1989 Sep;91(1):163–169. doi: 10.1104/pp.91.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Genge S., Pilger D., Hiller R. G. The relationship between chlorophyll b and pigment-protein complex II. Biochim Biophys Acta. 1974 Apr 23;347(1):22–30. doi: 10.1016/0005-2728(74)90196-0. [DOI] [PubMed] [Google Scholar]
- Johanningmeier U. Possible control of transcript levels by chlorophyll precursors in Chlamydomonas. Eur J Biochem. 1988 Nov 1;177(2):417–424. doi: 10.1111/j.1432-1033.1988.tb14391.x. [DOI] [PubMed] [Google Scholar]
- King J., Khanna V. A Nitrate Reductase-less Variant Isolated from Suspension Cultures of Datura innoxia (Mill.). Plant Physiol. 1980 Oct;66(4):632–636. doi: 10.1104/pp.66.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRoche J., Bennett J., Falkowski P. G. Characterization of a cDNA encoding for the 28.5-kDa LHCII apoprotein from the unicellular marine chlorophyte, Dunaliella tertiolecta. Gene. 1990 Nov 15;95(2):165–171. doi: 10.1016/0378-1119(90)90358-x. [DOI] [PubMed] [Google Scholar]
- Leong T. Y., Goodchild D. J., Anderson J. M. Effect of Light Quality on the Composition, Function, and Structure of Photosynthetic Thylakoid Membranes of Asplenium australasicum (Sm.) Hook. Plant Physiol. 1985 Jul;78(3):561–567. doi: 10.1104/pp.78.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney M. A., Hoober J. K., Marks D. B. Kinetics of Chlorophyll Accumulation and Formation of Chlorophyll-Protein Complexes during Greening of Chlamydomonas reinhardtii y-1 at 38 degrees C. Plant Physiol. 1989 Nov;91(3):1100–1106. doi: 10.1104/pp.91.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortain-Bertrand A., Bennett J., Falkowski P. G. Photoregulation of the Light-Harvesting Chlorophyll Protein Complex Associated with Photosystem II in Dunaliella tertiolecta: Evidence that Apoprotein Abundance but Not Stability Requires Chlorophyll Synthesis. Plant Physiol. 1990 Sep;94(1):304–311. doi: 10.1104/pp.94.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U., Gounaris K., Barber J. Dynamics of Photosystem II and Its Light Harvesting System in Response to Light Changes in the Halotolerant Alga Dunaliella salina. Plant Physiol. 1987 Sep;85(1):194–198. doi: 10.1104/pp.85.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1990 Oct;2(10):1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugarman P. M., Appleman D. Chlorophyll Synthesis in Chlorella II. Effect of Glucose and Light Intensity on the Lag Phase. Plant Physiol. 1966 Dec;41(10):1701–1708. doi: 10.1104/pp.41.10.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukenik A., Bennett J., Mortain-Bertrand A., Falkowski P. G. Adaptation of the Photosynthetic Apparatus to Irradiance in Dunaliella tertiolecta: A Kinetic Study. Plant Physiol. 1990 Apr;92(4):891–898. doi: 10.1104/pp.92.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]