Abstract
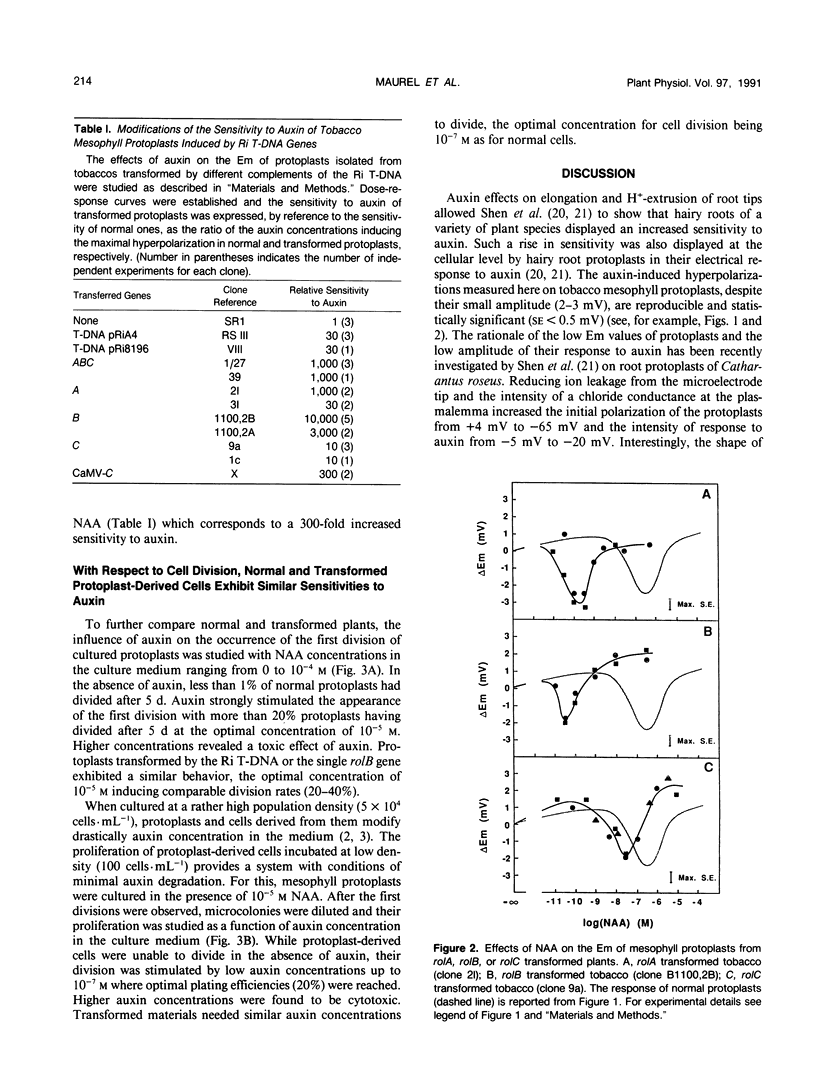
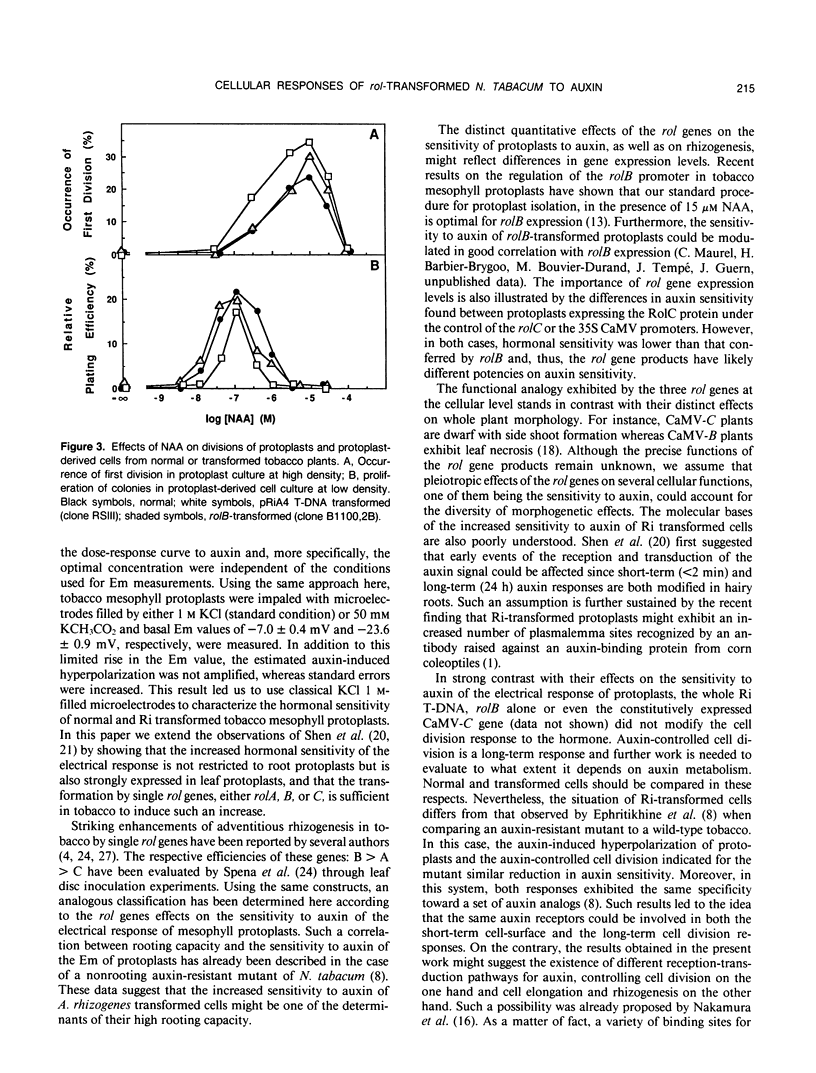
Two kinds of cellular responses to auxin, the hyperpolarization of protoplasts and the division of protoplast-derived cells, were compared in Nicotiana tabacum plants transformed by different T-DNA fragments of Agrobacterium rhizogenes strain A4. Using transmembrane potential difference measurements to characterize hormonal sensitivity of mesophyll protoplasts, we found a 30-fold increase in sensitivity to auxin in protoplasts transformed by the whole Ri A4 T-DNA. Furthermore, the rol genes of the Ri A4 TL-DNA, together or as single genes, were able to increase the sensitivity to auxin by factors up to 104. The different effects of the single rol genes on the sensitivity of mesophyll protoplasts to auxin, rolB being the most powerful, were consistent with their respective rhizogenic effects on leaf fragments (A Spena, T Schmülling, C Koncz, J Schell [1987] EMBO J 6: 3891-3899). No difference was seen concerning the effects of auxin on division of cells derived from normal or transformed protoplasts. These results suggest that only some cellular responses to auxin could be selectively altered by rol genes. They also show that rol-transformed tobaccos can be a model system to study auxin action in plants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caboche M., Aranda G., Poll A. M., Huet J. C., Leguay J. J. Auxin Conjugation by Tobacco Mesophyll Protoplasts : Correlations between Auxin Cytotoxicity under Low Density Growth Conditions and Induction of Conjugation Processes at High Density. Plant Physiol. 1984 May;75(1):54–59. doi: 10.1104/pp.75.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardarelli M, Mariotti D, Pomponi M, Spanò L, Capone I, Costantino P. Agrobacterium rhizogenes T-DNA genes capable of inducing hairy root phenotype. Mol Gen Genet. 1987 Oct;209(3):475–480. doi: 10.1007/BF00331152. [DOI] [PubMed] [Google Scholar]
- Durand-Tardif M., Broglie R., Slightom J., Tepfer D. Structure and expression of Ri T-DNA from Agrobacterium rhizogenes in Nicotiana tabacum. Organ and phenotypic specificity. J Mol Biol. 1985 Dec 5;186(3):557–564. doi: 10.1016/0022-2836(85)90130-5. [DOI] [PubMed] [Google Scholar]
- Ephritikhine G., Barbier-Brygoo H., Muller J. F., Guern J. Auxin effect on the transmembrane potential difference of wild-type and mutant tobacco protoplasts exhibiting a differential sensitiity to auxin. Plant Physiol. 1987 Apr;83(4):801–804. doi: 10.1104/pp.83.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P. J. Plant hormone mutants. Trends Genet. 1988 Jun;4(6):157–162. doi: 10.1016/0168-9525(88)90021-2. [DOI] [PubMed] [Google Scholar]
- Maurel C., Brevet J., Barbier-Brygoo H., Guern J., Tempé J. Auxin regulates the promoter of the root-inducing rolB gene of Agrobacterium rhizogenes in transgenic tobacco. Mol Gen Genet. 1990 Aug;223(1):58–64. doi: 10.1007/BF00315797. [DOI] [PubMed] [Google Scholar]
- Nakamura C., Van Telgen H. J., Mennes A. M., Ono H., Libbenga K. R. Correlation between Auxin Resistance and the Lack of a Membrane-Bound Auxin Binding Protein and a Root-Specific Peroxidase in Nicotiana tabacum. Plant Physiol. 1988 Nov;88(3):845–849. doi: 10.1104/pp.88.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmülling T., Schell J., Spena A. Promoters of the rolA, B, and C genes of Agrobacterium rhizogenesare differentially regulated in transgenic plants. Plant Cell. 1989 Jul;1(7):665–670. doi: 10.1105/tpc.1.7.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmülling T., Schell J., Spena A. Single genes from Agrobacterium rhizogenes influence plant development. EMBO J. 1988 Sep;7(9):2621–2629. doi: 10.1002/j.1460-2075.1988.tb03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W. H., Davioud E., David C., Barbier-Brygoo H., Tempé J., Guern J. High Sensitivity to Auxin is a Common Feature of Hairy Root. Plant Physiol. 1990 Oct;94(2):554–560. doi: 10.1104/pp.94.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W. H., Petit A., Guern J., Tempé J. Hairy roots are more sensitive to auxin than normal roots. Proc Natl Acad Sci U S A. 1988 May;85(10):3417–3421. doi: 10.1073/pnas.85.10.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkar V. P., Pythoud F., White F. F., Nester E. W., Gordon M. P. rolA locus of the Ri plasmid directs developmental abnormalities in transgenic tobacco plants. Genes Dev. 1988 Jun;2(6):688–697. doi: 10.1101/gad.2.6.688. [DOI] [PubMed] [Google Scholar]
- Spanò L., Mariotti D., Cardarelli M., Branca C., Costantino P. Morphogenesis and Auxin Sensitivity of Transgenic Tobacco with Different Complements of Ri T-DNA. Plant Physiol. 1988 Jun;87(2):479–483. doi: 10.1104/pp.87.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spena A., Schmülling T., Koncz C., Schell J. S. Independent and synergistic activity of rol A, B and C loci in stimulating abnormal growth in plants. EMBO J. 1987 Dec 20;6(13):3891–3899. doi: 10.1002/j.1460-2075.1987.tb02729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepfer D. Transformation of several species of higher plants by Agrobacterium rhizogenes: sexual transmission of the transformed genotype and phenotype. Cell. 1984 Jul;37(3):959–967. doi: 10.1016/0092-8674(84)90430-6. [DOI] [PubMed] [Google Scholar]
- White F. F., Taylor B. H., Huffman G. A., Gordon M. P., Nester E. W. Molecular and genetic analysis of the transferred DNA regions of the root-inducing plasmid of Agrobacterium rhizogenes. J Bacteriol. 1985 Oct;164(1):33–44. doi: 10.1128/jb.164.1.33-44.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L. The use of transgenic plants to study plant gene expression. Trends Genet. 1988 Jan;4(1):13–18. doi: 10.1016/0168-9525(88)90122-9. [DOI] [PubMed] [Google Scholar]
- Zambryski P., Tempe J., Schell J. Transfer and function of T-DNA genes from agrobacterium Ti and Ri plasmids in plants. Cell. 1989 Jan 27;56(2):193–201. doi: 10.1016/0092-8674(89)90892-1. [DOI] [PubMed] [Google Scholar]


