Abstract
Background:
An individual’s diagnostic subtype may fail to predict the efficacy of a given type of treatment for anomia. Classification by conceptual-semantic impairment may be more informative.
Aims:
This study examined the effects of conceptual-semantic impairment and diagnostic subtype on anomia treatment effects in primary progressive aphasia (PPA) and Alzheimer’s disease (AD).
Methods & Procedures:
At baseline, the picture and word versions of the Pyramids and Palm Trees and Kissing and Dancing tests were used to measure conceptual-semantic processing. Based on norming that was conducted with unimpaired older adults, participants were classified as being impaired on both the picture and word versions (i.e., modality-general conceptual-semantic impairment), the picture version (Objects or Actions) only (i.e., visual-conceptual impairment), the word version (Nouns or Verbs) only (i.e., lexical-semantic impairment), or neither the picture nor the word version (i.e., no impairment). Following baseline testing, a lexical treatment and a semantic treatment were administered to all participants. The treatment stimuli consisted of nouns and verbs that were consistently named correctly at baseline (Prophylaxis items) and/or nouns and verbs that were consistently named incorrectly at baseline (Remediation items). Naming accuracy was measured at baseline, and it was measured at three, seven, eleven, fourteen, eighteen, and twenty-one months.
Outcomes & Results:
Compared to baseline naming performance, lexical and semantic treatments both improved naming accuracy for treated Remediation nouns and verbs. For Prophylaxis items, lexical treatment was effective for both nouns and verbs, and semantic treatment was effective for verbs, but the pattern of results was different for nouns -- the effect of semantic treatment was initially nonsignificant or marginally significant, but it was significant beginning at 11 Months, suggesting that the effects of prophylactic semantic treatment may become more apparent as the disorder progresses. Furthermore, the interaction between baseline Conceptual-Semantic Impairment and the Treatment Condition (Lexical vs. Semantic) was significant for verb Prophylaxis items at 3 and 18 Months, and it was significant for noun Prophylaxis items at 14 and 18 Months.
Conclusions:
The pattern of results suggested that individuals who have modality-general conceptual-semantic impairment at baseline are more likely to benefit from lexical treatment, while individuals who have unimpaired conceptual-semantic processing at baseline are more likely to benefit from semantic treatment as the disorder progresses. In contrast to conceptual-semantic impairment, diagnostic subtype did not typically predict the treatment effects.
Keywords: anomia, treatment, primary progressive aphasia, Alzheimer’s disease, conceptual, semantic
Introduction
Anomia is a common deficit in neurodegeneration, including primary progressive aphasia (PPA; Westbury & Bub, 1997) and typical Alzheimer’s disease (AD; Appell et al., 1982). While the typical presentation of AD involves impairment in the learning and recall of new information (McKhann et al., 2011), individuals with typical AD may also develop language deficits, and these individuals have benefited from treatment for anomia (Altmann & McClung, 2008; Morelli et al., 2011; Noonan et al., 2012). PPA, which has been associated with frontotemporal lobar degeneration (FTLD) and atypical AD (Mesulam et al., 2014), is a clinical syndrome that involves progressive language impairment (Gorno-Tempini et al., 2011; Mesulam, 1982). Behavior and other aspects of cognition, such as visuospatial skills and episodic memory, are relatively preserved during the initial phases of the illness.
PPA Variants
Three main variants of PPA have been identified (Gorno-Tempini et al., 2011). The two core features of the semantic variant (svPPA) are impaired confrontation naming and single-word comprehension deficits. Both of these features are required for diagnosis. At least three of the following features are also required for diagnosis: 1) Impaired object knowledge; 2) surface dyslexia or dysgraphia; 3) spared repetition; 4) spared speech production. In a majority of cases, svPPA has been associated with TDP-43 positive FTLD (Leyton et al., 2016; Mesulam et al., 2014; Snowden et al., 2007).
The two core features of the logopenic variant (lvPPA) are impaired single-word retrieval and impaired repetition of sentences and phrases. Both of these features are required for diagnosis. At least three of the following features are also required for diagnosis: 1) Phonological speech errors; 2) Spared single-word comprehension and object knowledge; 3) Spared motor speech; 4) Spared grammar. In a majority of cases, lvPPA has been associated with atypical AD (Leyton et al., 2016; Mesulam et al., 2014; Rabinovici et al., 2008).
The two core features of the nonfluent/agrammatic variant (nfvPPA) are effortful, halting speech with apraxia and agrammatic language production. Only one of these features is required for diagnosis. At least two of the following features are also required for diagnosis: 1) Impaired comprehension of syntactically complex sentences; 2) spared single-word comprehension; 3) spared object knowledge. In a majority of cases, nfvPPA has been associated with tau-positive inclusions in FTLD, corticobasal degeneration, or progressive supranuclear palsy (Hodges et al., 2004; Knibb et al., 2006; Mesulam et al., 2014).
Anomia in PPA and AD
The patterns of atrophy in PPA and AD and the types of paraphasias present in the different subtypes of PPA suggest that anomia results from varied underlying deficits in PPA and AD. The specific underlying deficit may determine the type of treatment that will be effective in rehabilitation programs for anomia (see Nickels, 2002). The semantic variant of PPA involves bilateral atrophy of the anterior temporal lobe (Gorno-Tempini et al., 2004; Gorno-Tempini et al., 2011; Mummery et al., 2000), an area that has been associated with conceptual-semantic processing (Mummery et al., 2000; Binney et al., 2010; Lambon Ralph et al., 2010; Migliaccio et al., 2016; Rogalski et al., 2011; Rogers et al., 2006). In the left hemisphere, atrophy may extend to mid and posterior portions of the middle temporal gyrus and inferior temporal gyrus (Mesulam et al., 2012; Leyton et al., 2016), areas that have been associated with the interface between lexical and semantic information (Migliaccio et al., 2016; Hickok & Poeppel, 2004; Hickok & Poeppel, 2007; Indefrey, 2011; Indefrey & Levelt, 2004). At the behavioral level, conceptual impairment, lexical-semantic deficits, and semantic paraphasic errors occur in svPPA (Hodges et al., 1994; Mesulam et al., 2009; Neary et al., 1998). Thus, anomia in svPPA appears to be caused by degraded conceptual representations and/or degraded lexical-semantic connections.
The logopenic variant of PPA involves atrophy of the left posterior superior temporal lobe and the left inferior parietal lobe (Gorno-Tempini et al., 2004; Josephs et al., 2013; Rohrer et al., 2010), areas that have been associated with phonological processing and phonological short-term memory (Hickok & Poeppel, 2007; Baldo & Dronkers, 2006; Baldo et al., 2012; Buchsbaum et al., 2011; Gorno-Tempini et al., 2008; Leff et al., 2009). At the behavioral level, phonemic paraphasic errors are likely to occur in lvPPA (Gorno-Tempini et al., 2008; Henry & Gorno-Tempini, 2010). Thus, anomia in lvPPA may be caused by degraded phonological representations and/or difficulty maintaining these representations. However, as lvPPA progresses, atrophy spreads to areas of the temporal lobe that are associated with lexical-semantic and conceptual processing (Leyton et al., 2016), and semantic impairment may appear in cases of lvPPA (Roncero et al., 2020). Therefore, during the later stages of the syndrome, anomia in lvPPA may be due in part to lexical-semantic and/or conceptual impairment, in addition to the phonological impairment that develops during the earlier stages of the syndrome.
The nonfluent/agrammatic variant of PPA has been associated with atrophy in several left hemisphere areas, including the inferior frontal gyrus, insula, and premotor and supplementary motor areas (Grossman et al., 1996; Josephs et al., 2006; Nestor et al., 2003). These areas have been associated with phonological speech encoding (Indefrey, 2011) and articulation (Hickok & Poeppel, 2007; Indefrey, 2011). At the behavioral level, phonemic paraphasic errors and speech apraxia are likely to occur in nfvPPA (Ash et al., 2010, 2013). Thus, anomia in nfvPPA may be caused by difficulty producing the correct phonological output. However, compared to noun naming, impaired verb naming has been found to emerge earlier in nfvPPA (Cotelli et al., 2006; Hillis et al., 2004; Hillis et al., 2002; Thompson et al., 2012), suggesting that verb anomia in nfvPPA may be due in part to grammatical or conceptual-semantic impairment (Meyer et al., 2020).
Typical AD involves degeneration that originates within the medial temporal lobe (Braak & Braak, 1995; Delacourte et al., 1999). In AD, the underlying cause of anomia remains unclear. Anomia in AD may be due to degradation of the semantic network (Chertkow & Bub, 1990; Hodges & Patterson, 1995; Hodges et al., 1992; Caputi et al., 2016; Domoto-Reilly et al., 2012; Frings et al., 2011), impaired lexical retrieval (Nebes et al., 1984), or a combination of the two (Rogers & Friedman, 2008; Joubert et al., 2010; Reilly et al., 2011). Impairment of strategic or controlled processing may also play a role (Rich et al., 2002; Moreaud et al., 2001; Nebes et al., 1989).
Anomia Treatment
Anomia treatment protocols are of three major types. Phonological treatment aims to strengthen the word’s phonological representation through tasks such as word repetition or phonological manipulation (see Jokel et al., 2016; Madden et al., 2017). Orthographic treatment seeks to strengthen the word’s orthographic representation via tasks such as reading and writing the word (e.g., Meyer et al., 2015b). Semantic treatment aims to strengthen the word’s conceptual representation through tasks such as semantic feature analysis (Boyle & Coelho, 1995; Reilly, 2016; e.g., “Is this a tool?” “Where do you find this object?” “What do you do with this object?”).
In studies of anomia treatment in PPA, a within-subjects design has not typically been utilized (exceptions, which are discussed below, include Jokel et al., 2016; Krajenbrink et al., 2020; Meyer et al., 2019; Suárez-González et al., 2018). As a result, it can be difficult to determine if differences in the observed effects of different types of treatment are actually due to disparities in treatment efficacy, rather than individual differences. Furthermore, treatment studies for anomia in PPA and AD have typically focused on the remediation of words that cannot be named at baseline. However, individuals with neurodegenerative aphasia will continue to decline over time, and additional words will be lost from an individual’s functional vocabulary. Therefore, prophylactic treatment of words that can be named at baseline may be necessary in order to prevent decline for these items. Several studies have demonstrated that prophylactic treatment results in maintenance of naming accuracy for trained words over time, while accuracy for matched untrained items declines over time (Flurie et al., 2020; Jokel et al., 2006, 2010; Meyer et al., 2015b, 2019).
In a recent study (Meyer et al., 2019), we utilized a within-subjects design that included two types of treatment (orthographic and phonological) to examine the prophylaxis and remediation of anomia in PPA. Where feasible, a portion of the items assigned to each condition were consistently named correctly by the participant during the initial evaluation (prophylaxis items), while the remaining items in each condition were consistently named incorrectly during the initial evaluation (remediation items). The analysis of the group treatment effects included 26 participants with prophylaxis items and 11 participants with remediation items.
The orthographic and phonological treatments both resulted in significant treatment effects for prophylaxis and remediation items in svPPA and lvPPA, suggesting that the two treatments have similar levels of efficacy in these two subtypes, possibly because both treatments strengthened lexical-semantic connections through repeated activation. With the exception of one participant who also had remediation items, participants with nfvPPA only had prophylaxis items (because of their high baseline naming accuracy for the noun stimuli), and they showed significant treatment effects for these items.
A few studies have utilized a within-subjects design to compare phonological, orthographic, or lexical treatment to semantic treatment in svPPA or semantic dementia, and these studies have produced mixed results. Dressel et al. (2010) found that semantic treatment was initially more effective than phonological treatment in a single participant with semantic dementia, but this advantage was not maintained at follow-up. Jokel et al. (2016) found that phonological and semantic treatments were both effective in svPPA, and only one of the four participants showed a significantly larger effect for semantic treatment, compared to phonological treatment. Suárez-González et al. (2018) compared a conceptual-semantic treatment (Conceptual Enrichment training) to an orthographic treatment (reading the name of the target word). The Conceptual Enrichment treatment sought to strengthen the semantic network of the target item by presenting the target image along with semantically-related images. Both treatments improved naming in a participant with svPPA, but the conceptual-semantic treatment also resulted in task generalization and longer maintenance of the treatment effect. In contrast, Krajenbrink et al. (2020) found that phonological/orthographic treatment that included writing of the target word improved spoken naming accuracy in a participant with svPPA, while neither Conceptual Enrichment training nor phonological/orthographic treatment without writing resulted in significant improvement in spoken naming. Thus, there is currently no consensus that a particular type of treatment is more effective within svPPA or the other subtypes of PPA.
Challenges in PPA Subtype Classification
Another problem is related to PPA subtype classification. When the international criteria (Gorno-Tempini et al., 2011) have been utilized for subtype classification, between 10% and 41% of PPA cases have been found to be unclassifiable (Mesulam et al., 2014; Botha et al., 2015; Harris et al., 2013; Sajadi et al., 2012; Wicklund et al., 2014). A classification failure occurs when an individual with PPA simultaneously meets the criteria for two subtypes or does not meet the criteria for any subtype (Mesulam et al., 2014; Mesulam et al., 2012; Mesulam et al., 2009; Sajadi et al., 2012; Louwersheimer et al., 2016; Sajadi et al., 2014). This ambiguity regarding subtypes is not surprising, because the progression of the disease process is not identical in all patients, and they enroll in studies at different stages of their disease. Pure symptoms may occur in the initial stages of the disease, but as the disease progresses, other symptoms follow. Indeed, a more global language impairment is the common end-state of all subtypes (Rogalski et al., 2011).
A New Approach to Anomia Treatment Selection in PPA and AD
Given the aforementioned difficulties related to subtype classification and the constantly evolving set of symptoms, we hypothesize that a different approach to anomia treatment selection will be more useful. In the current study, we sought to determine if the specific type of lexical or conceptual impairment is a better predictor of anomia treatment efficacy than the participant’s diagnostic subtype. Three potential causes of naming errors are lexical-semantic impairment (i.e., a disconnection between the concept and corresponding word), visual-conceptual impairment (i.e., a disconnection between the concept and its visual representation), and conceptual-semantic impairment (i.e., a difficulty with the concept that can be measured in both visual and linguistic modalities). For some individuals with PPA or AD, lexical-semantic processing may be more impaired than visual-conceptual processing, while other individuals may show the opposite pattern (Butler et al., 2009). Indeed, some individuals with svPPA have been found to have greater lexical-semantic than visual-conceptual impairment (Mesulam et al., 2013).
One way to evaluate the specific type of semantic and/or conceptual impairment for nouns and objects is through administration of the Word and Picture versions of the Pyramids and Palm Trees test (PPT; Howard & Patterson, 1992). Impaired performance on the Word version and unimpaired performance on the Picture version would suggest that a lexical-semantic deficit for nouns is present, while the opposite pattern would suggest that a visual-conceptual deficit for objects is present. Furthermore, impairment on both versions of the PPT would indicate that a modality-general conceptual-semantic impairment for objects/nouns is present. Similarly, the specific type of lexical-semantic, visual-conceptual, or modality-general conceptual-semantic impairment for actions and verbs can be evaluated via the Picture and Word versions of the Kissing and Dancing test (KD; Bak & Hodges, 2003).
In the current study, the Word and Picture versions of the PPT and KD were administered to all participants at baseline. We predicted that participants with a lexical-semantic impairment would benefit more from lexical (phonological/orthographic) treatment than conceptual treatment, because lexical treatment was expected to strengthen the connection between words and their meanings (see Meyer et al., 2019), while conceptual treatment may be unnecessary if concepts are not declining. Furthermore, we predicted that participants with a visual-conceptual impairment or a modality-general conceptual-semantic impairment would benefit more from a treatment that focuses on conceptual representations, such as semantic feature analysis (Boyle & Coelho, 1995; Reilly, 2016). For prophylaxis items, which were consistently named correctly at baseline, conceptual representations may not be damaged at baseline. For these items, the goal of the semantic treatment is to strengthen the representations before they become degraded. For remediation items, which were consistently named incorrectly at baseline, conceptual representations may be damaged, but they might not be completely degraded, and semantic treatment could also strengthen these representations.
Anomia Treatment for Verbs
In PPA, only a few anomia treatment studies have included treatment stimuli from grammatical categories other than nouns (see Beales et al., 2016; Croot et al., 2019; Fenner et al., 2019; Paek et al., 2021; Sheppard et al., 2022; Taylor-Rubin et al., 2021). In the current study, we included noun and verb stimuli for all participants. While verb-naming impairment may emerge earlier in nfvPPA (Cotelli et al., 2006; Hillis et al., 2004; Hillis et al., 2002; Thompson et al., 2012), verb-naming impairment has also been found in typical AD (Cotelli et al., 2006), and it has been found in lvPPA and svPPA (Meyer et al., 2020; Riello et al., 2018; Thompson et al., 2012). Thus, remediation and/or prophylaxis of anomia for verbs may be beneficial in all variants of PPA, as well as typical AD.
Method
Participants
This study was approved by the Institutional Review Boards of Georgetown University and Johns Hopkins University, and informed consent was obtained from all participants. Potential participants were referred by neurologists, clinical neuropsychologists, and speech-language pathologists within the Washington, DC and Baltimore, MD metropolitan areas. The inclusion criteria were a clinical diagnosis of PPA or typical (amnestic) AD, English fluency since childhood, at least 10 years of education, age of at least 40 years, and no history of other neurological or psychiatric disorders. PPA subtyping was based on the international criteria (Gorno-Tempini et al., 2011) and each participant’s baseline assessment results. Participants with any subtype of PPA were included, as well as participants with unclassifiable PPA.
Forty-three individuals with PPA or AD enrolled in the treatment study. Three participants withdrew during the initial evaluation, and three participants withdrew during the initial treatment period, before any measurement of treatment effects had occurred. Of the remaining 37 participants, 11 had lvPPA, 5 had svPPA, 7 had nfvPPA, 10 had unclassifiable PPA, and 4 had typical AD (see Table 1 for demographic information). All of the participants with typical AD reported word-finding difficulty, and three out of four had baseline impairment on the Boston Naming Test (BNT; Kaplan et al., 2001; see Table 2).
Table 1.
Demographic Information and Treatment Items
| Participant | Age | Education | Sex | Symptom Duration | Time Post-Diagnosis | Noun Proph | Noun Remed | Verb Proph | Verb Remed |
|---|---|---|---|---|---|---|---|---|---|
| LV1 | 75 | 20 | M | 62 months | 12 months | X | X | ||
| LV2 | 74 | 12 | F | 29 months | 5 months | X | X | X | |
| LV3 | 70 | 18 | F | 74 months | 3 months | X | X | ||
| LV4 | 57 | 13 | F | 68 months | 4 months | X | X | X | X |
| LV5 | 67 | 18 | F | 40 months | 3 months | X | X | ||
| LV6 | 70 | 12 | F | 43 months | 2 months | X | X | ||
| LV7 | 73 | 16 | M | 13 months | 1 month | X | X | X | |
| LV8 | 76 | 18 | F | 76 months | 4 months | X | X | ||
| LV9 | 75 | 18 | F | 45 months | 4 months | X | X | ||
| LV10 | 75 | 16 | M | 33 months | 5 months | X | X | X | X |
| LV11 | 77 | 17 | F | 48 months | 30 months | X | X | X | |
| SV1 | 76 | 16 | F | 20 months | 8 months | X | X | X | X |
| SV2 | 67 | 18 | F | 112 months | 2 months | X | X | X | X |
| SV3 | 55 | 16 | F | 19 months | 1 month | X | X | X | X |
| SV4 | 69 | 20 | F | 23 months | 5 months | X | X | X | |
| SV5 | 70 | 16 | F | 40 months | 4 months | X | X | X | |
| NFV1 | 79 | 18 | F | 57 months | 9 months | X | X | ||
| NFV2 | 76 | 16 | F | 28 months | 4 months | X | X | ||
| NFV3 | 68 | 18 | F | 38 months | 23 months | X | X | ||
| NFV4 | 74 | 12 | M | 34 months | 4 months | X | X | ||
| NFV5 | 63 | 20 | F | 100 months | 13 months | X | |||
| NFV6 | 66 | 16 | F | 43 months | 6 months | X | X | ||
| NFV7 | 67 | 20 | M | 16 months | 3 months | X | X | ||
| UPPA1 | 78 | 16 | M | 74 months | 2 months | X | X | X | X |
| UPPA2 | 71 | 18 | M | 99 months | 40 months | X | X | ||
| UPPA3 | 55 | 16 | F | 92 months | 2 months | X | X | X | |
| UPPA4 | 62 | 15 | F | 41 months | 5 months | X | X | X | |
| UPPA5 | 74 | 20 | F | 16 months | 4 months | X | X | X | |
| UPPA6 | 76 | 16 | F | 29 months | 5 months | X | X | X | |
| UPPA7 | 80 | 16 | F | 35 months | 11 months | X | X | ||
| UPPA8 | 72 | 19 | M | 35 months | 5 months | X | X | ||
| UPPA9 | 62 | 16 | F | 50 months | 40 months | X | X | ||
| UPPA10 | 75 | 18 | M | 50 months | 2 months | X | X | ||
| AD1 | 86 | 16 | M | 52 months | 2 months | X | X | ||
| AD2 | 62 | 16 | M | 86 months | 8 months | X | X | ||
| AD3 | 58 | 18 | M | 96 months | 91 months | X | X | ||
| AD4 | 82 | 14 | F | 26 months | 2 months | X | X |
Note. LV: logopenic variant; SV: semantic variant; NFV: nonfluent/agrammatic variant; UPPA: unclassifiable primary progressive aphasia; AD: Alzheimer’s disease; Proph: Prophylaxis items; Remed: Remediation items.
Table 2.
Baseline Assessment Results
| Participant | O/N CSI | A/V CSI | FBI/66 | MMSE/30 | BNT/60 | WPM/48 | BDAE AA/7 | BDAE PL/7 | BDAE GF/7 | BDAE ES/10 | BDAE SR/10 | PR/10 | Reading Difference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LV1 | Neither | Actions | 28.5 | 27 | 25 | 48 | 6 | 7 | 7 | 10 | 9 | 10 | −2 |
| LV2 | Both | Both | 13 | 10 | 3 | 36 | 7 | 7 | 7 | 7 | 6 | 3 | 0 |
| LV3 | Objects | Neither | 7 | 22 | 43 | 48 | 6 | 7 | 7 | 6 | 8 | 6 | −2 |
| LV4 | Both | Both | 9.5 | 20 | 24 | 48 | 7 | 7 | 7 | 4 | 1 | 3 | −4 |
| LV5 | Neither | Neither | 5 | 28 | 31 | 48 | 7 | 7 | 7 | 10 | 9 | 8 | −1 |
| LV6 | Neither | Actions | 3 | 24 | 29 | 48 | 7 | 7 | 7 | 10 | 8 | 8 | −2 |
| LV7 | Both | Actions | 11 | 29 | 34 | 47 | 7 | 7 | 6 | 7 | 7 | 8 | −1 |
| LV8 | Objects | Actions | 4 | 23 | 31 | 48 | 6 | 7 | 7 | 8 | 7 | 7 | 0 |
| LV9 | Objects | Neither | 7.5 | 20 | 43 | 47 | 7 | 7 | 7 | 6 | 8 | 7 | −1 |
| LV10 | Both | Both | 7 | 18 | 23 | 47 | 7 | 6 | 7 | 9 | 9 | 6 | −2 |
| LV11 | Neither | Neither | 5 | 22 | 30 | 47 | 7 | 7 | 7 | 8 | 9 | 6 | −2 |
| SV1 | Both | Both | 15 | 20 | 23 | 39 | 7 | 7 | 7 | 4 | 10 | 10 | −3 |
| SV2 | Both | Both | 0 | 23 | 6 | 46 | 7 | 7 | 7 | 10 | 9 | 8 | −3 |
| SV3 | Both | Both | 27 | 6 | 29 | 45 | 7 | 6 | 7 | 8 | 6 | 6 | −4 |
| SV4 | Both | Both | 21 | 13 | 18 | 46 | 7 | 7 | 6 | 4 | 7 | 9 | −2 |
| SV5 | Both | Actions | 8 | 25 | 23 | 46 | 7 | 7 | 7 | 7 | 5 | 2 | −2 |
| NFV1 | Neither | Verbs | 10 | 26 | 47 | 48 | 7 | 6 | 6 | 9 | 9 | 4 | 0 |
| NFV2 | Neither | Neither | 4 | 30 | 47 | 48 | 5 | 5 | 4 | 10 | 5 | 1 | 0 |
| NFV3 | Objects | Both | 16 | 27 | 44 | 48 | 4 | 6 | 6 | 9 | 7 | 5 | −2 |
| NFV4 | Objects | Both | 15 | 18 | 32 | 47 | 6 | 6 | 5 | 8 | 6 | NA | 1 |
| NFV5 | Neither | Neither | 15 | 25 | 23 | 48 | 4 | 4 | 3 | 6 | 0 | 0 | −3 |
| NFV6 | Neither | Neither | 3 | 26 | 42 | 48 | 6 | 7 | 6 | 8 | 7 | 4 | 0 |
| NFV7 | Neither | Actions | 15 | 26 | 57 | 48 | 5 | 7 | 6 | 9 | 10 | 9 | −1 |
| UPPA1 | Both | Actions | 8 | 26 | 13 | 46 | 7 | 7 | 7 | 9 | 3 | 3 | −4 |
| UPPA2 | Both | Neither | 12.5 | 25 | 8 | 45 | 7 | 7 | 7 | 10 | 3 | 4 | −3 |
| UPPA3 | Both | Actions | 14 | 12 | 33 | 45 | 7 | 7 | 7 | 4 | 2 | 2 | −2 |
| UPPA4 | Both | Actions | 44 | 18 | 18 | 44 | 7 | 6 | 6 | 4 | 5 | 4 | −5 |
| UPPA5 | Both | Both | 33 | 8 | 9 | 17 | 7 | 6 | 6 | 2 | 1 | NA | −6 |
| UPPA6 | Objects | Actions | 11 | 14 | 37 | 46 | 7 | 7 | 6 | 6 | 6 | 9 | 0 |
| UPPA7 | Both | Both | NA | 25 | 48 | 40 | 6 | 7 | 6 | 5 | 9 | 9 | 1 |
| UPPA8 | Objects | Actions | 19 | 21 | 56 | 48 | 6 | 7 | 6 | 10 | 7 | 10 | 0 |
| UPPA9 | Both | Both | 5 | 8 | 7 | 44 | 4 | 4 | 4 | NA | 0 | 0 | −2 |
| UPPA10 | Neither | Neither | 31 | 27 | 52 | 48 | 7 | 7 | 7 | 10 | 10 | NA | 0 |
| AD1 | Both | Actions | 8 | 29 | 31 | 37 | 6 | 7 | 6 | 10 | 9 | 9 | 0 |
| AD2 | Neither | Actions | 26.5 | 25 | 57 | 48 | 7 | 7 | 7 | 10 | 10 | 10 | 0 |
| AD3 | Both | Both | 15 | 1 | 2 | 31 | 5 | 5 | 6 | 4 | 0 | 0 | −4 |
| AD4 | Both | Both | 15 | 17 | 34 | 46 | 7 | 7 | 6 | 3 | 8 | NA | −1 |
Note. LV: logopenic variant; SV: semantic variant; NFV: nonfluent/agrammatic variant; UPPA: unclassifiable primary progressive aphasia; AD: Alzheimer’s disease; O/N CSI: object/noun conceptual-semantic impairment; A/V CSI: action/verb conceptual-semantic impairment; FBI: Frontal Behavioral Inventory; MMSE: Mini-Mental State Examination; BNT: Boston Naming Test; WPM: Word-Picture Matching; BDAE: Boston Diagnostic Aphasia Examination; AA: Articulatory Agility; PL: Phrase Length; GF: Grammatical Form; ES: Embedded Sentences; SR: Sentence Repetition; PR: Pseudoword Repetition; Reading Difference: Low Frequency Irregular Words minus Low Frequency Regular Words (a more negative score indicates greater surface alexia); NA: not administered.
The majority (9/10) of the participants with unclassifiable PPA had deficits in multiple language domains, which resulted in these participants either simultaneously meeting the criteria for two subtypes, or not meeting the criteria for any subtype. One participant (UPPA10) did not meet the criteria for any subtype because he had relatively mild language impairment.
During the baseline evaluation sessions, participants completed a battery of language and cognitive tests (see Table 2). The picture versions of the PPT (Howard & Patterson, 1992) and KD (Bak & Hodges, 2003) were used to measure visual-conceptual processing for objects and actions, respectively, while the word versions of the PPT and KD were used to measure lexical-semantic processing for nouns and verbs, respectively. Additional tests included the BNT (Kaplan et al., 2001), Auditory Word-Picture Matching (Rogers & Friedman, 2008), Reading of Regular and Irregular Words (Meyer et al., 2018), Pseudoword Repetition (five-syllable pseudowords; Meyer et al., 2015a), and the MMSE (Folstein et al., 1975). Selected subtests from the BDAE (Goodglass et al., 2001) were also administered, including Picture Description, Embedded Sentences, Verbal Agility, and Sentence Repetition. A modified version of the Frontal Behavioral Inventory (FBI; Kertesz et al., 1997) was completed by a caregiver. The modified FBI omitted the Concreteness, Verbal Apraxia, and Alien Hand questions.
Treatment Materials
For each participant, 120 pictures of nouns were selected from a set of 284 pictures, and 60 pictures of verbs were selected from a set of 116 pictures. In norming conducted with unimpaired controls, these pictures had high name agreement. The selected items were those that were named correctly by the participant during three baseline oral naming tests (Prophylaxis Items), and/or those that were named incorrectly during all three of these tests (Remediation Items). Depending on their baseline naming performance, some participants had both Prophylaxis and Remediation items, while other participants had only Prophylaxis items or only Remediation items (see Table 1). For both nouns and verbs, each participant had two sets of treated items: Lexical Treatment Condition (LTC) and Semantic Treatment Condition (STC).
For nouns, in order to facilitate the measurement of item generalization within semantic categories, each participant also had two sets of untrained items [Untrained Lexical Condition (ULC) and Untrained Semantic Condition (USC)]. Each untrained set was matched with one of the treated sets on frequency (Baayen et al., 1995), semantic category, and length (number of syllables, phonemes, and letters). Semantic categories included Animals (e.g., cow), Appliances (e.g., toaster), Body Parts (e.g., arm), Clothing (e.g., shoe), Food (e.g., banana), Furniture (e.g., desk), Musical Instruments (e.g., guitar), Items from Nature (e.g., cloud), Common Objects (e.g., candle), People (e.g., doctor), Structures (e.g., bridge), Tools (e.g., hammer), and Vehicles (e.g., truck).
For verbs, the selected items were divided into three sets [LTC, STC, and the Untrained Condition (UC)], and the three sets were matched on frequency and length as described above, as well as transitivity (intransitive or transitive) and verb category. Verb categories included Object Action (e.g., lifting), Body Action (e.g., clapping), Body Action Motion (e.g., marching), and Mental State (e.g., thinking).
For each selected noun or verb picture, there were three different exemplars. Exemplar 1 was tested twice during the baseline evaluation and was utilized during treatment. Exemplar 2 was tested once at baseline, was not trained, and was used to assess stimulus generalization during post-treatment and follow-up testing. Exemplar 3 was used as a foil during treatment.
Procedure
The baseline evaluation occurred over the course of six to eight sessions, with one or two sessions per week. Each participant’s language abilities were tested comprehensively, and each participant’s treatment items were selected. Baseline performance for the selected items was also assessed with a Naming during Scene Description task.
The treatment timeline is presented in Table 3. During the first month of treatment, there were two sessions per week, and each session included both types of treatment. During the subsequent five months, one treatment session occurred per month, and shorter practice sessions occurred two times per week. The purpose of the monthly treatment sessions was to check in with the participant and caregiver and to verify that the treatment tasks were being practiced according to the protocol.
Table 3.
Treatment Timeline
| Baseline | During Month 1 | During Months 2–6 | During Month 7 | During Month 8 | During Months 9–13 | During Month 14 | During Month 15 | During Months 16–20 | During Month 21 | During Month 22 |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Evaluation | 2 Treatment Sessions Per Week | Home Practice 2 Times Per Week, 1 Treatment Session Per Month | Break (No Practice, Treatment , or Testing) | First Post- Treatment Evaluation | Home Practice 2 Times Per Week, 1 Treatment Session Per Month | Break (No Practice, Treatment , or Testing) | Second Post- Treatment Evaluation | Home Practice 2 Times Per Week, 1 Treatment Session Per Month | Break (No Practice, Treatment , or Testing) | Third Post- Treatment Evaluation |
The post-treatment evaluation began one month after the end of the initial home practice period. Participants resumed practice and monthly treatment sessions after the post-treatment evaluation, and they took a one-month break before each follow-up evaluation, which began at the end of months 14 and 21. Thus, each participant typically completed a total of 24 assessment sessions, 23 treatment sessions, and 120 practice sessions.
Each participant received both of the treatments described below, with different items in each treatment set. The order of the treatments was counterbalanced across sessions.
Lexical Treatment Condition (LTC).
Stimuli were presented in the following sequence, which is depicted in Figure 1: 1) Picture alone, 1.5 seconds. 2) The picture remained on the screen, and the participant was presented with the word’s orthographic and phonological features, and was then instructed to write the word. For example, for the item elephant, the following features/cues were presented: “This word starts with the letter ‘e.’” This word has 3 syllables.” “Copy elephant.” Each cue was presented in written and auditory form. 3) The word (in written and auditory form) was presented with the picture, and the participant was asked to read/repeat the word. 4) To help ensure that participants attended to the picture stimuli, two recognition slides were presented in succession, with the words “Did you see this?,” the word, and the correct or incorrect picture exemplar. Instructions specified that the identical exemplar of the picture should be present for a “Yes” response.
Figure 1.
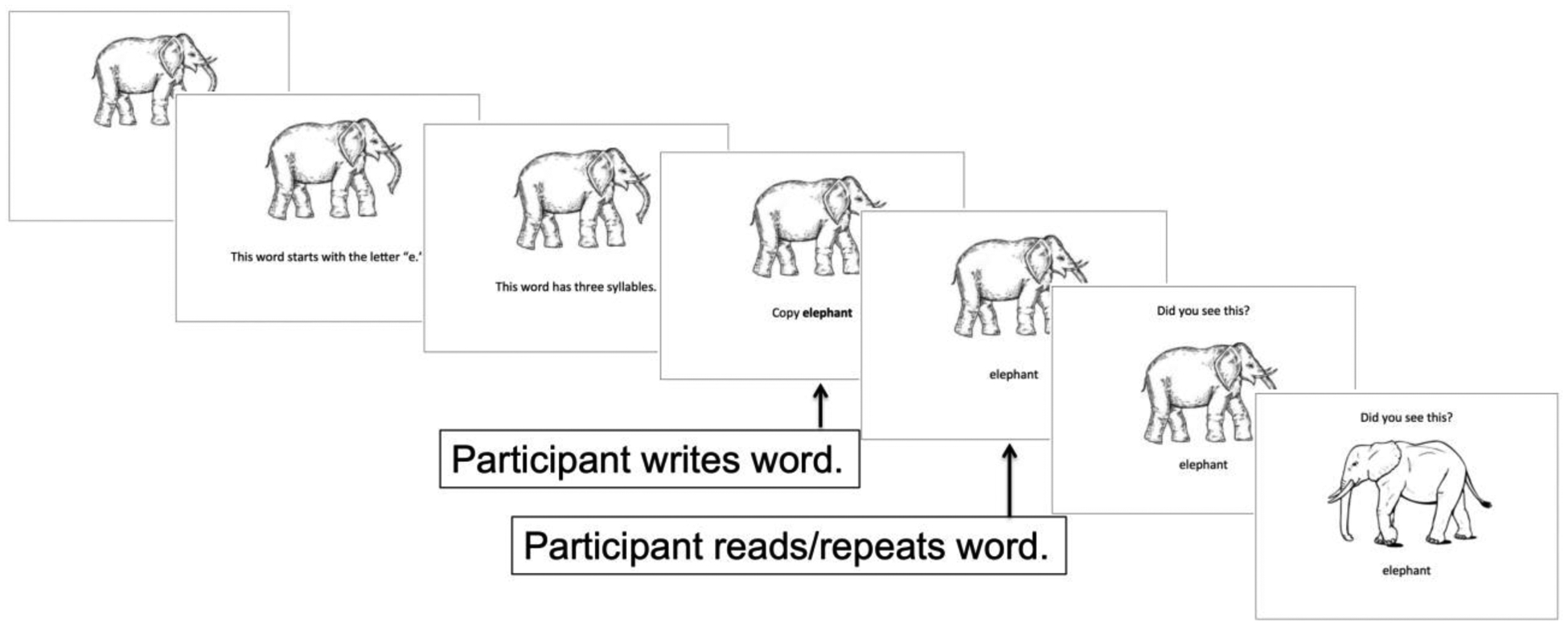
Example item from the Lexical Treatment Condition.
Semantic Treatment Condition (STC).
Stimuli were presented in the following sequence, which is depicted in Figure 2: 1) Picture alone, 1.5 seconds. 2) The picture remained on the screen, and the participant was presented with the word’s semantic features, similar to the Error-Reduced procedure employed by Reilly (2016). The third cue always included the target word. For example, for the noun elephant, the following features/cues were presented: “This has a trunk.” “It is gray.” An elephant is large.” For the verb kicking, the following features/cues were presented: “People do this for sports.” They do this with their leg.” “He is kicking the girl with his foot.” For both nouns and verbs, each feature was presented in written and auditory form. 3–4) Steps 3–4 were identical to those described above for LTC.
Figure 2.
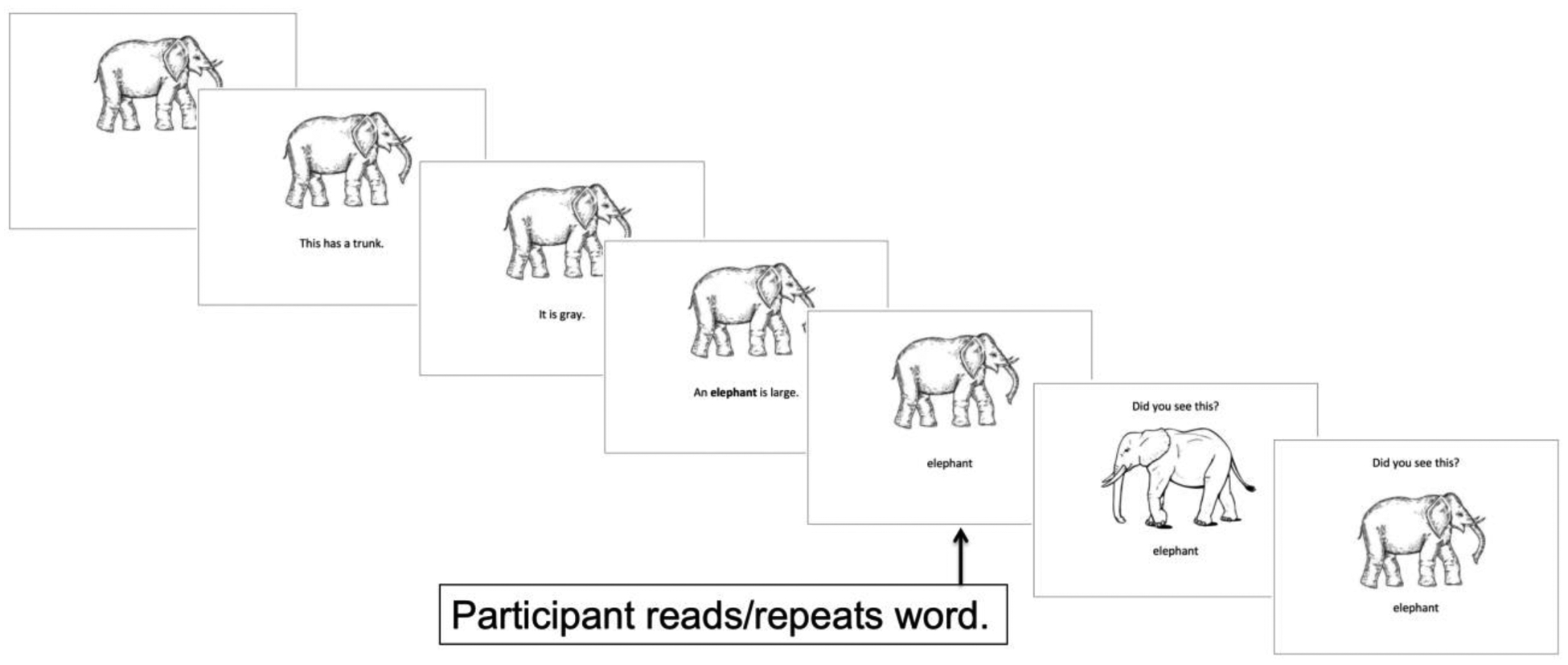
Example item from the Semantic Treatment Condition.
Practice Sessions.
The practice sessions were identical to the treatment sessions, except that the recognition test (step 4) was omitted. For each participant, a caregiver was provided with instructions for each type of treatment, and the experimenter explained the instructions to the caregiver. Participants performed the practice sessions with the caregiver two times per week, and the caregiver ensured that the participant practiced each set appropriately.
Four participants who did not have a caregiver available (LV1, LV6, NFV5, UPPA7) performed the home practice with one of the experimenters two times per week, using videoconferencing. Three additional participants (AD1, NFV7, and SV1) had a caregiver available, but declined to complete the home practice with the caregiver or the experimenter.
Post-Treatment and Follow-Up Testing.
Naming accuracy for Exemplar 2 was measured at 3, 7, 11, 14, 18, and 21 months. The tests for months 3, 11, and 18 were administered at the beginning of the monthly treatment sessions.
Participants also completed a Naming during Scene Description task at 7, 14, and 21 Months. In this task, a series of visual scenes was presented, and each scene contained one of the participant’s trained or untrained noun items. Participants were asked to briefly describe each scene. This test was used to assess task generalization.
Telerehabilitation.
All but three of the 37 participants participated remotely, either because they were unable to travel to the laboratory for the required number of sessions, or because of in-person restrictions that began during the COVID-19 pandemic. These participants were provided with a laptop, an echo-cancellation microphone, and a signature pad. For these participants, treatment sessions and the majority of the evaluation sessions were conducted via videoconferencing.
Data Analysis.
For each participant, naming accuracy was calculated as a proportion, separately for each type of item (prophylaxis or remediation) and each trained or untrained condition, and the proportions were arcsin transformed to control for possible violations of the normality assumption. For remediation items, we used the paired-samples t-test to examine the change in naming accuracy from baseline. Trained and untrained items were analyzed separately, in order to examine item generalization within the untrained condition.
For prophylaxis items, the accuracy data were analyzed in two different ways. In the first set of analyses, the paired-samples t-test was used to determine if accuracy for trained items was greater than accuracy for untrained items within each treatment condition.
In the second set of analyses, mixed-design ANCOVA was used to examine the interaction between Conceptual-Semantic Impairment and the size of the treatment effect (trained minus untrained) for each Treatment Condition (Lexical vs. Semantic). Diagnostic Subtype, Symptom Duration, and BDAE Severity Rating were entered as covariates.
The variable of Conceptual-Semantic Impairment included four possible categories: modality-general conceptual-semantic impairment, visual-conceptual impairment, lexical-semantic impairment, and no impairment. Based on norming of the picture and word versions of the PPT and KD that was conducted with unimpaired older adults, participants were classified as being impaired (scores greater than 2 standard deviations below the mean) on both the picture and word versions (i.e., modality-general conceptual-semantic impairment), the picture version (Objects or Actions) only (i.e., visual-conceptual impairment), the word version (Nouns or Verbs) only (i.e., lexical-semantic impairment), or neither the picture nor the word version (i.e., no impairment; see Table 2 and Figure 3). Diagnostic Subtype included five categories: lvPPA, svPPA, nfvPPA, unclassifiable PPA, and AD.
Figure 3.
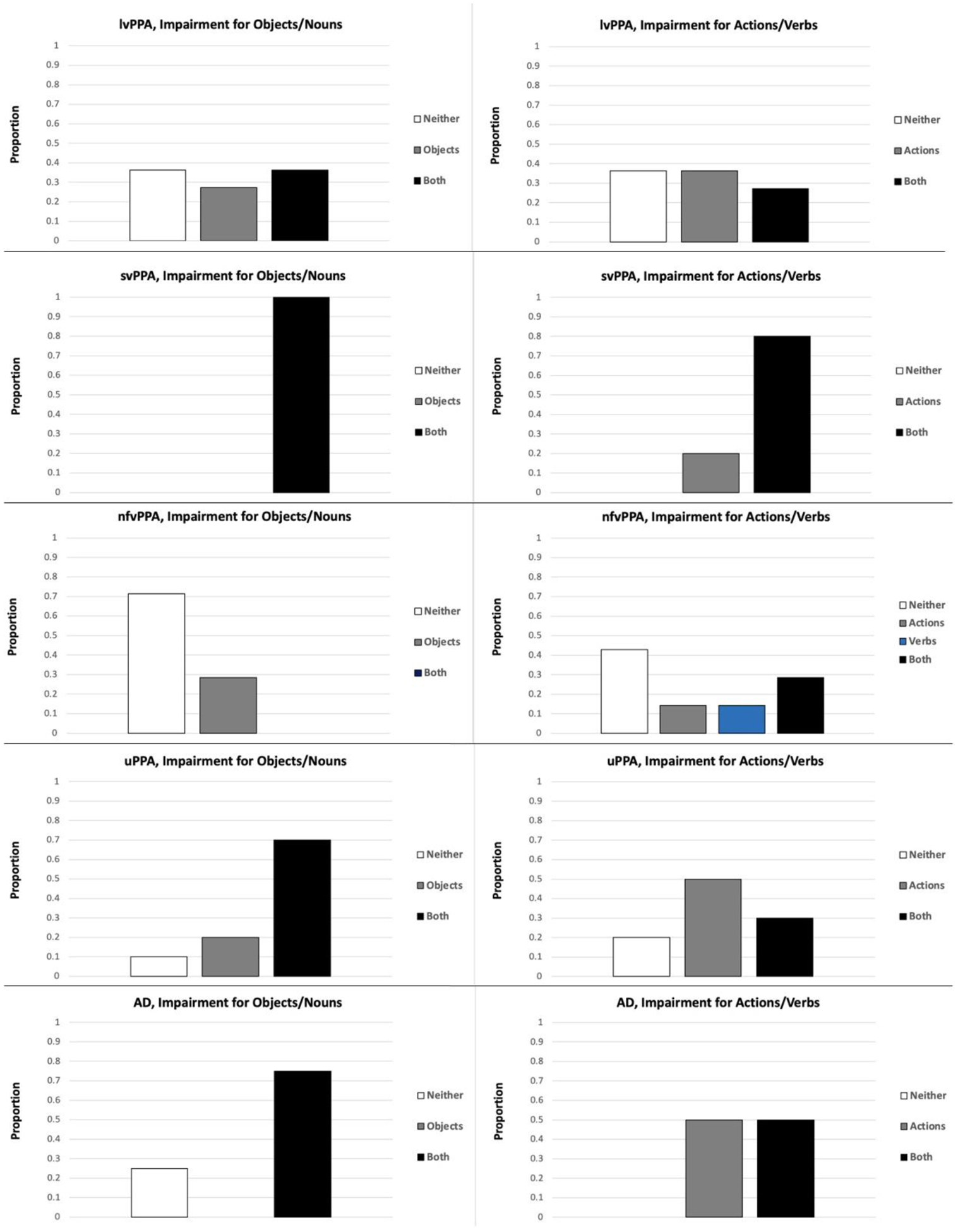
Conceptual-Semantic Impairment, by Diagnostic Subtype. lvPPA: logopenic variant primary progressive aphasia (N = 11); svPPA: semantic variant primary progressive aphasia (N = 5); nfvPPA: nonfluent/agrammatic variant primary progressive aphasia (N = 7); uPPA: unclassifiable primary progressive aphasia (N = 10); AD: Alzheimer’s disease (N = 4).
One participant (NFV1) was excluded from the ANCOVA analyses for verbs because she was the only participant who was impaired on wKD, but not pKD.
Results
Prophylaxis Items, Exemplar 2 (Stimulus Generalization).
Accuracy for the untrained exemplar was measured at 3, 7, 11, 14, 18, and 21 Months. At 3 Months, accuracy for trained items was significantly greater than accuracy for matched untrained items in LTC for nouns, t(32) = 3.44, p = .002, and in both LTC and STC for verbs [LTC: t(27) = 2.68, p = .012; STC: t(27) = 2.51, p = .018; see Figure 4]. The same pattern of results was observed at 7 Months [LTC for Nouns: t(30) = 3.42, p = .002; LTC for Verbs: t(25) = 4.07, p < .001; STC for Verbs: t(25) = 4.82, p < .001; see Figure 4]. At 11 Months, accuracy for trained items was significantly greater than untrained items in LTC for both nouns and verbs [LTC for Nouns: t(27) = 3.24, p = .003; LTC for Verbs: t(22) = 3.12, p = .005; see Figure 4]. In STC, the treatment effect was significant for nouns, t(27) = 2.40, p = .024, and nonsignificant for verbs, t(22) = 0.93, p = .364. At 14 Months, accuracy for trained items was significantly greater than untrained items for nouns and verbs in both LTC and STC [LTC, Nouns: t(24) = 4.41, p < .001; STC, Nouns: t(24) = 2.61, p = .015; LTC, Verbs: t(22) = 3.06, p = .006; STC, Verbs: t(22) = 2.96, p = .007; see Figure 4]. The same pattern of results was observed at 18 Months [LTC, Nouns: t(20) = 3.04, p = .006; STC, Nouns: t(20) = 2.85, p = .010; LTC, Verbs: t(18) = 2.64, p = .017; STC, Verbs: t(18) = 4.07, p < .001; see Figure 5]. At 21 Months, the treatment effect in LTC was significant for nouns, t(16) = 2.27, p = .038, and nonsignificant for verbs, t(14) = 1.96, p = .070. In STC, the treatment effect was significant for both nouns and verbs, STC for Nouns: t(16) = 3.65, p = .002; STC for Verbs: t(14) = 2.69, p = .018.
Figure 4.
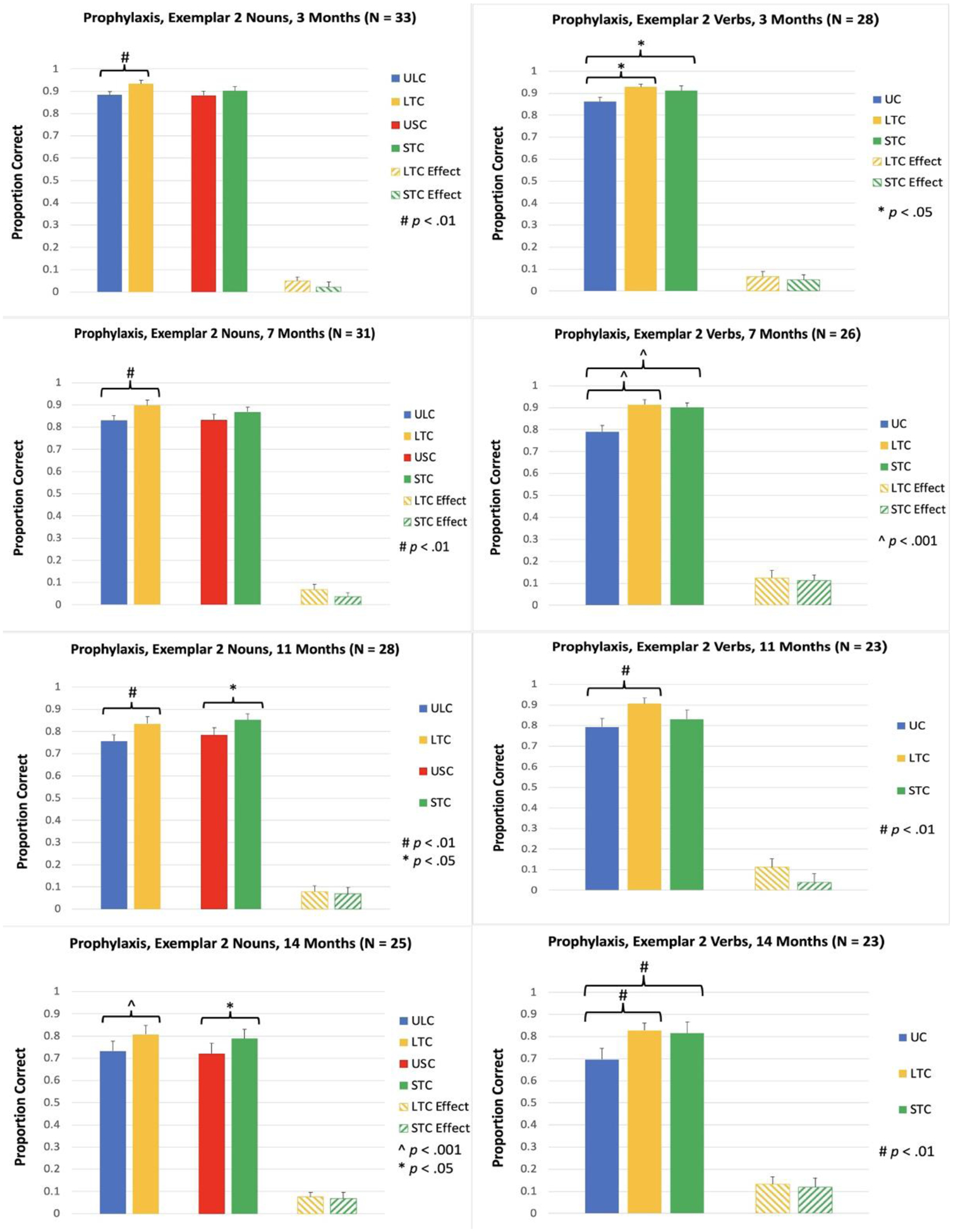
Proportion of items named correctly for Prophylaxis items, Exemplar 2, at 3, 7, 11, and 14 Months. ULC: Untrained Lexical Condition; LTC: Lexical Treatment Condition; USC: Untrained Semantic Condition; STC: Semantic Treatment Condition; UC: Untrained Condition; Effect: trained minus untrained.
Figure 5.
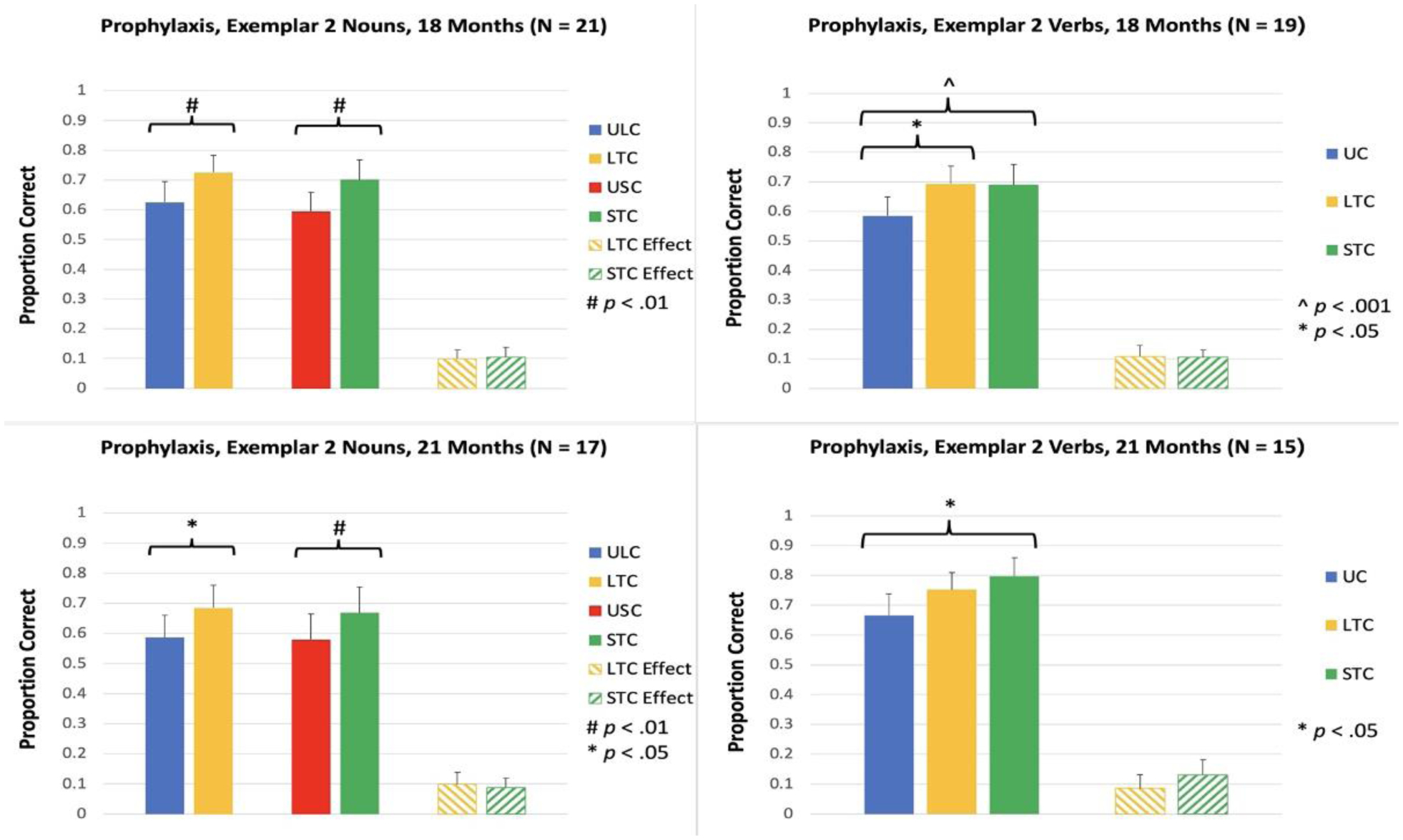
Proportion of items named correctly for Prophylaxis items, Exemplar 2, at 18 and 21 Months. ULC: Untrained Lexical Condition; LTC: Lexical Treatment Condition; USC: Untrained Semantic Condition; STC: Semantic Treatment Condition; UC: Untrained Condition; Effect: trained minus untrained.
Prophylaxis Items, Naming during Scene Description (Task Generalization).
We assessed naming of the noun items during a scene description task at 7, 14, and 21 Months. In LTC, the decline in naming accuracy was not significantly different between the trained and matched untrained items at any time point [LTC, 7 Months: t(26) = 2.04, p = .052; LTC, 14 Months: t(22) = 1.93, p = .067; LTC, 21 Months: t(14) = 0.92, p = .374; see Figure 6]. In STC, the change in naming accuracy for trained and matched untrained items was not significantly different at 7 Months, t(26) = 1.51, p = .142, but at 14 and 21 Months, the decline in accuracy for STC was significantly smaller than the decline for matched untrained items, STC, 14 Months: t(22) = 3.28, p = .003; STC, 21 Months: t(14) = 2.55, p = .023
Figure 6.
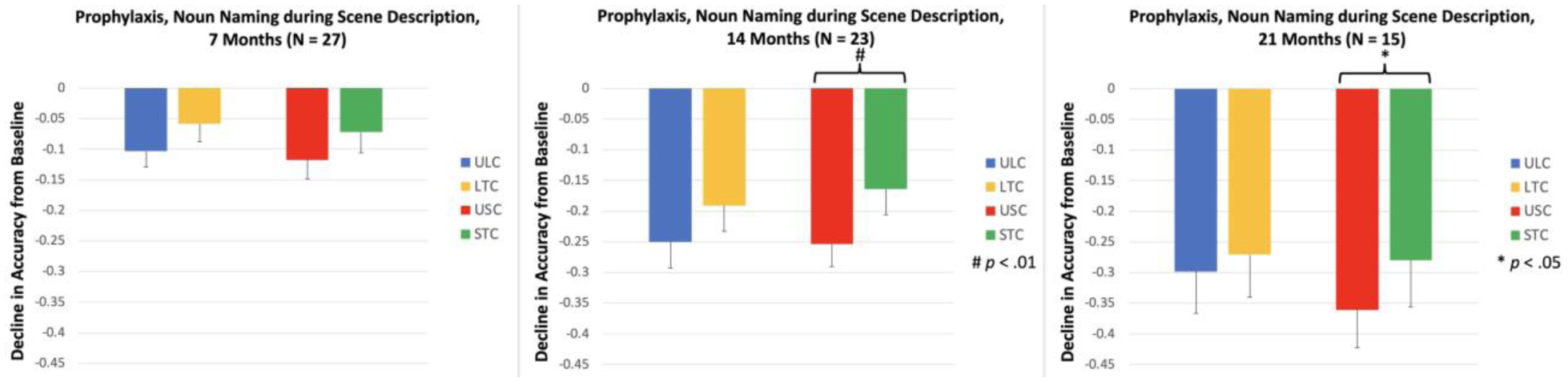
Decline in the proportion of items named correctly for Prophylaxis items, Naming during Scene Description task. ULC: Untrained Lexical Condition; LTC: Lexical Treatment Condition; USC: Untrained Semantic Condition; STC: Semantic Treatment Condition.
Prophylaxis Items, ANCOVA.
At 3 Months, there was a significant interaction between the Treatment Condition (lexical vs. semantic) and Conceptual-Semantic Impairment for Exemplar 2 Verbs, F(2, 21) = 5.17, p = .015. As can be seen in Figure 7, the Lexical treatment effect was significantly larger for participants with modality-general conceptual-semantic impairment, compared to participants with unimpaired conceptual-semantic processing (p = .007) and participants with visual-conceptual impairment (p = .005). In addition, within-subjects analysis showed that participants with modality-general conceptual-semantic impairment had a larger Lexical treatment effect, compared to their Semantic treatment effect [(p = .007); see Figure 8].
Figure 7.
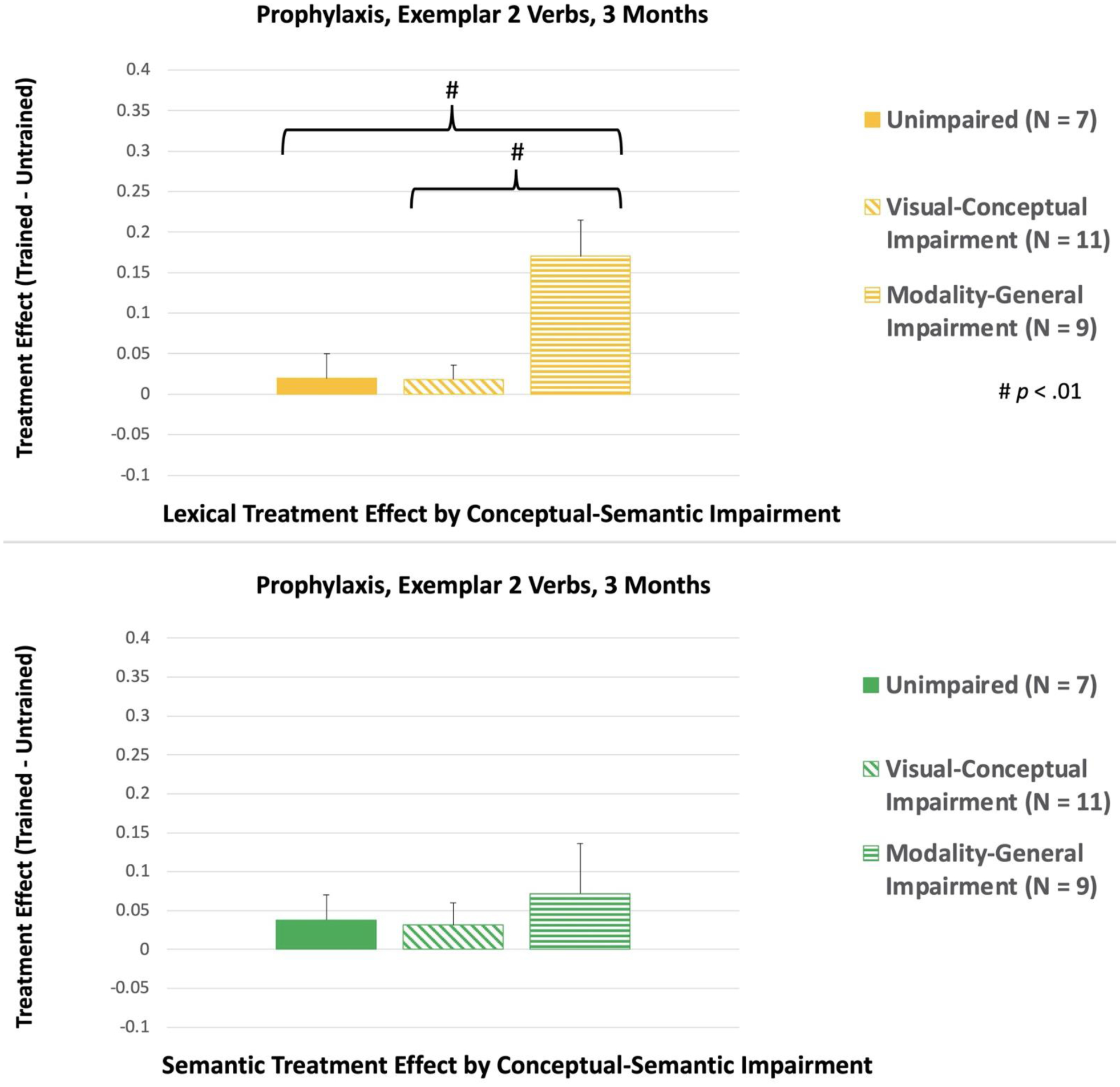
Between-subjects comparisons of the treatment effects for Exemplar 2, Prophylaxis Verbs at 3 Months. LTC: Lexical Treatment Condition; STC: Semantic Treatment Condition.
Figure 8.
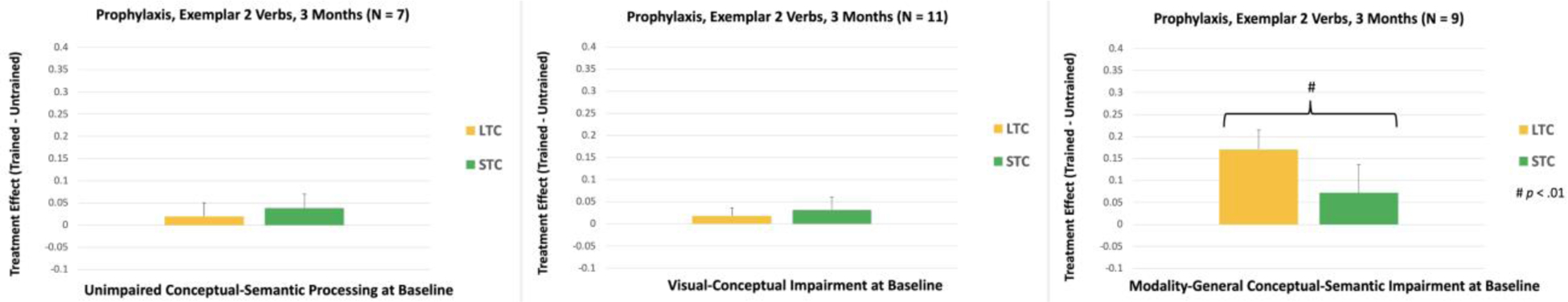
Within-subjects comparisons of the treatment effects for Exemplar 2, Prophylaxis Verbs at 3 Months. LTC: Lexical Treatment Condition; STC: Semantic Treatment Condition.
There were no significant interaction effects at 7 or 11 Months. However, at 14 Months, there was a significant Treatment Condition x Conceptual-Semantic Impairment interaction for Exemplar 2 Nouns, F(2, 19) = 6.32, p = .008. Participants with unimpaired conceptual-semantic processing at baseline had a larger Semantic treatment effect than participants with modality-general conceptual-semantic impairment [(p = .007); see Figure 9]. Furthermore, within-subjects analysis showed that participants with unimpaired conceptual-semantic processing at baseline had a larger Semantic treatment effect, compared to their Lexical treatment effect [(p = .021); see Figure 10]. In contrast, participants with modality-general conceptual-semantic impairment at baseline showed a larger Lexical treatment effect, compared to the Semantic treatment effect (p = .019). For participants with visual-conceptual impairment at baseline, the two treatment effects were not significantly different.
Figure 9.
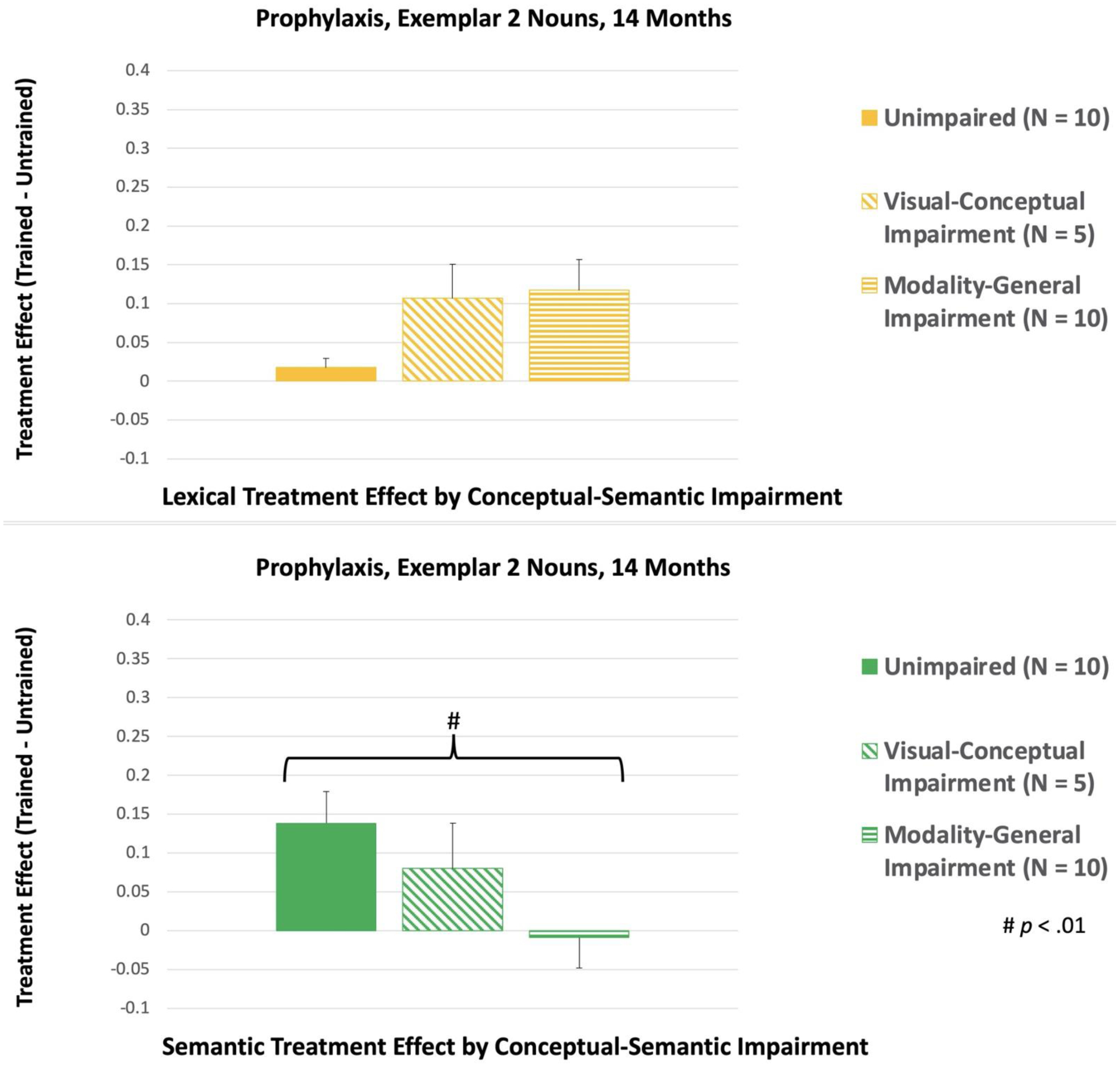
Between-subjects comparisons of the treatment effects for Exemplar 2, Prophylaxis Nouns at 14 Months. LTC: Lexical Treatment Condition; STC: Semantic Treatment Condition.
Figure 10.
Within-subjects comparisons of the treatment effects for Exemplar 2, Prophylaxis Nouns at 14 Months. LTC: Lexical Treatment Condition; STC: Semantic Treatment Condition.
At 18 Months, there were four significant interaction effects. For Exemplar 2 Nouns, the Treatment Condition x Conceptual-Semantic Impairment interaction was significant, F(2, 15) = 4.01, p = .040. However, none of the follow-up comparisons were significant. The Treatment Condition x BDAE Severity Rating interaction was also significant for Exemplar 2 Nouns, F(1, 15) = 20.66, p < .001. To explore this interaction, we examined the correlation between BDAE Severity and the treatment effect for each condition. Neither correlation was significant, Lexical: N = 21, τb = −.349, p = .057; Semantic: N = 21, τb = .289, p = .112.
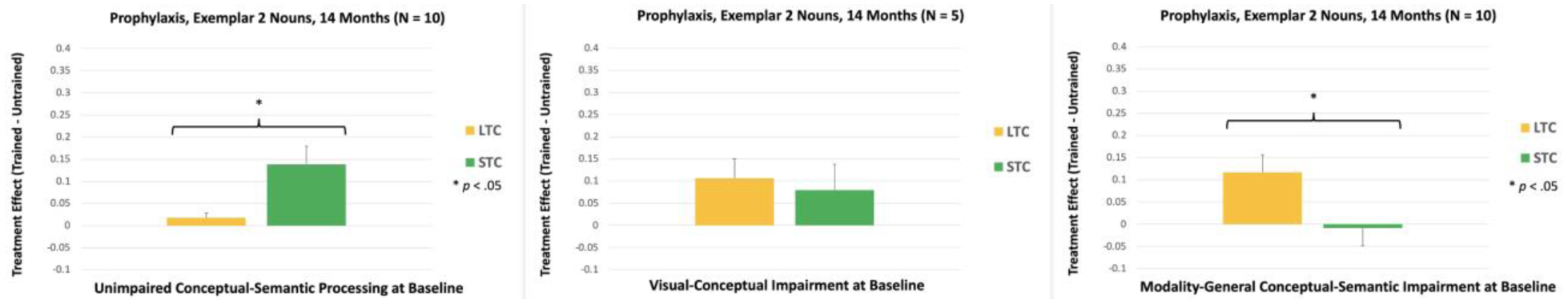
For Exemplar 2 Verbs, the Treatment Condition x Conceptual-Semantic Impairment interaction was significant, F(2, 12) = 5.52, p = .020. Within-subjects analysis showed that participants with unimpaired conceptual-semantic processing at baseline and participants with visual-conceptual impairment at baseline had a larger Semantic treatment effect, compared to their Lexical treatment effect [Unimpaired: p = .038; Visual-Conceptual Impairment: p = .023; see Figure 11]. In contrast, participants with modality-general conceptual-semantic impairment had a larger Lexical treatment effect, compared to their Semantic treatment effect (p = .022).
Figure 11.
Within-subjects comparisons of the treatment effects for Exemplar 2, Prophylaxis Verbs at 18 Months. LTC: Lexical Treatment Condition; STC: Semantic Treatment Condition.
Also at 18 Months, the Treatment Condition x Diagnostic Subtype interaction was significant for Exemplar 2 Verbs, F(1, 12) = 14.24, p = .003. For lvPPA and nfvPPA, there were 7 and 6 participants per Subtype, respectively, and follow-up comparisons were not significant (all p’s > .05). For svPPA, uPPA, and AD, there were three or fewer participants for each Diagnostic Subtype, and we used nonparametric analysis to explore the significant interaction. Fisher’s exact test was used to compare the Lexical and Semantic conditions for each participant. None of these comparisons were significant (all p’s > .05).
At 21 Months, the Treatment Condition x Symptom Duration interaction was significant for Exemplar 2 Nouns, F(1, 11) = 5.26, p = .043. In the Lexical condition, the correlation between the treatment effect and Symptom Duration was not significant, r(15) = .152, p = .559. In contrast, the correlation was significant in the Semantic condition, r(15) = −.492, p = .045, and the treatment effect decreased as symptom duration increased.
For the Naming during Scene Description task, there were no significant interaction effects at any time point.
Remediation Items, Exemplar 2 (Stimulus Generalization).
Accuracy for the untrained exemplar was measured at 3, 7, 11, 14, 18 and 21 Months. Compared to the baseline naming accuracy of 0% for these items, the increase in naming accuracy was significant in LTC and STC for both nouns and verbs at 3 Months [LTC, Nouns: t(16) = 7.07, p < .001; STC, Nouns: t(16) = 7.69, p < .001; LTC, Verbs: t(14) = 6.94, p < .001; STC, Verbs: t(14) = 4.72, p < .001; see Figure 12], 7 Months [LTC, Nouns: t(14) = 6.66, p < .001; STC, Nouns: t(14) = 3.56, p = .003; LTC, Verbs: t(11) = 4.23, p = .001; STC, Verbs: t(11) = 4.44, p < .001; see Figure 12], 11 Months [LTC, Nouns: t(12) = 8.51, p < .001; STC, Nouns: t(12) = 4.43, p < .001; LTC, Verbs: t(9) = 5.35, p < .001; STC, Verbs: t(9) = 4.51, p = .001; see Figure 12], 14 Months [LTC, Nouns: t(10) = 5.79, p < .001; STC, Nouns: t(10) = 2.96, p = .014; LTC, Verbs: t(8) = 4.36, p = .002; STC, Verbs: t(8) = 2.83, p = .022; see Figure 12], 18 Months [LTC, Nouns: t(8) = 5.12, p < .001; STC, Nouns: t(8) = 2.60, p = .031; LTC, Verbs: t(7) = 4.13, p = .004; STC, Verbs: t(7) = 2.62, p = .034; see Figure 13], and 21 Months [LTC, Nouns: t(6) = 2.86, p = .029; STC, Nouns: t(6) = 2.73, p = .034; LTC, Verbs: t(5) = 2.58, p = .049; STC, Verbs: t(5) = 2.68, p = .044; see Figure 13].
Figure 12.
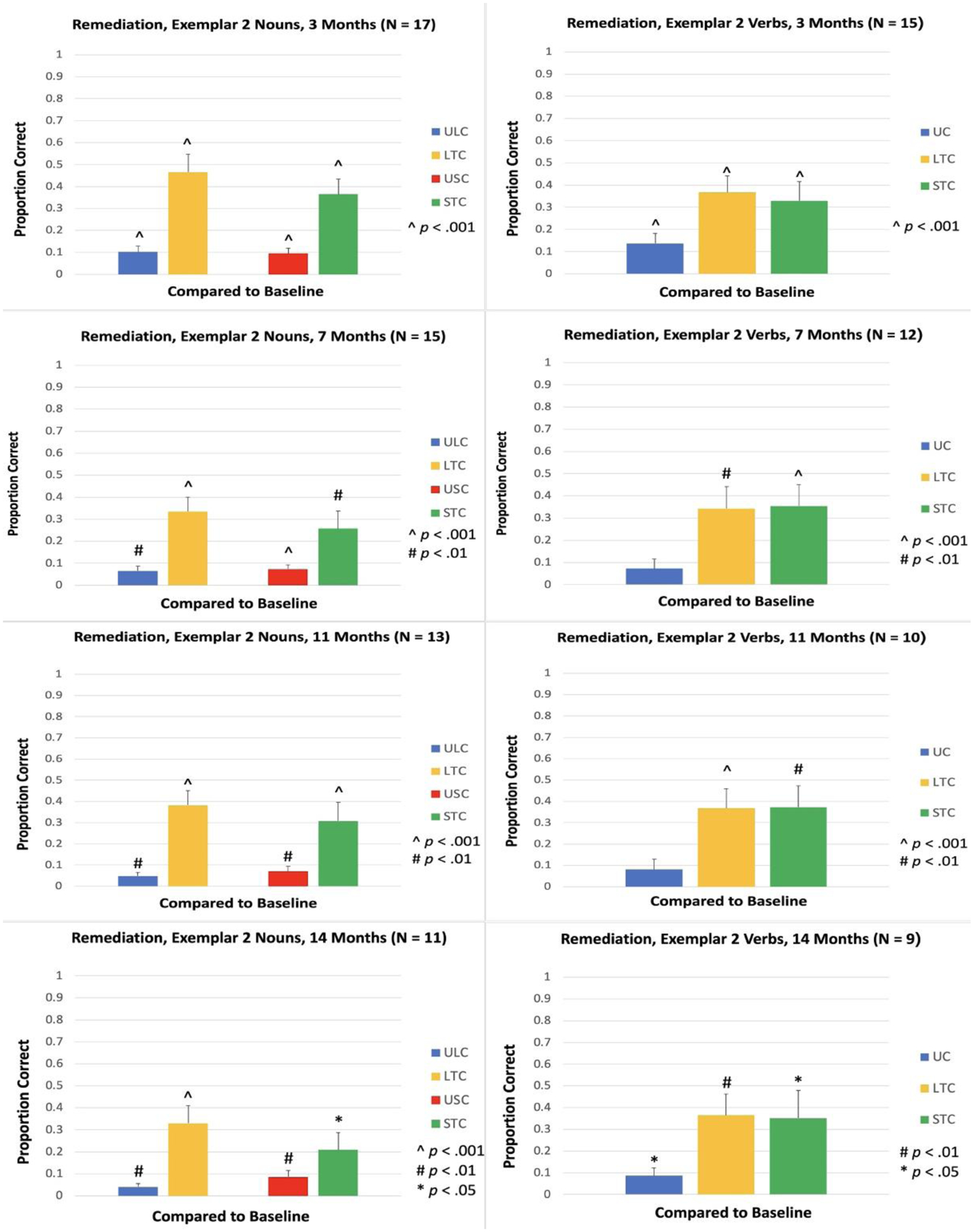
Proportion of items named correctly for Remediation items, Exemplar 2, at 3, 7, 11, and 14 Months. ULC: Untrained Lexical Condition; LTC: Lexical Treatment Condition; USC: Untrained Semantic Condition; STC: Semantic Treatment Condition; UC: Untrained Condition.
Figure 13.
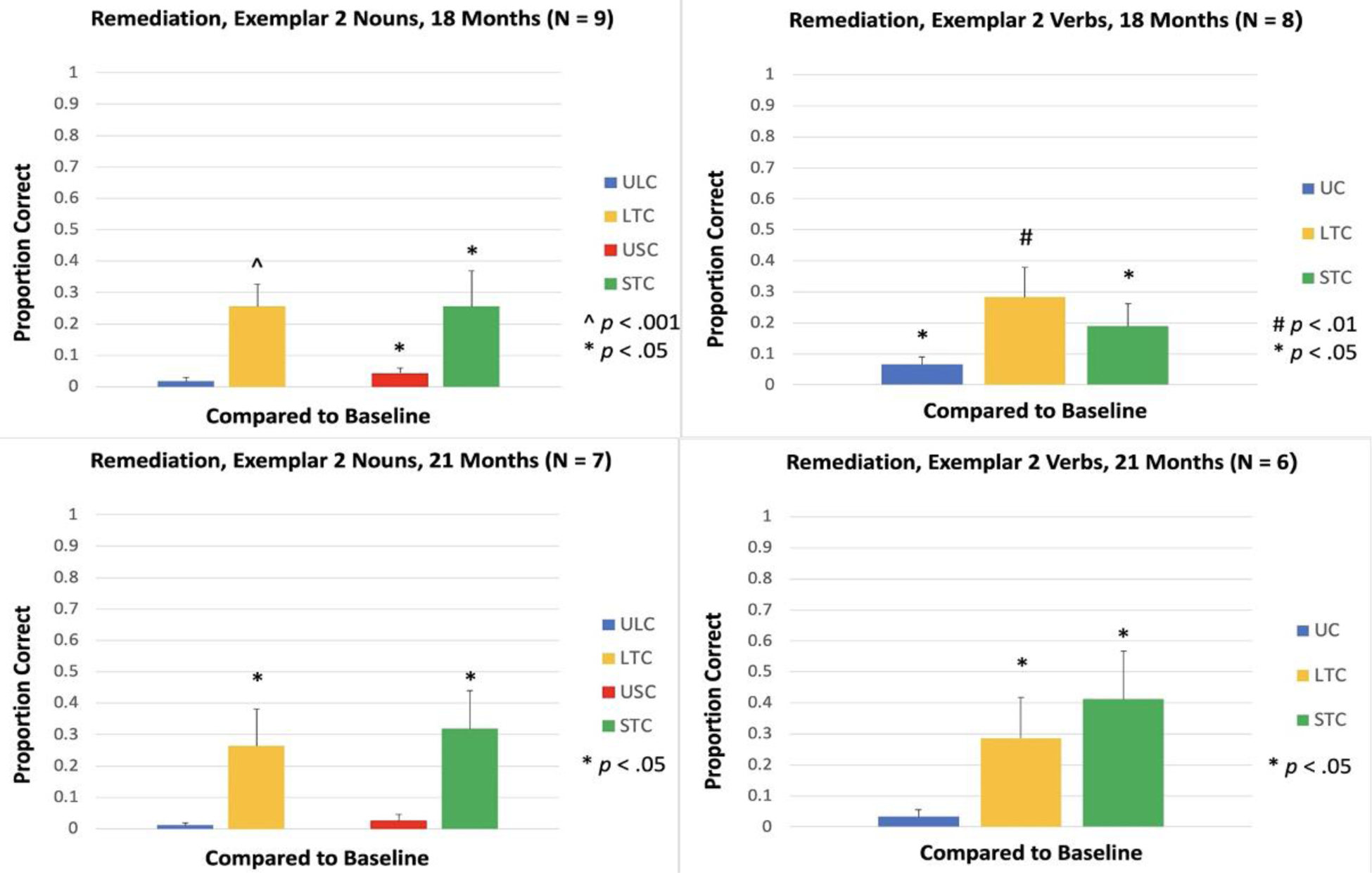
Proportion of items named correctly for Remediation items, Exemplar 2, at 18 and 21 Months. ULC: Untrained Lexical Condition; LTC: Lexical Treatment Condition; USC: Untrained Semantic Condition; STC: Semantic Treatment Condition; UC: Untrained Condition.
Remediation, Item Generalization.
For nouns, there was an increase in naming accuracy for matched untrained items in both conditions (ULC and USC), and the increase in accuracy was significant at 3, 7, 11, and 14 Months [ULC, 3 Months: t(16) = 5.32, p < .001; USC, 3 Months: t(16) = 4.76, p < .001; ULC, 7 Months: t(14) = 3.69, p = .002; USC, 7 Months: t(14) = 4.30, p < .001; ULC, 11 Months: t(12) = 3.10, p = .009; USC, 11 Months: t(12) = 3.39, p = .005; ULC, 14 Months: t(10) = 3.33, p = .008; USC, 14 Months: t(10) = 3.70, p = .004; see Figure 12]. At 18 Months, the increase was significant in USC, but not ULC [ULC: t(8) = 1.50, p = .171; USC: t(8) = 2.94, p = .019; see Figure 13]. At 21 Months, the increase was not significant in either condition [ULC: t(6) = 1.54, p = .174; USC: t(6) = 1.48, p = .189; see Figure 13].
For verbs, there was a significant increase in accuracy for items in the untrained condition (UC) at 3 Months [t(14) = 4.55, p < .001; see Figure 12], 14 Months [t(8) = 3.21, p = .012; see Figure 12], and 18 Months [t(7) = 3.20, p = .015; see Figure 13], but the increase was not significant at 7 Months [t(11) = 1.81, p = .098; see Figure 12], 11 Months [t(9) = 1.85, p = .098; see Figure 12], or 21 Months [t(5) = 1.58, p = .175; see Figure 13].
Discussion
Compared to baseline naming performance, Lexical and Semantic treatments both improved naming accuracy for treated Remediation nouns and verbs, including stimulus generalization at every time point. Naming accuracy also improved for semantically-matched untrained Remediation nouns in both treatment conditions, providing evidence for item generalization within semantic categories. However, item generalization was less reliable over time for verbs (see Figures 12 and 13), possibly because the verb categories (e.g., Object Actions; Body Actions) were less specific than the noun categories (e.g., Animals; Appliances).
For Prophylaxis items, Lexical Treatment was effective for both nouns and verbs, including stimulus generalization at every time point, except for verbs at 21 Months. Semantic Treatment was effective at every time point for verbs, with one exception (11 Months). The pattern of results was different for nouns -- the effect of Semantic Treatment was initially nonsignificant (at 3 and 7 Months), but it was significant at 11, 14, 18, and 21 Months, suggesting that the effects of prophylactic Semantic Treatment may become more apparent as the disorder progresses over time, possibly because conceptual-semantic deficits begin to develop in additional participants.
Similarly, for nouns in the Semantic Treatment condition, there was evidence of generalization to a Naming during Scene Description task when it was administered at later time points (14 and 21 Months), with significantly less decline in naming accuracy for trained items, compared to matched untrained items. In contrast, for this task, the effect of Lexical Treatment was marginally significant at 7 and 14 Months and nonsignificant at 21 Months.
For nouns, Diagnostic Subtype did not interact with Treatment Condition (Lexical vs. Semantic) at any time point. The lack of an interaction between Diagnostic Subtype and the Treatment Effect is consistent with findings of similar treatment outcomes in participants with lvPPA and svPPA (Henry et al., 2019; Meyer, Tippett, & Friedman, 2018), and the current study extends this finding to include nfvPPA, unclassifiable PPA, and AD. On the other hand, in the current study there was a significant interaction between Diagnostic Subtype and Treatment Condition for Exemplar 2 Verbs at 18 Months. However, follow-up comparisons did not reveal any significant differences between Lexical and Semantic treatment for any Diagnostic Subtype.
In contrast to Diagnostic Subtype, there were multiple significant interactions that involved the variable of Conceptual-Semantic Impairment. The first significant interaction occurred at 3 Months. At this time point, participants with baseline modality-general conceptual-semantic impairment for actions/verbs showed a significantly greater treatment effect for Exemplar 2 Prophylaxis Verbs in the Lexical condition, compared to their treatment effect in the Semantic condition, and also compared to the Lexical treatment effect for the other two subgroups (unimpaired conceptual-semantic processing and visual-conceptual impairment).
Furthermore, the interaction between Conceptual-Semantic Impairment and the Treatment Effect (lexical vs. semantic) was significant for Exemplar 2 Prophylaxis Nouns at 14 Months, and for Exemplar 2 Prophylaxis Verbs at 18 Months. For both of these interactions, the pattern of results suggested that individuals who have modality-general conceptual-semantic impairment at baseline are more likely to benefit from lexical treatment over time, while individuals who have unimpaired conceptual-semantic processing at baseline are more likely to benefit from semantic treatment as the disorder progresses. These findings are consistent with the argument that treatment is more likely to be beneficial when it is designed to capitalize on spared language abilities (Henry et al., 2019). Participants who have modality-general conceptual-semantic impairment at baseline may have difficulty comprehending some of the semantic cues used during treatment, while spared phonological and orthographic abilities could allow them to benefit from the cues used during lexical treatment, which may strengthen lexical-semantic connections through repeated activation (Meyer et al., 2019). Participants who have unimpaired conceptual-semantic processing at baseline may be more likely to comprehend the semantic cues, which could strengthen intact semantic representations before they begin to decline.
For Exemplar 2 Prophylaxis Verbs at 18 Months, participants with visual-conceptual impairment at baseline had a significantly larger treatment effect in the Semantic condition, compared to their treatment effect in the Lexical condition. This finding suggests that individuals who have visual-conceptual impairment (without lexical-semantic impairment) are able to comprehend the semantic cues, which may strengthen visual-conceptual connections or strengthen intact semantic representations before they begin to decline.
In contrast to the findings of the current study, Suárez-González et al. (2018) found that a participant with svPPA, who was impaired on both versions of the PPT, showed greater benefit from conceptual-semantic treatment, compared to orthographic treatment. Although both types of treatment improved naming for noun items, the conceptual-semantic treatment also resulted in task generalization and longer maintenance of the treatment effect. In Jokel et al. (2016), two of the participants with svPPA were impaired on both versions of PPT. One of these participants had similar treatment effects in the semantic and phonological conditions, while the other participant showed a significantly greater benefit from semantic treatment. In the current study, a few participants who were impaired on both versions of PPT or KD had a numerically larger treatment effect in the semantic treatment condition on at least one task at 14 Months. However, this pattern was the exception rather than the rule, and it remains unclear why some participants exhibit this pattern. One possible explanation involves the potential roles of taxonomic and thematic processing impairments on the conceptual-semantic treatment response. Taxonomic features are organized around hierarchical, categorical relations; they are space- and time-independent (e.g., a dog is an animal, and it has fur). In contrast, thematic features are related to experience, events, and scenarios; the relationship is spatial, temporal, or functional (e.g., dogs can be found at the park, and they catch frisbees). Treatments that utilize semantic feature analysis typically include features that are taxonomically and thematically related to target words, without distinguishing between the two. We posit that measures of taxonomic and thematic proficiency at baseline will predict which of these two feature types will yield better treatment outcomes in PPA and AD. This hypothesis will be tested in future work.
Unexpectedly, in the current study no participants were impaired on wPPT without also being impaired on pPPT, and only one participant was impaired on wKD without also being impaired on pKD. One explanation for these findings is that impairment on wPPT or wKD is actually indicative of a broader, modality-general conceptual-semantic impairment, such that impairment on the word version of one of these tasks will typically coincide with impairment on the corresponding picture version. Another possible explanation for these findings is that participants verbally recode the picture stimuli on the pPPT and pKD, which could be beneficial if words provide a direct route to the activation of conceptual representations (Lupyan & Thompson-Schill, 2012). Participants with a lexical-semantic deficit, as indicated by impaired performance on wPPT or wKD, may have difficulty verbally recoding the picture stimuli, which could lead to impaired performance on pPPT or pKD.
All participants with svPPA had modality-general conceptual-semantic impairment for objects/nouns, and 4 out of 5 with svPPA had conceptual-semantic impairment for actions/verbs. In previous studies that have utilized these tasks, greater variability in performance across the four tasks has been found in svPPA (Jokel et al., 2016) and semantic dementia (Bak & Hodges, 2003). Jokel et al. found that 2 out of 4 participants with svPPA were only impaired on a subset of the 4 tasks, while Bak and Hodges found that 6 out of 14 participants with semantic dementia were only impaired on a subset of the 4 tasks. Furthermore, the pattern of broad conceptual-semantic impairment for both objects/nouns and actions/verbs was not unique to svPPA in the current study – this pattern also occurred in three participants with lvPPA, three participants with unclassifiable PPA, and two participants with AD.
In contrast to the other four diagnostic subtypes that were included in this study, no participant with nfvPPA exhibited a pattern of broad conceptual-semantic impairment for both objects/nouns and actions/verbs. Instead, the greatest conceptual impairment in nfvPPA occurred in two participants with a visual-conceptual deficit for objects and a modality-general conceptual-semantic impairment for actions/verbs, while the other five participants with nfvPPA either showed no evidence of lexical-semantic or visual-conceptual impairment, or they had isolated impairment for verbs or actions. Additionally, unlike the other four diagnostic subtypes, no participant with nfvPPA had remediation items for nouns or verbs. At baseline, participants with nfvPPA typically had either consistently high naming accuracy or naming performance that was variable across the three naming tests, resulting in a dearth of candidates for remediation. Therefore, a focus on prophylactic treatment appears to be particularly important in nfvPPA.
At 21 Months, there was a significant interaction between Treatment Condition and Symptom Duration for Exemplar 2 Nouns. For the Lexical treatment, the correlation between the treatment effect and Symptom Duration was not significant. In contrast, for the Semantic treatment there was a significant relationship between the treatment effect and Symptom Duration, with a smaller effect as Symptom Duration increased. Notably, this interaction was only significant at the final time point of the study. This finding underscores the importance of beginning semantic treatment during the earlier stages of the disorder.
One limitation of this study is the relatively small number of participants for some Diagnostic Subtypes (e.g., svPPA and typical AD). Replication with a larger number of participants may be necessary for these subtypes. Another potential limitation is that the alternating treatment design that was utilized in this study poses some risk of each treatment facilitating accuracy for items in the other trained and untrained conditions (e.g., the Semantic treatment could generalize to items in LTC or ULC). In order to facilitate the measurement of item generalization, the trained Lexical and Semantic noun items were selected from different semantic categories, and the trained noun items were matched with untrained items on semantic category and length. There was a small but significant improvement for untrained noun Remediation items in both treatment conditions (Lexical and Semantic), and the size of the improvement was similar in the two conditions. These findings suggest that item generalization occurred in both treatment conditions, but the generalization effect was not large in either condition.
In conclusion, the findings of this study indicate that lexical and semantic treatments are both effective in the prophylaxis and remediation of anomia for nouns and verbs in PPA and AD. However, the effects of semantic treatment take longer to emerge for Prophylaxis nouns, and differences between impairment subgroups become more apparent over time, highlighting the importance of measuring treatment effects over an extended period of time. Participants who have modality-general conceptual-semantic impairment at baseline eventually show greater benefit from lexical treatment, while individuals who have unimpaired conceptual-semantic processing at baseline eventually show greater benefit from semantic treatment. In contrast to conceptual-semantic impairment, diagnostic subtype does not typically predict treatment effects. From a clinical perspective, the findings of this study suggest that conceptual-semantic impairment should be measured in both the visual and lexical modalities prior to the initiation of anomia treatment for patients with PPA or typical AD. In order to facilitate the maintenance of each patient’s functional vocabulary, patients with modality-general conceptual-semantic impairment should be enrolled in lexical treatment, while patients who have unimpaired conceptual-semantic processing should be enrolled in semantic treatment.
General aphasia batteries can identify impairments in auditory and reading comprehension, repetition, and oral and written verbal expression, and can establish overall severity of language impairment, but are not sufficient to uncover the specific patterns of deficits in phonology, syntax or semantics that are required to guide treatment. Supplementary tests are required to assess these domains (Europa et al., 2020; Henry et al., 2018). The Pyramids and Palm Trees and Kissing and Dancing tests can be used to measure conceptual-semantic impairment for Objects/Nouns and Actions/Verbs, respectively. Administration of these tasks is straightforward, and a short version of the PPT (Breining et al., 2015) is available and well-suited to the time constraints of clinical practice.
Acknowledgments
This study was supported by the NIDCD and NIA under grant number R01DC011317. ClinicalTrials.gov ID: NCT02675270
Footnotes
The authors report no conflicts of interest.
References
- Altmann LJP, & McClung JS (2008). Effects of semantic impairment on language use in Alzheimer’s disease. Seminars in Speech and Language, 29, 18–31. DOI: 10.1055/s-2008-1061622 [DOI] [PubMed] [Google Scholar]
- Appell J, Kertesz A, & Fisman M (1982). A study of language functioning in Alzheimer patients. Brain & Language, 17, 73–91. DOI: 10.1016/0093-934X(82)90006-2 [DOI] [PubMed] [Google Scholar]
- Ash S, McMillan C, Gunawardena D, Avants B, Morgan B, Khan A, et al. (2010). Speech errors in progressive non-fluent aphasia. Brain & Language, 113, 13–20. DOI: 10.1016/j.bandl.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, Evans E, O’Shea J, Powers J, Boller A, Weinberg D, et al. (2013). Differentiating primary progressive aphasias in a brief sample of connected speech. Neurology, 81, 329–336. DOI: 10.1212/WNL.0b013e31829c5d0e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, & Gulikers L (1995). The CELEX Lexical Database (Release 2) [CD-ROM]. Philadelphia: Linguistic Data Consortium, University of Pennsylvania. [Google Scholar]
- Bak TH, & Hodges JR (2003). Kissing and dancing - a test to distinguish the lexical and conceptual contributions to noun/verb and action/object dissociation. Preliminary results in patients with frontotemporal dementia. Journal of Neurolinguistics, 16, 169–181. DOI: 10.1016/S0911-6044(02)00011-8 [DOI] [Google Scholar]
- Baldo JV, & Dronkers NF (2006). The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology, 20, 529–538. DOI: 10.1037/0894-4105.20.5.529 [DOI] [PubMed] [Google Scholar]
- Baldo JV, Katseff S, & Dronkers NF (2012). Brain regions underlying repetition and auditory-verbal short-term memory deficits in aphasia: Evidence from voxel-based lesion symptom mapping. Aphasiology, 26, 338–354. DOI: 10.1080/02687038.2011.602391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales A, Cartwright J, Whitworth A, & Panegyres PK (2016). Exploring generalisation processes following lexical retrieval intervention in primary progressive aphasia. International Journal of Speech-Language Pathology, 18, 299–314. DOI: 10.3109/17549507.2016.1151936 [DOI] [PubMed] [Google Scholar]
- Beeson PM, King RM, Bonakdarpour B, Henry ML, Cho H, & Rapcsak SZ (2011). Positive effects of language treatment for the logopenic variant of primary progressive aphasia. Journal of Molecular Neuroscience, 45, 724–736. DOI: 10.1007/s12031-011-9579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Embleton KV, Jefferies E, Parker GJ, & Lambon Ralph MA (2010). The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: Evidence from a novel direct comparison of distortion-corrected fMRi, rTMS, and semantic dementia. Cerebral Cortex, 20, 2728–2738. DOI: 10.1093/cercor/bhq019 [DOI] [PubMed] [Google Scholar]
- Botha H, Duffy JR, Whitwell JL, Strand EA, Machulda MM, Schwarz CG, et al. (2015). Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex, 69, 220–236. DOI: 10.1016/j.cortex.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M, & Coelho CA (1995). Application of semantic feature analysis as a treatment for aphasic dysnomia. American Journal of Speech-Language Pathology, 4, 94–98. DOI: 10.1044/1058-0360.0404.94 [DOI] [Google Scholar]
- Braak H, & Braak E (1995). Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiology of Aging, 16, 271–284. DOI: 10.1016/0197-4580(95)00021-6 [DOI] [PubMed] [Google Scholar]
- Breining BL, Lala T, Martínez Cuitiño M, Manes F, Peristeri E, Tsapkini K, et al. (2015). A brief assessment of object semantics in primary progressive aphasia. Aphasiology, 29, 488–505. DOI: 10.1080/02687038.2014.973360 [DOI] [Google Scholar]
- Buchsbaum BR, Baldo J, Okada K, Berman KF, Dronkers N, D’Esposito M, & Hickok G (2011). Conduction aphasia, sensory-motor integration, and phonological short-term memory – An aggregate analysis of lesion and fMRI data. Brain and Language, 119, 119–128. DOI: 10.1016/j.bandl.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CR, Brambati SM, Miller BL, Gorno-Tempini M (2009). The neural correlates of verbal and nonverbal semantic processing deficits in neurodegenerative disease. Cognitive and Behavioral Neurology, 22, 73–80. DOI: 10.1097/WNN.0b013e318197925d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputi N, Di Giacomo D, Aloisio F, Passafiume D (2016). Deterioration of semantic associative relationships in mild cognitive impairment and Alzheimer Disease. Applied Neuropsychology: Adult, 23, 186–195. DOI: 10.1080/23279095.2015.1030020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertkow H & Bub D (1990). Semantic memory loss in dementia of Alzheimer’s type. What do various measures measure? Brain, 113, 397–417. DOI: 10.1093/brain/113.2.397 [DOI] [PubMed] [Google Scholar]
- Cotelli M, Borroni B, Manenti R, Alberici A, Calabria M, Agosti C, et al. (2006). Action and object naming in frontotemporal dementia, progressive supranuclear palsy, and corticobasal degeneration. Neuropsychology, 20, 558–565. DOI: 10.1037/0894-4105.20.5.558 [DOI] [PubMed] [Google Scholar]
- Croot K, Raiser T, Taylor-Rubin C, Ruggero L, Ackl N, Wlasich E, et al. (2019). Lexical retrieval treatment in primary progressive aphasia: An investigation of treatment duration in a heterogeneous case series. Cortex, 115, 133–158. DOI: 10.1016/j.cortex.2019.01.009 [DOI] [PubMed] [Google Scholar]
- Delacourte A, David JP, Sergeant N, Buee L, Wattez A, Vermersch P, et al. (1999). The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology, 52, 1158–1165. DOI: 10.1212/wnl.52.6.1158 [DOI] [PubMed] [Google Scholar]
- Domoto-Reilly K, Sapolsky D, Brickhouse M, Dickerson BC, & ADNI (2012). Naming impairment in Alzheimer’s disease is associated with left anterior temporal lobe atrophy. NeuroImage, 63, 348–355. DOI: 10.1016/j.neuroimage.2012.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel K, Huber W, Frings L, Kummerer D, Saur D, Mader I, et al. (2010). Model-oriented naming therapy in semantic dementia: A single-case fMRI study. Aphasiology, 24, 1537–1558. DOI: 10.1080/02687038.2010.500567 [DOI] [Google Scholar]
- Europa E, Iaccarino L, Perry DC, Weis E, Welch AE, Rabinovici GD, et al. (2020). Diagnostic Assessment in Primary Progressive Aphasia: An Illustrative Case Example. Am J Speech Lang Pathol, 29, 1833–1849. DOI: 10.1044/2020_AJSLP-20-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner AS, Webster KT, Ficek BN, Frangakis CE & Tsapkini K (2019). Written Verb Naming Improves After tDCS Over the Left IFG in Primary Progressive Aphasia. Front. Psychol, 10, 1396. DOI: 10.3389/fpsyg.2019.01396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurie M, Ungrady M & Reilly J (2020). Evaluating a Maintenance-Based Treatment Approach to Preventing Lexical Dropout in Progressive Anomia. J. Speech Lang. Hear. Res 63, 4082–4095. DOI: 10.1044/2020_JSLHR-20-00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). Mini-Mental State: A practical method for grading the cognitive state of outpatients for the clinician. Journal of Psychiatric Research, 12, 189–198. DOI: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Frings L, Kloppel S, Teipel S, Peters O, Frolich L, Pantel J, et al. (2011). Left anterior temporal lobe sustains naming in Alzheimer’s dementia and mild cognitive impairment. Curr. Alzheimer Res 8, 893–901. DOI: 10.2174/156720511798192673 [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, & Barresi B (2001). Boston Diagnostic Aphasia Examination (3rd Edition). Austin: Pro-Ed. [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A et al. (2008). The logopenic/phonological variant of primary progressive aphasia. Neurology, 71, 1227–1234. DOI: 10.1212/01.wnl.0000320506.79811.da [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55, 335–346. DOI: 10.1002/ana.10825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76, 1006–1014. DOI: 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, et al. (1996). Progressive non-fluent aphasia: language, cognitive and PET measures contrasted with probable Alzheimer’s disease. Journal of Cognitive Neuroscience, 8,135–154. DOI: 10.1162/jocn.1996.8.2.135 [DOI] [PubMed] [Google Scholar]
- Harris JM, Gall C, Thompson JC, Richardson AMT, Neary D, du Plessis D, et al. (2013). Classification and pathology of primary progressive aphasia. Neurology, 81, 1832–1839. DOI: 10.1212/01.wnl.0000436070.28137.7b [DOI] [PubMed] [Google Scholar]
- Henry ML, & Gorno-Tempini ML (2010). The logopenic variant of primary progressive aphasia. Current Opinion in Neurology, 23, 633–637. DOI: 10.1097/WCO.0b013e32833fb93e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Grasso SM (2018). Assessment of Individuals with Primary Progressive Aphasia. Semin Speech Lang., 39, 231–241. DOI: 10.1055/s-0038-1660782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Hubbard HI, Grasso SM, Dial HR, Beeson PM, Miller BL, et al. (2019). Treatment for Word Retrieval in Semantic and Logopenic Variants of Primary Progressive Aphasia: Immediate and Long-Term Outcomes. J. Speech Lang. Hear. Res, 62, 2723–2749. DOI: 10.1044/2018_JSLHR-L-18-0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2004). Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition, 92, 67–99. DOI: 10.1016/j.cognition.2003.10.011 [DOI] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8, 393–402. DOI: 10.1016/j.jcomdis.2012.06.004 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Oh S, & Ken L (2004). Deterioration of naming nouns versus verbs in primary progressive aphasia. Annals of Neurology, 55, 268–275. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, & Caramazza A (2002). Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. Journal of Cognitive Neuroscience, 14, 1099–1108. DOI: 10.1002/ana.10812 [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. (2004). Clinicopathological correlates in frontotemporal dementia. Annals of Neurology, 56, 399–406. [DOI] [PubMed] [Google Scholar]
- Hodges JR & Patterson K (1995). Is semantic memory consistently impaired early in the course of Alzheimer’s disease? Neuroanatomical and diagnostic implications. Neuropsychologia, 33, 441–459. DOI: 10.1002/ana.20203 [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, & Tyler LK (1994). Loss of semantic memory: Implications for the modularity of mind. Cognitive Neuropsychology, 11, 505–542. DOI: 10.1080/02643299408251984 [DOI] [Google Scholar]
- Hodges JR, Salmon DP, & Butters N (1992). Semantic memory impairment in Alzheimer s disease: failure of access or degraded knowledge? Neuropsychologia, 30, 301–314. DOI: 10.1016/0028-3932(92)90104-t [DOI] [PubMed] [Google Scholar]
- Howard D, & Patterson K (1992). The pyramids and palm trees test: A test of semantic access from words and pictures. Bury St. Edmunds, UK: Thames Valley Test Company. [Google Scholar]
- Indefrey P (2011). The spatial and temporal signatures of word production components: a critical update. Frontiers in Psychology, 2, 1–16. DOI: 10.3389/fpsyg.2011.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, & Levelt WJM (2004). The spatial and temporal signatures of word production components. Cognition, 92, 101–144. DOI: 10.1016/j.cognition.2002.06.001 [DOI] [PubMed] [Google Scholar]
- Jokel R, Kielar A, Anderson ND, Black SE, Rochon E, Graham S, et al. (2016). Behavioural and neuroimaging changes after naming therapy for semantic variant primary progressive aphasia. Neuropsychologia, 89, 191–216. DOI: 10.1016/j.neuropsychologia.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Jokel R, Rochon E, & Anderson ND (2010). Errorless learning of computer-generated words in a patient with semantic dementia. Neuropsychological Rehabilitation, 20, 16–41. DOI: 10.1080/09602010902879859 [DOI] [PubMed] [Google Scholar]
- Jokel R, Rochon E, & Leonard C (2006). Treating anomia in semantic dementia: Improvement, maintenance, or both? Neuropsychological Rehabilitation, 16(3), 241–256. DOI: 10.1080/09602010500176757 [DOI] [PubMed] [Google Scholar]
- Josephs KA, Dickson DW, Murray ME, Senjem ML, Parisi JE, Petersen RC, et al. (2013). Quantitative neurofibrillary tangle density and brain volumetric MRI analyses in Alzheimer’s disease presenting as logopenic progressive aphasia. Brain and Language, 127, 127–134. DOI: 10.1016/j.bandl.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, et al. (2006). Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain, 129, 1385–1398. DOI: 10.1093/brain/awl078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert S, Brambati SM, Ansado J, Barbeau EJ, Felician O, Didic M, et al. (2010). The cognitive and neural expression of semantic memory impairment in mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia, 48, 978–988. DOI: 10.1016/j.neuropsychologia.2009.11.019 [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (2001). Boston Naming Test (2nd edition). Philadelphia: Lippincott, Williams, & Wilkins. [Google Scholar]
- Kertesz A, Davidson W, & Fox H (1997). Frontal behavioral inventory: Diagnostic criteria for frontal lobe dementia. Canadian Journal of Neurological Sciences, 24, 29–36. DOI: 10.1017/s0317167100021053 [DOI] [PubMed] [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, & Hodges JR (2006). Clinical and pathological characterization of progressive aphasia. Annals of Neurology, 59, 156–165. DOI: 10.1002/ana.20700 [DOI] [PubMed] [Google Scholar]
- Krajenbrink T, Croot K, Taylor-Rubin C & Nickels L (2020). Treatment for spoken and written word retrieval in the semantic variant of primary progressive aphasia. Neuropsychol. Rehabil, 30, 915–947. DOI: 10.1080/09602011.2018.1518780 [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Cipolotti L, Manes F, & Patterson K (2010). Taking both sides: Do unilateral anterior temporal lobe lesions disrupt semantic memory. Brain, 133, 3243–3255. DOI: 10.1093/brain/awq264 [DOI] [PubMed] [Google Scholar]
- Leff AP, Schofield TM, Crinion JT, Seghier ML, Grogan A, Green DW, et al. (2009). The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain, 132, 3401–3410. DOI: 10.1093/brain/awp273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton CE, Britton AK, Hodges JR, Halliday GM, & Kril JJ (2016). Distinctive pathological mechanisms involved in primary progressive aphasias. Neurobiology of Aging, 38, 82–92. DOI: 10.1016/j.neurobiolaging.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Louwersheimer E, Keulen MA, Steenwijk MD, Wattjes MP, Jiskoot LC, Vrenken H, et al. (2016). Heterogeneous language profiles in patients with primary progressive aphasia due to Alzheimer’s disease. Journal of Alzheimer’s Disease, 51, 581–590. DOI: 10.3233/JAD-150812 [DOI] [PubMed] [Google Scholar]
- Lupyan G, & Thompson-Schill SL (2012). The evocative power of words: Activation of concepts by verbal and nonverbal means. Journal of Experimental Psychology: General, 141, 170–186. DOI: 10.1037/a0024904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden EB, Robinson RM, & Kendall DL (2017). Phonological treatment approaches for spoken word production in aphasia. Seminars in Speech and Language, 38, 62–74. DOI: 10.1055/s-0036-1597258 [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 7, 263–269. DOI: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM (1982). Slowly progressive aphasia without generalized dementia. Annals of Neurology, 11, 592–598. DOI: 10.1002/ana.410110607 [DOI] [PubMed] [Google Scholar]
- Mesulam M, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, et al. (2009). Neurology of anomia in the semantic variant of primary progressive aphasia. Brain, 132, 2553–2565. DOI: 10.1093/brain/awp138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH (2014). Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain, 137, 1176–1192. DOI: 10.1093/brain/awu024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, et al. (2013). Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain, 136, 601–618. DOI: 10.1093/brain/aws336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Thompson C, Rogalski E, & Weintraub S (2012). Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain, 135, 1537–1553. DOI: 10.1093/brain/aws080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Snider SF, Campbell RE, & Friedman RB (2015a). Phonological short-term memory in logopenic variant primary progressive aphasia and mild Alzheimer’s disease. Cortex, 71, 183–189. DOI: 10.1016/j.cortex.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Snider SF, Eckmann CB, & Friedman RB (2015b). Prophylactic treatments for anomia in the logopenic variant of primary progressive aphasia: Cross-language transfer. Aphasiology, 29, 1062–1081. DOI: 10.1080/02687038.2015.1028327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Snider SF, McGowan SA, Tippett DC, Hillis AE, & Friedman RB (2020). Grammatical ability predicts relative action naming impairment in primary progressive aphasia. Aphasiology, 34, 664–674. DOI: 10.1080/02687038.2020.1734527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Tippett DC, & Friedman RB (2018). Prophylaxis and remediation of anomia in the semantic and logopenic variants of primary progressive aphasia. Neuropsychological Rehabilitation, 28, 352–368. DOI: 10.1080/09602011.2016.1148619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Tippett DC, Turner RS & Friedman RB (2019). Long-Term maintenance of anomia treatment effects in primary progressive aphasia. Neuropsychological Rehabilitation, 29, 1439–1463. DOI: 10.1080/09602011.2018.1425146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio R, Boutet C, Valabregue R, Ferrieux S, Nogues M, Lehericy S, et al. (2016). The brain network of naming: A lesson from primary progressive aphasia. PLoS ONE 11(2): e0148707. DOI: 10.1371/journal.pone.0148707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreaud O, David D, Charnallet A, & Pellat J (2001). Are semantic errors actually semantic? Evidence from Alzheimer’s disease. Brain & Language, 77, 176–186. DOI: 10.1006/brln.2000.2427 [DOI] [PubMed] [Google Scholar]
- Morelli CA, Altmann LJP, Kendall D, Fischler I, & Heilman KM (2011). Effects of semantic elaboration and typicality on picture naming in Alzheimer’s disease. Journal of Communication Disorders, 44, 413–428. DOI: 10.1016/j.jcomdis.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, & Hodges JR (2000). A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Annals of Neurology, 47, 36–45. DOI: [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, … Benson DF (1998). Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology, 51, 1546–1554. DOI: 10.1212/wnl.51.6.1546 [DOI] [PubMed] [Google Scholar]
- Nebes RD, Brady CB, & Huff FJ (1989). Automatic and attentional mechanisms of semantic priming in Alzheimer’s disease. Journal of Clinical Experimental Neuropsychology, 11, 219–230. DOI: 10.1080/01688638908400884 [DOI] [PubMed] [Google Scholar]
- Nebes RD, Martin DC, & Horn LC (1984). Sparing of semantic memory in Alzheimer’s disease. Journal of Abnormal Psychology, 93, 321–330. DOI: 10.1037/0021-843X.93.3.321 [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, & Hodges JR (2003). Progressive non-fluent aphasia is associated with hypometabolism centered on the left anterior insula. Brain, 126, 2406–2418. DOI: 10.1093/brain/awg240 [DOI] [PubMed] [Google Scholar]
- Nickels L (2002). Therapy for naming disorders: Revisiting, revising, and reviewing. Aphasiology, 16, 935–979. DOI: 10.1080/02687030244000563 [DOI] [Google Scholar]
- Noonan KA, Pryer LR, Jones RW, Burns AS, & Lambon Ralph MA (2012). A direct comparison of errorless and errorful therapy for object name relearning in Alzheimer’s disease. Neuropsychological Rehabilitation, 22, 215–234. DOI: 10.1080/09602011.2012.655002 [DOI] [PubMed] [Google Scholar]
- Paek EJ, Murray LL & Newman SD (2021). Effects of concurrent action and object naming treatment on naming skills and functional brain activation patterns in primary progressive aphasia: An fMRI study with a case-series design. Brain Lang, 218, 104950. DOI: 10.1016/j.bandl.2021.104950 [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. (2008). Aβ Amyloid and Glucose Metabolism in Three Variants of Primary Progressive Aphasia. Annals of Neurology, 64, 388–401. DOI: 10.1002/ana.21451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J (2016). How to constrain and maintain a lexicon for the treatment of progressive semantic naming deficits: Principles of item selection for formal semantic therapy. Neuropsychological Rehabilitation, 26, 126–156. DOI: 10.1080/09602011.2014.1003947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J, Peelle JE, Antonucci SM, & Grossman M (2011). Anomia as a marker of distinct semantic memory impairments in Alzheimer’s disease and semantic dementia. Neuropsychology, 25, 413–426. DOI: 10.1037/a0022738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich JB, Park NW, Dopkins S, & Brandt J (2002). What do Alzheimer’s disease patients know about animals? It depends on task structure and presentation format. Journal of the International Neuropsychological Society, 8, 83–94. DOI: 10.1017/S1355617701020082 [DOI] [PubMed] [Google Scholar]
- Riello M, Faria AV, Ficek B, Webster K, Onyike CU, Desmond J, Tsapkini K (2018). The role of language severity and education in explaining performance on object and action naming in primary progressive aphasia. Frontiers in Aging Neuroscience, 10. doi:10.3389/fnagi.2018.00346. DOI: 10.3389/fnagi.2018.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, et al. (2011). Anatomy of language impairments in primary progressive aphasia. Journal of Neuroscience, 31, 3344–3350. DOI: 10.1523/JNEUROSCI.5544-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, & Friedman RB (2008). The underlying mechanisms of semantic memory loss in Alzheimer’s disease and semantic dementia. Neuropsychologia, 46, 12–21. DOI: 10.1016/j.neuropsychologia.2007.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Hocking J, Noppeney U, Mechelli A, Gorno-Tempini ML, Patterson K, et al. (2006). Anterior temporal cortex and semantic memory: Reconciling findings from neuropsychology and functional imaging. Cognitive Affective and Behavioral Neuroscience, 6, 201–213. DOI: 10.3758/cabn.6.3.201 [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, et al. (2010). Progressive logopenic/phonological aphasia: Erosion of the language network. Neuroimage, 49, 984–993. DOI: 10.1016/j.neuroimage.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncero C, Nikelski J, Probst S, Fernandez A, Thiel A, & Chertkow H (2020). The semantic storage loss score: An Algorithm for measuring an individual’s level of semantic storage loss due to temporal lobe damage in neurodegenerative disease. PLOS ONE, 15, e0235810. DOI: 10.1371/journal.pone.0235810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadi SA, Patterson K, Arnold RJ, Watson PC, & Nestor PJ (2012). Primary progressive aphasia: a tale of two syndromes and the rest. Neurology, 78, 1670–1677. DOI: 10.1212/WNL.0b013e3182574f79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadi SA, Patterson K, Nestor PJ (2014). Logopenic, mixed, or Alzheimer-related aphasia? Neurology, 82, 1–5. DOI: 10.1212/WNL.0000000000000271 [DOI] [PubMed] [Google Scholar]
- Sheppard SM, Goldberg EB, Sebastian R, Walker A, Meier EL, & Hillis AE (2022). Transcranial Direct Current Stimulation Paired With Verb Network Strengthening Treatment Improves Verb Naming in Primary Progressive Aphasia: A Case Series. American Journal of Speech-Language Pathology, 23. doi:10.1044/2022_AJSLP-21–00272. DOI: 10.1044/2022_AJSLP-21-00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-González A, Savage SA, & Caine D (2018). Successful short-term re-learning and generalization of concepts in semantic dementia. Neuropsychol. Rehabil, 28, 1095–1109. DOI: 10.1080/09602011.2016.1234399 [DOI] [PubMed] [Google Scholar]
- Taylor-Rubin C, Nickels L & Croot K (2022). Exploring the effects of verb and noun treatment on verb phrase production in primary progressive aphasia: A series of single case experimental design studies. Neuropsychol. Rehabil, 32, 1121–1163. DOI: 10.1080/09602011.2021.1879174 [DOI] [PubMed] [Google Scholar]
- Thompson CK, Lukic S, King MC, Mesulam MM, & Weintraub S (2012). Verb and noun deficits in stroke-induced and primary progressive aphasia: The Northwestern Naming Battery. Aphasiology, 26, 632–655. DOI: 10.1080/02687038.2012.676852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbury C, & Bub D (1997). Primary progressive aphasia: A review of 112 cases. Brain and Language, 60, 381–406. DOI: 10.1006/brln.1997.1840 [DOI] [PubMed] [Google Scholar]
- Wicklund MR, Duffy JR, Strand EA, Machulda MM, Whitwell JL, & Josephs KA (2014). Quantitative application of the primary progressive aphasia consensus criteria. Neurology, 82, 1119–1126. DOI: 10.1212/WNL.0000000000000261 [DOI] [PMC free article] [PubMed] [Google Scholar]


