Abstract
Elucidation of a signal transduction pathway essential to lipopolysaccharide (LPS)-induced macrophage activation has the capacity to provide new targets for the treatment of septic shock. In this regard, activation of the transcription factor NF-κB is commonly thought to be critical to LPS-stimulated macrophage inflammatory mediator production, although certain immunological, genetic, and molecular evidence suggests that other factors are involved. To address this issue, we hypothesized that the degree of LPS-induced NF-κB mobilization should correlate with the murine endotoxicity of the species of LPS used for in vitro study. Therefore, using d-galactosamine-sensitized mice, we assessed the lethal potencies of eight LPS preparations from Escherichia, Salmonella, Klebsiella, Bacteroides, Pseudomonas, Neisseria, and Rhodobacter species as well as that of the endotoxin substructure lipid X. The lethal potencies of these LPS preparations varied by >160-fold. Treatment of RAW 264.7 cells with the same LPS preparations induced levels of tumor necrosis factor alpha (TNF-α) and NO production that correlated with the LPS 50% lethal dose. The combined analysis of the levels of these two mediators produced in response to LPS in RAW cells was found to be a strong predictor of murine endotoxic lethality. Interestingly, while relatively nontoxic in mice, Rhodobacter capsulatus LPS stimulated RAW cell NF-κB-like DNA binding protein mobilization and TNF-α production to levels comparable to those of more toxic species of LPS but was unable to induce NO generation in RAW cells. These data indicate that neither NF-κB activation nor TNF-α production alone is a dependable predictor of LPS lethality. Additionally, cotreatment of RAW cells with the potent inflammatory mediator ADP had no effect on the ability of R. capsulatus LPS to stimulate NO production but significantly enhanced induction of NO production by the toxic species of LPS. In contrast, cotreatment of RAW cells or peritoneal macrophages with gamma interferon (IFN-γ) normalized the abilities of both toxic and nontoxic LPS preparations to induce NO production, suggesting that selected preparations of LPS may preferentially generate an IFN-γ-like signal that accounts for enhanced toxicity. In sum, the activation of NF-κB does not correspond to LPS lethality, thereby complicating models of macrophage activation that highlight NF-κB alone as a signal transduction factor necessary for LPS-mediated toxicity.
The failure of cytokine neutralization strategies to protect septic patients from death has heightened interest in the study of receptors that bind lipopolysaccharide (LPS) and LPS-induced macrophage signal transduction (6, 37). An essential LPS signaling pathway remains to be elucidated, however, as redundancy exists at multiple levels. For example, of the LPS receptors, surface expression of CD14 by macrophages confers the greatest sensitivity to LPS (38), and CD14-deficient mice are more resistant to septic shock (15); however, other LPS receptors with more clearly defined signaling capacity exist (23). Subsequent to receptor occupation, multiple signal transduction pathways are activated in LPS-stimulated macrophages. These pathways include the involvement of heterotrimeric and low-molecular-weight G proteins, phospholipases A, C, and D, nonreceptor tyrosine kinases, protein kinase C, and several members of the mitogen-activated protein kinase family leading to enhanced rates of inflammatory mediator transcription and translation (6, 33). In sum, no study to date has demonstrated an absolute requirement for any particular macrophage signal transduction component for LPS-mediated toxicity despite an intense focus on the issue.
Recently, several studies have suggested the existence of a signal transduction component which binds lipid A and is essential to LPS-induced macrophage activation (5, 18, 19). Whether this component is associated with CD14 on the cell surface or is expressed intracellularly remains to be determined. The approach of these previous studies was to use LPS preparations of various biological activities to compare the abilities of these preparations to generate macrophage inflammatory mediators in vitro to their abilities to bind LPS receptors or induce macrophage activation of the transcription factor NF-κB. One limitation of these studies, however, was that they could not control for the variability in biological activity of their LPS preparations due to factors such as purity, solubility, and aggregation state. This renders their comparison between in vitro parameters inherently less reliable, particularly with respect to the binding studies. Hence, careful attention to defining the toxicity of an LPS preparation may overcome such concerns by providing an internal control, thus potentially enabling the identification of signal transduction pathways that are induced in macrophages preferentially by toxic species of LPS.
Some data suggest that an important hallmark of LPS-induced macrophage signal transduction is the stimulation of a pathway(s) that ultimately leads to the activation of the transcription factor NF-κB (4, 5, 12); however, several lines of evidence to the contrary exist. For example, macrophages derived from an LPS-hyporesponsive strain of mice (C3H/HeJ) activate NF-κB without producing tumor necrosis factor alpha (TNF-α) or other LPS-induced inflammatory mediators (8). Additionally, NF-κB is not required for TNF-α transcription (13), and the primary mode of regulation of this cytokine is at a posttranscriptional level (14). To address this issue, we hypothesized that the degree of activation of NF-κB by LPS treatment of macrophages in vitro corresponds to the murine lethality of the LPS preparation. We therefore assessed the biological activity of eight preparations of LPS and lipid X and controlled for variation in biological activity due to preparation purity, solubility, and aggregation state by determining the murine 50% lethal dose (LD50) of the stock solutions used for in vitro experiments. Furthermore, we demonstrated the validity of using LPS-treated RAW 264.7 cells for these studies by showing that the production of TNF-α and NO by LPS treatment of this cell line predicts endotoxin lethality in mice, particularly when these mediators are analyzed together. Finally, we showed that mobilization of an NF-κB-like DNA binding protein in LPS-stimulated RAW cells did not correspond to LPS toxicity. Because exogenous gamma interferon (IFN-γ), but not ADP, synergized with the nontoxic Rhodobacter capsulatus LPS to induce RAW cell and peritoneal macrophage NO production, we suggest a model whereby toxic preparations of LPS may preferentially activate a signaling pathway leading to the production of a factor that exhibits an IFN-γ-like activity.
MATERIALS AND METHODS
Animals and cell culture.
Six-week-old male C57Bl/6 mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and were cared for according to University of Wisconsin School of Medicine and National Institutes of Health guidelines. To obtain resident peritoneal macrophages, mice were sacrificed by cervical dislocation and the abdominal skin was reflected. Hank’s buffered salt solution (HBSS) containing 10 U of heparin/ml was injected into the peritoneal cavity (5 ml/mouse), and the mice were shaken for 2 min. Peritoneal cells were collected in a syringe and washed once in HBSS-heparin at 4°C. Erythrocytes were lysed by a 6-min incubation at 4°C in hypotonic Gey’s solution (130 mM NH4Cl, 5 mM KCl, 2 mM Na2HPO4, 0.2 mM KH2PO4, 5 mM glucose, 25 μM phenol red, 0.2 mM MgCl2, 25 μM MgSO4, 0.1 mM CaCl2, and 1 mM NaHCO3). Two volumes of medium were added followed by centrifugation at 200 × g for 5 min. The cells were resuspended, plated, and maintained in RPMI 1640 (BioWhittaker, Walkersville, Md.) supplemented with 10% Cosmic calf serum (HyClone Laboratories, Inc., Logan, Utah), 2 mM l-glutamine (BioWhittaker), 2 mM sodium pyruvate, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 0.25 μg of amphotericin B/ml in a humidified environment at 37°C with 5% CO2. After 1 h of incubation, the adherent peritoneal cells were washed three times with medium and immediately used for experimentation.
Murine RAW 264.7 macrophages were obtained from the American Type Culture Collection (Rockville, Md.) and were maintained at low densities (<75% confluence) for less than 20 passages in RPMI 1640 supplemented with 5% Cosmic calf serum plus l-glutamine, sodium pyruvate, and the antibiotics at the concentrations stated above. RAW cells were plated at the indicated densities 18 to 24 h prior to initiation of the experiments.
Endotoxins.
Eight preparations of LPS were used in this study as well as lipid X, which is a nontoxic lipid A substructure that has LPS-antagonistic properties (27). Phenol-extracted preparations of LPS from Escherichia coli O111:B4, Salmonella minnesota, and Klebsiella pneumoniae were obtained from Sigma (St. Louis, Mo.). The other preparations were generous gifts and included LPS preparations from Neisseria meningitidis A1 (C.-M. Tsai, Center for Biologics Evaluation and Research, Food and Drug Administration, Bethesda, Md.), Bacteroides fragilis NCTC 9434 and Pseudomonas aeruginosa PAC 605 (U. Zähringer, Forschungsinstitut, Borstel, Germany), E. coli Re (K. Takayama, William S. Middleton Memorial VA Hospital, Madison, Wis.), R. capsulatus (H. Mayer, Max-Planck Institute, Freiburg, Germany), and synthetic lipid X (P. Stütz, Sandoz, Vienna, Austria). Additionally, synthetic R. capsulatus lipid A was obtained from William Christ (Eisai Research Institute, Andover, Mass.). Pyrogen-free water was used to make a 20 mM HEPES solution at pH 7.4. Each of the LPS preparations was individually resuspended in 20 mM HEPES at a concentration of 10 mg/ml, sonicated, and then diluted to a stock concentration of 0.3 mg/ml in 20 mM HEPES. The level of protein contamination of these stocks was determined by the micro-bicinchoninic acid (BCA) method. Finally, the stock solutions were sonicated again at the beginning of each experiment.
Mass spectrometry.
The metal (Mg, Ca, Na, and Fe) and phosphorus contents of the LPS preparations were determined by inductively coupled plasma-mass spectrometry, performed at the University of Wisconsin—Madison Extension Soil and Plant Analysis Laboratory. After background subtraction, the values reported reflect the metal and phosphorus concentrations (μM) associated with 300 μg of LPS/ml.
LD50 determination.
Murine sensitization to LPS was achieved by intraperitoneal injection of d-galactosamine (18 mg in 100 μl of phosphate-buffered saline [PBS]) as described previously (22). Eight twofold serial dilutions of LPS were made for each preparation in PBS. The target LPS concentrations of these dilutions ranged from 12.5 ng/ml to 1.6 μg/ml or from 125 ng/ml to 16 μg/ml, depending on the estimated lethality of the species used. Intravenous injections by the retro-orbital route were performed with a tuberculin syringe, a 25-gauge needle, and a 100-μl injection volume. Mortality was tabulated 48 h postinjection, and all of the mice surviving to this time point lived in good health for at least 1 week until sacrifice. Thirty mice were used per LPS preparation, and the LD50s were determined by the Reed-Muench method (28).
TNF-α ELISA.
RAW 264.7 cells were plated at 105 cells/well on a 24-well plate for 18 to 24 h and stimulated for 4 h by replacement of the old medium with 400 μl of supplemented RPMI 1640 containing 0 or 1 μg of LPS/ml. Culture supernatants were measured for TNF-α content by using a sandwich enzyme-linked immunosorbent assay (ELISA). Briefly, the primary capture antibody, a rat anti-murine TNF-α monoclonal antibody (clone MP6-XT22; Pharmingen, San Diego, Calif.), was added to a 96-well Pro Bind ELISA plate (Becton Dickinson, Lincoln Park, N.J.) at a concentration of 1 μg/ml in PBS and incubated overnight at 4°C. After being blocked with 1% bovine serum albumin, the culture supernatants were diluted in PBS, added to the ELISA plate, and incubated for at least 1 h at 37°C. A TNF-α standard curve was generated by using a recombinant murine standard (Genzyme, Cambridge, Mass.). Secondary antibodies (rabbit anti-murine TNF-α polyclonal antisera; Genzyme) and tertiary antibodies (goat anti-rabbit immunoglobulin G-horseradish peroxidase conjugate; Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) were plated at 1:1,000 and 1:5,000 dilutions, respectively. Nine 5-min washes with PBS plus 0.02% Tween 20 after the secondary and tertiary antibody incubations consistently resulted in background absorbance readings of less than 0.2 optical density units. Antibody detection involved the use of the TMB Microwell Peroxidase Substrate System (Kirkegaard & Perry, Gaithersburg, Md.) with 1 M H3PO4 as the stop reagent and 415 nm as the detection wavelength.
NO assay.
RAW 264.7 cells were plated at 105 cells/well (24-well plate) for 18 to 24 h prior to stimulation, whereas peritoneal macrophages were plated at 5 × 105 cells/well for 1 h prior to washing and treatment. Experiments were initiated by replacing the old medium with 400 μl of supplemented RPMI with or without LPS (1 μg/ml) and with or without IFN-γ (a generous gift of E. Balish, University of Wisconsin, Madison; 20 U/ml for experiments using RAW cells and 1 U/ml for experiments using peritoneal macrophages) followed by incubation at 37°C for 20 h. Nitrite, a stable metabolite of NO, was measured in culture supernatants with the Griess reagent, and concentrations were compared to a standard curve of sodium nitrite, as described previously (7).
Nuclear extract preparation and NF-κB electrophoretic mobility shift assay.
RAW cells were plated at 2.5 × 106 cells/plate onto 10-cm-diameter petri dishes and incubated for 18 to 24 h prior to the start of the experiment. LPS treatments were performed with 5 ml of fresh medium with or without 1 μg of LPS/ml at 37°C for 1 h. The cells were harvested by being scraped into PBS containing 2% serum and were washed twice with this buffer. Nuclear extracts were prepared as described previously (5). Briefly, cell pellets were resuspended in 400 μl of buffer A containing 10 mM Tris (pH 7.8), 5 mM MgCl2, 10 mM KCl, 0.3 mM EGTA, 0.3 M sucrose, 10 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol, 1 μg of aprotinin/ml, and 1 μg of leupeptin/ml. After 15 min of incubation at 4°C, these cells were lysed by adding Nonidet P-40 to a 0.5% final concentration and vortexing for 10 s. Nuclei were harvested by centrifugation at 7,200 × g for 10 s at 4°C. Pellets were then resuspended in 100 μl of buffer B containing 20 mM Tris (pH 7.8), 5 mM MgCl2, 320 mM KCl, 0.2 mM EGTA, 0.5 mM dithiothreitol, 1 μg of aprotinin/ml, and 1 μg of leupeptin/ml. Supernatants were collected and assayed for protein content by using a standard dye reagent (Bio-Rad, Richmond, Calif.) after centrifugation at 13,500 × g for 15 min at 4°C.
The NF-κB Binding Protein Detection System (Gibco BRL, Gaithersburg, Md.) was used according to the manufacturer’s instructions. Briefly, end labeling of a double-stranded oligonucleotide containing two consensus NF-κB binding elements (5′-GATCCAAGGGGACTTTCCATGGATCCAAGGGGACTTTCCATG) was performed with [γ-32P]ATP and polynucleotide kinase. Nuclear extracts (5 μg of nuclear protein) were incubated with labeled oligonucleotide (105 cpm) with or without a 100-fold excess of unlabeled oligonucleotide for 20 min at 25°C. The samples were separated on a 6% nondenaturing acrylamide gel and exposed to X-ray film for autoradiography.
GTPase activity.
Plasma membranes were prepared from RAW 264.7 cells by lysis and differential centrifugation (26, 35). GTPase activity was assayed for 5 min at 30°C in the presence of 2 μg of membrane protein, 3 μM ADP, 2 μM [γ-32P]GTP, 100 mM (NH4)2SO4, and 5 mM MgCl2, as described earlier (7, 26, 35).
Statistical analyses.
Statistical calculations were generally performed with the software package SPSS v. 6.0 (SPSS Inc, Chicago, Ill.). The production of LPS-induced mediators in vitro is expressed as the mean and standard error from six experiments unless otherwise indicated. Outliers were determined by using the maximum normal residual and the extreme Studentized deviate tests at the 1% level (32). Given the number of LPS species used in this study, Spearman’s correlation coefficients were used as a nonparametric test. Comparisons of various in vitro LPS parameters to LD50s were done for only six LPS species because several preparations (P. aeruginosa LPS, R. capsulatus LPS, and lipid X) were not lethal under the conditions initially tested (see Table 1). In contrast, mediator production induced by each of these three species was measurable in vitro; therefore, the comparisons between the levels of TNF-α and NO produced by RAW 264.7 cells in response to LPS included all nine of the LPS species tested. One-way analysis of variance and paired Student’s t tests were used to determine significant differences in the mediator levels between the treated groups and unstimulated controls. Linear regression was used to generate models of the relationship between TNF-α and NO production by LPS-treated macrophages.
TABLE 1.
Elemental analysis of the LPS preparationsa
| LPS source | Concn (μM)
|
||||
|---|---|---|---|---|---|
| Phosphorus | Calcium | Magnesium | Sodium | Iron | |
| S. minnesota | 410 | 230 | 180 | 50 | 0.4 |
| N. meningitidis A1 | 590 | <10 | <10 | 530 | 0.5 |
| E. coli O111:B4 | 400 | 170 | 260 | 60 | 0.6 |
| K. pneumoniae | 70 | 500 | 320 | 640 | 0.7 |
| B. fragilis NCTC 9434 | 200 | 210 | 60 | 3,100 | 10 |
| E. coli Re | 100 | 30 | 10 | 3,600 | 3.4 |
| P. aeruginosa PAC 605 | 300 | 180 | 20 | 10 | 10 |
| R. capsulatus | 150 | 170 | 20 | 2,400 | 0.2 |
| Lipid X | 360 | <10 | <10 | 680 | 0.4 |
The LPS preparations were analyzed for total phosphorus, calcium, magnesium, sodium, and iron contents by mass spectrometry. Data are concentrations of these elements in a 300-μg/ml solution of LPS. Additional characterization of these LPS stocks showed that the protein contents were below the level of detection by the micro-BCA method.
RESULTS
Characterization of the LPS preparations.
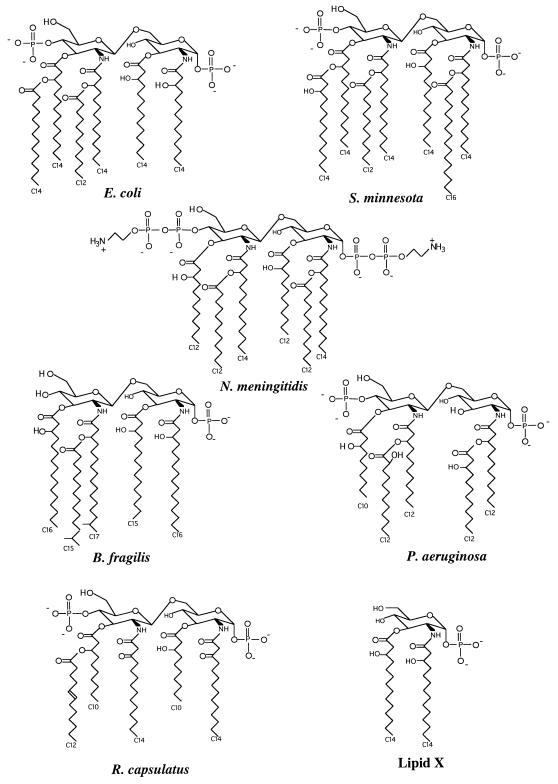
We collected the multiple preparations of LPS as lyophilized samples and resuspended them in the same buffer (20 mM HEPES, pH 7.4) with vigorous sonication in order to normalize the biochemical and immunological properties of the vehicle solution. The bioactivity of an LPS preparation is in part due to the chemical structure of its corresponding lipid A molecule, the endotoxic center of LPS (29); hence, the lipid A structures of many of the LPS preparations used in this study are displayed in Fig. 1 for comparison. Because LPS bioactivity can also vary according to purity, solubility, and aggregation state, we set out to characterize some of these parameters before proceeding with the biological studies. For example, the protein content of the individual LPS preparations was determined and was found to be below the detection threshold of the micro-BCA assay. Additionally, because phosphorus and divalent cation contents are thought to influence supramolecular structure and possibly the aggregation state of LPS (17, 30, 34), we quantified these elements in the LPS preparations using mass spectrometry with total sodium and iron contents as controls (Table 1). Despite large variations in ion content, the levels of these ions did not correlate with the biological activity of the LPS preparations with the exception of sodium. In this case, sodium content inversely correlated with the LPS LD50 (Tables 1 and 2) (Rs = 0.94, P = 0.01), although this likely reflects the coincidental contribution of the ion exchange column buffers used in the purification of some of the less toxic LPS samples. Although this analysis does not account for all potential impurities or differences in solubility, etc., we used the same suspension of each LPS preparation for all of the in vivo and in vitro experiments described below, thus eliminating procedural differences that would affect the relative contributions of these aspects which affect bioactivity.
FIG. 1.
Species differences in lipid A structure. Previously determined structures are presented for the lipid A molecules of E. coli, S. minnesota, N. meningitidis, B. fragilis, P. aeruginosa, and R. capsulatus (40). Additionally, the structure for lipid X is shown (40). Structural features that have been associated with a reduction in relative biological activity include the lack of a disaccharide or 4′ phosphate, the presence of five or fewer fatty acids, the occurrence of branched-chain or unsaturated fatty acids, and fatty acid chain lengths of fewer than 12 carbons (25).
TABLE 2.
LD50s for various LPS preparations in d-galactosamine-sensitized micea
| LPS source | LD50 (ng) |
|---|---|
| S. minnesota | 10 |
| N. meningitidis A1 | 16 |
| E. coli O111:B4 | 35 |
| K. pneumoniae | 51 |
| B. fragilis NCTC 9434 | 140 |
| E. coli Re | 1,000 |
| P. aeruginosa PAC 605 | >160 |
| R. capsulatus | >1,600 |
| Lipid X | No toxicity observed |
Eight serial twofold dilutions were made for each of the nine LPS preparations in PBS as described in Materials and Methods. Six-week-old male C57Bl/6 mice were given an intraperitoneal injection containing d-galactosamine (18 mg in 100 μl of PBS) followed by ether anesthesia and an intravenous injection by the retro-orbital route of 100 μl of PBS alone or containing various concentrations of LPS. Mortality at 48 h postinjection was tabulated, and the LD50 for each LPS was calculated by the Reed-Muench method. Estimations of LD50 are presented as greater than the highest dose if this dose was lethal to less than 50% of the mice.
LPS toxicity in mice.
To document the biological activities of these LPS preparations, we evaluated the endotoxicities of eight LPS species and lipid X in terms of their individual LD50s in d-galactosamine-sensitized mice. This analysis used 30 mice at 6 weeks of age per LPS species, all of which were treated on the same day. As shown in Table 2, the lethal potencies of these preparations differed by over 160-fold. Under these conditions LPS preparations from smooth Enterobacteriaceae species and N. meningitidis were more toxic than LPS isolated from either B. fragilis or E. coli Re. The LPS preparations from P. aeruginosa and R. capsulatus, as well as that of lipid X, were not lethal in the dilution range tested. In subsequent experiments, the same P. aeruginosa and R. capsulatus LPS preparations proved to be toxic but only at higher doses than those originally tested (i.e., ≥320 and ≥1,600 ng, respectively). These data were not included in the LD50 calculations for the preparations of P. aeruginosa and R. capsulatus LPS because they were generated on a different day with a different batch of mice. Lipid X, in subsequent experiments, was not toxic even at dosages of 25 μg/animal.
LPS-induced RAW cell production of TNF-α and NO correlates with the LD50 in mice.
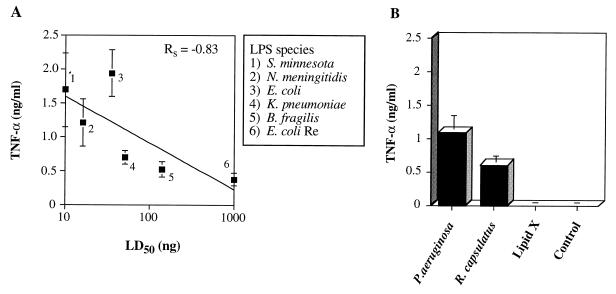
To determine whether RAW cells were an appropriate cell line in which to test our hypothesis that LPS-stimulated NF-κB activation correlates with endotoxicity, we first wanted to demonstrate that the production of NF-κB-regulated inflammatory mediators by LPS-treated RAW cells correlated with the LPS LD50. LPS-stimulated macrophage production of TNF-α is partially regulated by NF-κB (13), and this cytokine is thought to be central to the pathophysiology of endotoxic shock (6). Because this cytokine is produced rapidly upon LPS stimulation of macrophages (11), we measured TNF-α production at 4 h in an attempt to maximize differences between the levels produced by toxic and nontoxic species. Using lipid X and the eight LPS preparations in this study, the production of TNF-α by RAW 264.7 macrophages treated with LPS ranged from 1.9 ng/ml for E. coli LPS to background levels for lipid X (Fig. 2). These levels of TNF-α were found to generally correlate with LPS LD50s (Fig. 2A) (Rs = −0.83, P = 0.04). However, and quite surprisingly, one of the least toxic LPS preparations, i.e., R. capsulatus, induced RAW cells to produce levels of TNF-α significantly above that of the unstimulated control or that induced by lipid X (Fig. 2B) (P < 0.01 and P = 0.01, respectively). In fact, the amount of TNF-α produced by RAW cells treated with the relatively nontoxic R. capsulatus LPS for 4 h did not differ from the levels induced by the toxic K. pneumoniae LPS (P > 0.50) and was only slightly different from that generated by the highly toxic N. meningitidis LPS (P = 0.10). These results demonstrate that the processes involved in LPS-stimulated macrophage production of TNF-α do not completely differentiate between toxic and nontoxic LPS species, suggesting that whereas TNF-α may be essential for sepsis pathophysiology, its generation does not completely account for toxicity.
FIG. 2.
Correlation of LPS-induced RAW 264.7 macrophage TNF-α production to endotoxicity. RAW 264.7 macrophages were plated on a 24-well plate at a density of 105 per well in 1 ml of supplemented RPMI 1640 medium and maintained at 37°C and 5% CO2. The medium was replaced 20 h later with 400 μl of fresh RPMI 1640 medium alone or containing LPS at a final concentration of 1 μg/ml, and the cells were then incubated for 4 h at 37°C. Culture supernatants were diluted 1:10 with PBS and analyzed in triplicate for TNF-α content by ELISA (as detailed in Materials and Methods) using a recombinant murine TNF-α standard curve and background subtraction. The data are means and standard errors from six cultures for each LPS preparation, with the exception of P. aeruginosa PAC 605 LPS (n = 5). (A) TNF-α levels plotted against the LD50 data presented in Table 1. (B) TNF-α levels induced by the LPS preparations that were not toxic in the range tested.
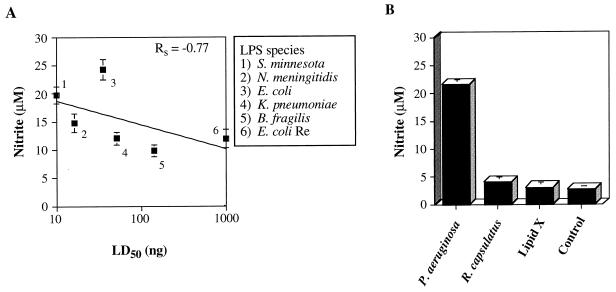
We therefore wanted to test the correlation of the in vitro production of another mediator to endotoxicity. Interleukin 6 (IL-6) was a potential candidate because the levels of this cytokine in human sera from septic patients are more predictive of prognosis than are levels of TNF-α (3). However, in a murine system, we have previously shown that IL-6 production does not correspond to lethality and is regulated by a different mechanism than are TNF-α, IL-1α, and NO (7, 26). In contrast, the role of NO in the pathogenesis of septic shock has been recently supported by the observation that humans produce NO during sepsis (31) and by data showing that the disruption of the inducible nitric oxide synthase (iNOS) gene renders mice less responsive to LPS in certain models of endotoxic shock (24). Despite a requirement of NF-κB activation for iNOS transcription (39), the kinetics of NO production are very different from those of TNF-α. Whereas TNF-α only requires 5 min of LPS stimulation to achieve maximal induction (11), RAW cells require at least 9 h of LPS treatment before maximal induction of NO is obtainable (7). For these reasons, we measured LPS-stimulated RAW cell NO production at 20 h to determine whether an inflammatory mediator produced later in the activation cascade would also correlate with LPS toxicity. We observed that most of the LPS preparations used in this study induced RAW 264.7 macrophages to produce nitrite, a stable metabolite of NO, when measured at 20 h. These NO levels correlated with LPS toxicity at less than the 0.1 level (Fig. 3A) (Rs = −0.77, P = 0.07). Additionally, R. capsulatus LPS did not induce RAW cells to generate levels of nitrite above those induced by the unstimulated control (Fig. 3B) (P = 0.21), in contrast to its ability to stimulate TNF-α production at 4 h (Fig. 2B). These results suggest that the measurement of LPS-stimulated NO production is more selective for toxic species of LPS than is the assessment of TNF-α production.
FIG. 3.
LPS-induced RAW 264.7 cell nitrite production shows some correlation to endotoxin lethality in mice. RAW 264.7 cells were plated and incubated prior to LPS stimulation according to the conditions specified in the legend for Fig. 2. Each of the endotoxins were used to stimulate RAW macrophages for 20 h and were employed at a concentration of 1 μg/ml in 400 μl of supplemented RPMI 1640 medium. Measurement of nitrite levels in the culture supernatants was performed in triplicate with the Griess reagent, as discussed in Materials and Methods. The data are means and standard errors from six cultures treated with each LPS preparation, with the exception of P. aeruginosa PAC 605 LPS (n = 5), and are presented in the same format as in Fig. 2.
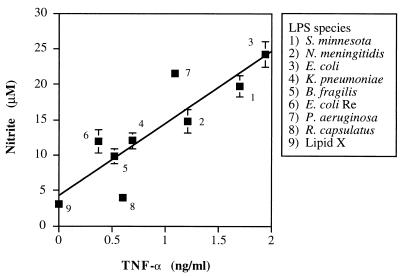
As evidenced by the failure of single anticytokine therapies to affect the outcome of septic shock (6), it is unlikely that the production of one mediator can account for all of the sepsis pathophysiology. However, the assessment of two or more mediators may have more predictive utility, particularly if their modes of regulation are distinct. We therefore compared the LPS-enhanced levels of TNF-α at 4 h to the amount of nitrite generated at 20 h in parallel RAW cell cultures. As shown in Fig. 4, a strong positive correlation (Rs = 0.88, P < 0.01, n = 9) was observed between the amount of TNF-α at 4 h and the amount of nitrite at 20 h in these LPS-treated macrophage supernatants, especially with regard to the toxic LPS preparations. Linear regression analysis yielded a highly significant positive slope (10 ± 2.2, P < 0.01) with an r2 residual value of 0.76. These data suggest that consideration of LPS-induced production of both TNF-α and NO together may be the most predictive indicator of endotoxin lethality in mice.
FIG. 4.
LPS-stimulated TNF-α production by RAW cells after 4 h of treatment largely predicts the levels of NO found at 20 h. TNF-α levels presented in Fig. 2 are shown plotted against the nitrite data from Fig. 3. Regression analyses of this plot yielded an Rs value of 0.88 (P < 0.01), a positive slope (10 ± 2.2), and an r2 residual value of 0.76.
Mobilization of NF-κB is not preferentially induced by toxic species of LPS.
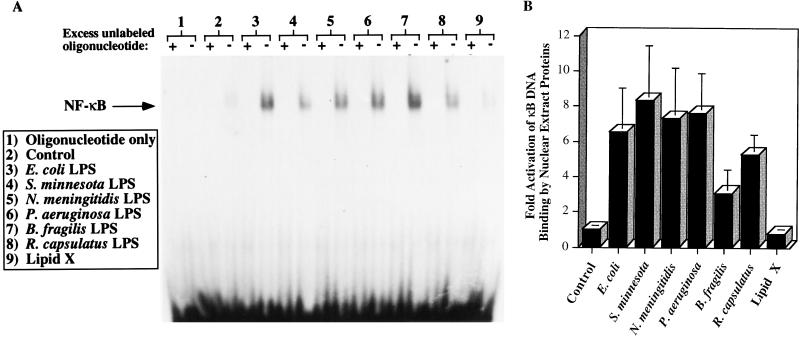
Because LPS-stimulated production of both TNF-α and NO correlates with the LD50 and is regulated in part by the action of NF-κB (13, 39), LPS-stimulated RAW cells are likely to be an appropriate cell line in which to test whether the toxicity of an LPS depends on the ability of the LPS preparation to activate this transcription factor. We therefore measured the capacity of nuclear extract proteins to bind to an oligonucleotide probe consisting of two tandemly repeated copies of the NF-κB consensus binding element. After a 1-h LPS stimulation of RAW cells, the nuclear extracts from all of the LPS-treated cultures contained increased levels of NF-κB-like DNA binding proteins relative to control samples (Fig. 5A). Densitometric quantification of data from at least three experiments is represented in Fig. 5B. Binding of the probe by these proteins can be prevented by adding a 100-fold excess of unlabeled oligonucleotide, which eliminated labeling of each nuclear extract, thus suggesting saturability. Lipid X was the only preparation that did not appear to mobilize the NF-κB-like DNA binding protein(s), consistent with its inability to stimulate RAW cell TNF-α and NO production. Of note, R. capsulatus LPS mobilizes this NF-κB-like DNA binding protein as efficiently as the highly toxic S. minnesota LPS, suggesting that this parameter alone is not an effective predictor of LPS toxicity.
FIG. 5.
R. capsulatus LPS and other LPS preparations except lipid X stimulate nuclear translocation of an NF-κB-like DNA binding protein in RAW 264.7 cells. RAW 264.7 cells were plated on 10-cm-diameter dishes at a density of 2.5 × 106/dish 24 h prior to treatment. The conditioned medium was removed and replaced with 5 ml of supplemented RPMI 1640 with and without the indicated endotoxins at a concentration of 1 μg/ml. After 1 h of incubation at 37°C, the cells were harvested and nuclear extracts were prepared as described in Materials and Methods. Nuclear extract protein (5 μg) was incubated with a labeled oligonucleotide containing two tandemly repeated consensus binding elements for NF-κB in the presence and absence of a 100-fold excess of unlabeled oligonucleotide. The samples were electrophoresed on a 6% nondenaturing polyacrylamide gel and visualized by autoradiography. (A) A representative experiment is shown. The labeling intensity was quantified by densitometry. (B) Data from panel A represented as the fold stimulation of LPS-induced RAW cell nuclear mobilization of an NF-κB-like DNA binding protein relative to that induced by the control medium. The data are means and standard errors from three cultures treated with LPS, with the exception of that for E. coli LPS (n = 4).
R. capsulatus LPS synergizes with IFN-γ, but not with ADP, to induce NO production.
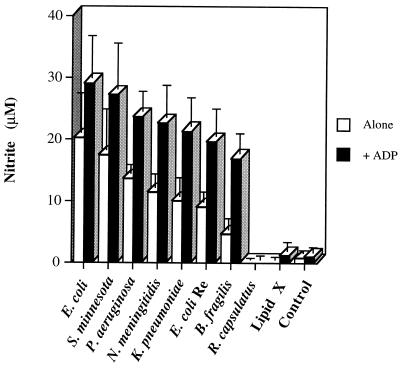
The inability of R. capsulatus LPS to induce RAW cell NO generation, despite its capacity to stimulate RAW cell NF-κB-like DNA binding protein mobilization and TNF-α production, suggests that R. capsulatus LPS may be able to synergize with secondary inflammatory mediators to induce macrophage NO production. Extracellular adenine nucleotides (e.g., ATP and ADP) are known to act as immune cell mediators, and they are present at inflammatory sites due to cell lysis, platelet aggregation, and release through channels (1, 6, 9). We have previously characterized an LPS-stimulable adenine nucleotide-dependent GTPase activity in RAW cell membranes that appears to be a purinergic receptor-linked G-protein-like activity and that has predicted the ability of various adenine nucleotides to either enhance or inhibit LPS-stimulated macrophage NO production (7, 35). This assay also identified 2-methyl-thio-ATP as a purinergic receptor modulator, and this derivative was subsequently shown to prevent LPS-induced TNF-α and IL-1α production as well as endotoxic death in mice (26). Whereas R. capsulatus LPS alone does not stimulate this GTPase, it does synergize with 3 μM ADP to induce a level of RAW membrane GTPase activity that is identical to that of the E. coli LPS control (data not shown). These data suggested that an adenine nucleotide-induced macrophage signal could potentially synergize with R. capsulatus LPS-stimulated NF-κB activation to generate RAW cell NO production. As shown in Fig. 6, exogenous ADP does synergize with most of the LPS species examined in terms of RAW macrophage nitrite induction. However, ADP treatment does not enhance the ability of R. capsulatus LPS or lipid X to generate NO (Fig. 6), relative to the ADP-only control, suggesting that the signals induced by extracellular adenine nucleotides cannot potentiate the activity of nontoxic species of LPS.
FIG. 6.
ADP cotreatment enhances RAW cell NO production induced by toxic LPS species but not by R. capsulatus LPS. RAW cells were plated and treated according to the conditions described in the legend for Fig. 3. The LPS species were used at a concentration of 1 μg/ml alone or in the presence of 100 μM ADP buffered in 20 mM HEPES, pH 7.4. Data are means and standard errors of nitrite measurements from three separate experiments.
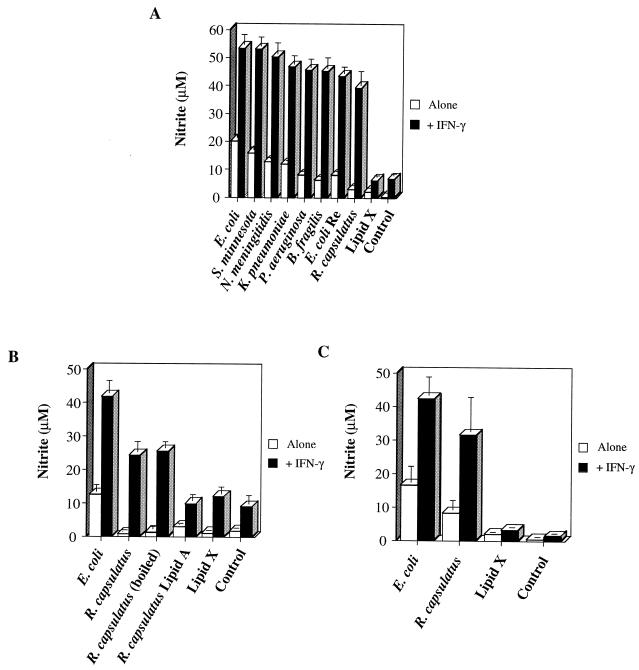
Besides adenine nucleotides, IFN-γ is another potent activator of macrophages that is known to synergize with E. coli LPS to induce NO production (2). Therefore, we evaluated the capacity of exogenous IFN-γ to affect stimulation of RAW cell NO production by the various LPS species. Treatment of RAW cells with each of the toxic LPS preparations in the presence of IFN-γ resulted in the synergistic production of nitrite when measured at 20 h (Fig. 7A). Most notably, IFN-γ cotreatment caused a 14- ± 2-fold enhancement of R. capsulatus LPS-induced nitrite production, compared with the 3- to 8-fold (± 1-fold) range of enhancement of nitrite production induced by the other LPS species. In fact, in the presence of IFN-γ, the level of nitrite production induced by R. capsulatus LPS did not differ from the respective amounts generated by the toxic preparations (P = 0.19). By contrast, the macrophage-stimulatory capacity of R. capsulatus LPS differed greatly from that of the monosaccharide lipid X (P < 0.01). Because this result was somewhat unexpected, several control experiments were subsequently performed. First, it was determined that the stimulatory capacity of this R. capsulatus LPS preparation was probably not due to protein contamination because protein was not detected by the micro-BCA method in this stock and boiling this LPS preparation for 10 min did not diminish its ability to induce RAW cells to make NO in the presence of IFN-γ (Fig. 7B). By contrast, synthetic R. capsulatus lipid A was unable to generate RAW cell NO production even in the presence of this cytokine (Fig. 7B), suggesting that the stimulatory capacity of R. capsulatus LPS requires its core polysaccharides. Moreover, this preparation of R. capsulatus LPS is capable of functional inhibition to a degree similar to that of a known inhibitor, lipid X. When 0.1 μg of E. coli LPS/ml was used to induce RAW cell NO production, the addition of either R. capsulatus LPS or lipid X at a 10:1 weight ratio facilitated a greater than 40% inhibition of nitrite generation. Finally, the synergism between IFN-γ and R. capsulatus LPS but not lipid X is also observed for the production of nitrite by primary macrophages in adherent murine peritoneal cell populations (Fig. 7C), suggesting that the ability of IFN-γ to potentiate nontoxic LPS species is not a phenomenon observed only in cell lines. In sum, IFN-γ cotreatment normalizes the ability of toxic and nontoxic LPS preparations to stimulate macrophage NO production.
FIG. 7.
IFN-γ cotreatment allows R. capsulatus LPS to induce nitrite production by RAW cells and peritoneal macrophages. (A and B) RAW cells were plated and treated according to the conditions described in the legend for Fig. 3. The LPS species were used at a concentration of 1 μg/ml alone or in the presence of 20 U of IFN-γ/ml. Where indicated, an aliquot of the R. capsulatus LPS was removed from its stock solution and boiled for 10 min prior to use for stimulating RAW cells. (C) peritoneal cells were plated in 24-well plates at a density of 5 × 105/well and were incubated for 1 h at 37°C. After three washes with medium, these cells were treated with the indicated LPS species (1 μg/ml) with or without 1 U of IFN-γ/ml for 20 h at 37°C. Data are means and standard errors of nitrite measurements from six (panel A) or three (panels B and C) separate experiments.
DISCUSSION
The purpose of this study was to determine whether LPS-induced mobilization of the transcription factor NF-κB in macrophages corresponds to LPS toxicity in mice. Our design was to identify an appropriate cell line for these studies while also providing an internal control to eliminate procedural differences that could allow factors such as LPS purity, solubility, and aggregation state to have a dynamic influence on LPS bioactivity. We demonstrate that despite an expected correlation between LPS-stimulated RAW cell production of TNF-α and LPS toxicity (Fig. 2), measurements of the in vitro production of this cytokine alone do not completely predict murine lethality. Specifically, the nontoxic preparation of R. capsulatus LPS induced levels of RAW cell TNF-α production that were identical to those generated by K. pneumoniae LPS, a preparation at least 30-fold more toxic. Future testing of the serum TNF-α levels induced by R. capsulatus LPS in mice might strengthen this assertion; however, similar observations have been made for humans, i.e., the TNF-α levels in the sera of septic patients are not predictive of mortality (3). Thus, predictions of LPS toxicity that are based on in vitro cytokine measurements need to include more than just TNF-α. In support of this idea, the production of NO by the various LPS preparations was shown to be correlated with LD50 (Fig. 3) and to be strongly correlated with LPS-induced TNF-α production (Fig. 4). The mechanisms by which LPS induces the production of these two mediators are at least partially independent because exogenous TNF-α alone is unable to stimulate RAW cell NO generation (unpublished data) and the TNF-α production induced by R. capsulatus LPS treatment of RAW cells failed to stimulate subsequent NO generation (Fig. 2 and 3). Hence, the combined assessment of LPS-induced RAW cell production of TNF-α and NO is the most predictive in vitro indicator of LPS lethality in d-galactosamine-sensitized mice. Moreover, RAW cells are an appropriate cell line for comparing LPS-induced signal transduction events to the endotoxicity of the LPS preparation.
Although the nuclear mobilization of NF-κB is rapidly stimulated by LPS treatment of macrophages and provides a useful assay to determine whether transfected LPS receptors are functional, the data in this study show that activation of NF-κB is not likely to be the most important hallmark of LPS signal transduction. Specifically, R. capsulatus LPS induced a level of NF-κB-like DNA binding protein mobilization similar to that generated by S. minnesota LPS (Fig. 5), despite a greater than 160-fold difference in the murine lethalities of these two preparations (Table 2). This assertion is supported by the fact that multiple immunological and environmental signals that do not mimic septic shock are able to activate NF-κB in macrophages and other cell types (20). Additionally, macrophages from an LPS-hyporesponsive strain of mice (C3H/HeJ) can mobilize NF-κB in response to LPS treatment and yet do not produce TNF-α or other inflammatory mediators in response to LPS treatment (8). One possibility that has been suggested is that activation of NF-κB needs to occur for several hours in order to acquire LPS-like effects (36). However, the data in Fig. 5 are consistent with the observations of others who found that R. sphaeroides LPS can induce NF-κB nuclear localization and DNA binding capacity for at least 8 h of treatment, which is a period sufficient for iNOS mRNA accumulation in macrophages stimulated by E. coli LPS (21). Collectively, these observations demonstrate that LPS-induced nuclear mobilization of NF-κB in macrophages is not sufficient for the production of all LPS-stimulated inflammatory mediators and does not correspond to LPS toxicity.
A separate signal transduction end point and/or pathway that is activated preferentially by toxic species of LPS must, therefore, exist. The ability of R. capsulatus LPS to induce NF-κB-like DNA binding protein mobilization and TNF-α production while failing to induce nitrite production suggests that iNOS transcription requires a second inflammatory signal that is not generated by nontoxic species of LPS. Extracellular adenine nucleotides were initially hypothesized to provide this signal because R. capsulatus LPS stimulated an adenine nucleotide-dependent G-protein-like activity in RAW cell membranes (data not shown). However, exogenous ADP only synergized with the toxic LPS preparations to induce RAW cell production of NO (Fig. 6B). Because extracellular adenine nucleotides have been shown to have a dramatic impact on LPS-stimulated macrophage TNF-α, IL-1α, and NO production (7, 26), these data suggest that the ability of extracellular adenine nucleotides to regulate LPS-induced macrophage production of NO may potentially be due to control at a posttranscriptional level.
In contrast, IFN-γ is known to regulate iNOS expression at the transcriptional level (39), and when added exogenously, this cytokine was able to synergize with R. capsulatus LPS to induce RAW cell production of NO (Fig. 7A). The extent of this synergy was comparable to that facilitated by the toxic LPS preparations. Control experiments showed that this phenomenon was not likely to be due to protein contamination of the R. capsulatus LPS preparation and that synergy also occurred when peritoneal macrophages were treated similarly (Fig. 7B and C). One possibility that these data suggest is that R. capsulatus LPS is unable to induce macrophage iNOS expression because it cannot stimulate macrophages to generate an autocrine factor that exhibits IFN-γ-like activity. IFN-γ does have an important role in LPS lethality; however, macrophages are thought to be incapable of producing this cytokine (16). Interestingly, two groups have shown that LPS-induced macrophage production of IFN-β is necessary and sufficient for NO generation (10, 41); i.e., LPS alone can induce macrophage NO production because of LPS-stimulated generation of endogenous IFN-β. Whether LPS-induced macrophage production of IFN-β correlates with endotoxicity remains to be determined, however.
In conclusion, this study has shown the importance of determining the lethality of an LPS preparation to provide an internal control for variability in LPS purity, solubility, and aggregation state. Specifically, this approach has allowed us to demonstrate that activation of NF-κB and the production of TNF-α by LPS treatment of RAW cells can be induced by nontoxic LPS preparations. These data suggest that extreme reductionist models of LPS-induced macrophage signal transduction or septic shock pathogenesis are incomplete. However, the continued use of multiple species of LPS with various biological activities should lead to the identification of other components essential to LPS-stimulated signal transduction and mediator production by macrophages. Finally, because the combined assessment of the production of two or more inflammatory mediators has provided the best models for predicting LPS toxicity in mice (Fig. 4) and humans (3), it is likely that the most effective therapeutics for septic shock will prove to be those which inhibit multiple LPS-induced end points.
ACKNOWLEDGMENTS
This project has been funded by Paul Bertics via an award from Gensia, Inc., a Shaw Scholar award, and NIH grants CA47881 and GM53271 and by Richard Proctor via gifts from Gensia, Inc. and the Medical School, Department of Medicine, and Graduate School of the University of Wisconsin. Loren Denlinger and Kristen Garis have been supported by University of Wisconsin Molecular Biosciences Training Grant T32-GM07215 (NIH). Julie Sommer was supported by the University of Wisconsin Biotechnology Training Program (NIH grant no. T32-GM08349).
We thank William Weidanz, Ralph Albrecht, Donna Paulnock, and Arnold Ruoho for their useful discussions and suggestions. We appreciate the technical support and comments provided by Greg Wiepz, Philip Fisette, and Dorothy Brar.
REFERENCES
- 1.Al-Awqati Q. Regulation of ion channels by ABC transporters that secrete ATP. Science. 1995;269:805–806. doi: 10.1126/science.7543697. [DOI] [PubMed] [Google Scholar]
- 2.Alley E W, Murphy W J, Russell S W. A classical enhancer element responsive to both lipopolysaccharide and interferon-gamma augments induction of the iNOS gene in mouse macrophages. Gene. 1995;158:247–251. doi: 10.1016/0378-1119(94)00892-v. [DOI] [PubMed] [Google Scholar]
- 3.Casey L C, Balk R A, Bone R C. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Delude R L, Fenton M J, Savedra R, Jr, Perera P-Y, Vogel S N, Thieringer R, Golenbock D T. CD14-mediated translocation of nuclear factor-kB induced by lipopolysaccharide does not require tyrosine kinase activity. J Biol Chem. 1994;269:22253–22260. [PubMed] [Google Scholar]
- 5.Delude R L, Savedra R, Jr, Zhao H, Thieringer R, Yamamoto S, Fenton M J, Golenbock D T. CD14 enhances cellular responses to endotoxin without imparting ligand-specific recognition. Proc Natl Acad Sci USA. 1995;92:9288–9292. doi: 10.1073/pnas.92.20.9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denlinger L C, Fisette P L, Garis K A, Daugherty S K, Bertics P J, Proctor R A. Adenine nucleotides prevent endotoxicity and can influence LPS signal transduction. In: Morrison D C, Ryan J L, editors. Novel therapeutic strategies in the treatment of sepsis. New York, N.Y: Marcel Dekker, Inc.; 1996. pp. 227–252. [Google Scholar]
- 7.Denlinger L C, Fisette P L, Garis K A, Kwon G, Vazquez-Torres A, Simon A D, Nguyen B, Proctor R A, Bertics P J, Corbett J A. Regulation of inducible nitric oxide synthase expression by macrophage purinoreceptors and calcium. J Biol Chem. 1996;271:337–342. doi: 10.1074/jbc.271.1.337. [DOI] [PubMed] [Google Scholar]
- 8.Ding A, Hwang S, Lander H M, Xie Q W. Macrophages derived from C3H/HeJ (Lpsd) mice respond to bacterial lipopolysaccharide by activating NF-kappa B. J Leukocyte Biol. 1995;57:174–179. doi: 10.1002/jlb.57.1.174. [DOI] [PubMed] [Google Scholar]
- 9.Dubyak G R, El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 10.Fujihara M, Ito N, Pace J L, Watanabe Y, Russell S W, Suzuki T. Role of endogenous interferon-b in lipopolysaccharide-triggered activation of the inducible nitric oxide synthase gene in a mouse macrophage cell line, J774. J Biol Chem. 1994;269:12773–12778. [PubMed] [Google Scholar]
- 11.Gallay P, Jongeneel C V, Barras C, Burnier M, Baumgartner J D, Glauser M P, Heumann D. Short time exposure to lipopolysaccharide is sufficient to activate human monocytes. J Immunol. 1993;150:5086–5093. [PubMed] [Google Scholar]
- 12.Gegner J A, Ulevitch R J, Tobias P S. Lipopolysaccharide (LPS) signal transduction and clearance. Dual roles for LPS binding protein and membrane CD14. J Biol Chem. 1995;270:5320–5325. doi: 10.1074/jbc.270.10.5320. [DOI] [PubMed] [Google Scholar]
- 13.Goldfeld A E, Doyle C, Maniatis T. Human tumor necrosis factor-α gene regulation by virus and lipopolysaccharide. Proc Natl Acad Sci USA. 1990;87:9769–9773. doi: 10.1073/pnas.87.24.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han J, Brown T, Beutler B. Endotoxin-responsive sequences control cachectin/tumor necrosis factor biosynthesis at the translational level. J Exp Med. 1990;171:465–475. doi: 10.1084/jem.171.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, Stewart C L, Goyert S M. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 16.Heinzel F P. The role of IFN-γ in the pathology of experimental endotoxemia. J Immunol. 1990;145:2920–2924. [PubMed] [Google Scholar]
- 17.Kato N, Ohta M, Kido N, Arakawa Y, Ito H, Naito S. Inhibitory effect of Ca(2+) on formation of Mg(2+)-mediated two-dimensional hexagonal lattice structure by an R-form lipopolysaccharide from Klebsiella pneumoniae. Microbiol Immunol. 1990;34:427–438. doi: 10.1111/j.1348-0421.1990.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 18.Kirikae F, Kirikae T, Qureshi N, Takayama K, Morrison D C, Nakano M. CD14 is not involved in Rhodobacter sphaeroides diphosphoryl lipid A inhibition of tumor necrosis factor alpha and nitric oxide induction by taxol in murine macrophages. Infect Immun. 1995;63:486–497. doi: 10.1128/iai.63.2.486-497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitchens R L, Munford R S. Enzymatically deacylated lipopolysaccharide (LPS) can antagonize LPS at multiple sites in the LPS recognition pathway. J Biol Chem. 1995;270:9904–9910. doi: 10.1074/jbc.270.17.9904. [DOI] [PubMed] [Google Scholar]
- 20.Kopp E B, Ghosh S. NF-kappa B and rel proteins in innate immunity. Adv Immunol. 1995;58:1–27. doi: 10.1016/s0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence O, Rachie N, Qureshi N, Bomsztyk K, Sibley C H. Diphosphoryl lipid A from Rhodobacter sphaeroides transiently activates NF-κB but inhibits lipopolysaccharide induction of kappa light chain and Oct-2 in the B-cell lymphoma line 70Z/3. Infect Immun. 1995;63:1040–1046. doi: 10.1128/iai.63.3.1040-1046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann V, Freudenberg M A, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine-treated mice. J Exp Med. 1987;165:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynn W A, Golenbock D T. Lipopolysaccharide antagonists. Immunol Today. 1992;13:271–276. doi: 10.1016/0167-5699(92)90009-V. [DOI] [PubMed] [Google Scholar]
- 24.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie Q, Sokol K, Hutchinson N, Chen H, Mudgett J S. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 25.Proctor R A, Denlinger L C, Bertics P J. Lipopolysaccharide and bacterial virulence. In: Roth J A, Bolin C A, Brogden K A, Minion F C, Wannemuehler M J, editors. Virulence mechanisms of bacterial pathogens. 2nd ed. Washington, D.C: ASM Press; 1995. pp. 173–194. [Google Scholar]
- 26.Proctor R A, Denlinger L C, Leventhal P S, Daugherty S K, van de Loo J-W, Tanke T, Firestein G S, Bertics P J. Protection of mice from endotoxic death by 2-methylthio-ATP. Proc Natl Acad Sci USA. 1994;91:6017–6020. doi: 10.1073/pnas.91.13.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Proctor R A, Will J A, Burhop K E, Raetz C R H. Protection of mice against lethal endotoxemia by a lipid A precursor. Infect Immun. 1986;52:905–907. doi: 10.1128/iai.52.3.905-907.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 29.Rietschel E T, Kirikae T, Schade F U, Mamat U, Schmidt G, Loppnow H, Ulmer A J, Zahringer U, Seydel U, de Padova F, Schreir M, Brade H. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 30.Seydel U, Labischinski H, Kastowsky M, Brandenburg K. Phase behavior, supramolecular structure, and molecular conformation of lipopolysaccharide. Immunobiology. 1993;187:191–211. doi: 10.1016/S0171-2985(11)80339-6. [DOI] [PubMed] [Google Scholar]
- 31.Shi Y, Li H Q, Shen C K, Wang J H, Qin S W, Liu R, Pan J. Plasma nitric oxide levels in newborn infants with sepsis. J Pediatr. 1993;123:435–438. doi: 10.1016/s0022-3476(05)81753-6. [DOI] [PubMed] [Google Scholar]
- 32.Snedecor G W, Cochran W G. Statistical methods. 8th ed. Ames: Iowa State University Press; 1989. [Google Scholar]
- 33.Sweet M J, Hume D A. Endotoxin signal transduction in macrophages. J Leukocyte Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 34.Takayama K, Mitchell D H, Din Z Z, Mukerjee P, Li C, Coleman D L. Monomeric Re lipopolysaccharide from Escherichia coli is more active than the aggregated form in the Limulus amoebocyte lysate assay and in inducing Egr-1 mRNA in murine peritoneal macrophages. J Biol Chem. 1994;269:2241–2244. [PubMed] [Google Scholar]
- 35.Tanke T, van de Loo J-W, Rhim H, Leventhal P S, Proctor R A, Bertics P J. Bacterial lipopolysaccharide-stimulated GTPase activity in RAW264.7 macrophage membranes. Biochem J. 1991;277:379–385. doi: 10.1042/bj2770379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson J E, Phillips R J, Erdjument-Bromage H, Tempst P, Ghosh S. I kappa-B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 37.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 38.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 39.Xie Q, Kashiwabara Y, Nathan C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 40.Zähringer U, Lindner B, Rietschel E T. Molecular structure of lipid A, the endotoxic center of bacterial lipopolysaccharides. Adv Carbohydr Chem Biochem. 1994;50:211–276. [PubMed] [Google Scholar]
- 41.Zhang X, Alley E W, Russell S W, Morrison D C. Necessity and sufficiency of beta interferon for nitric oxide production in mouse peritoneal macrophages. Infect Immun. 1994;62:33–40. doi: 10.1128/iai.62.1.33-40.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]