Abstract
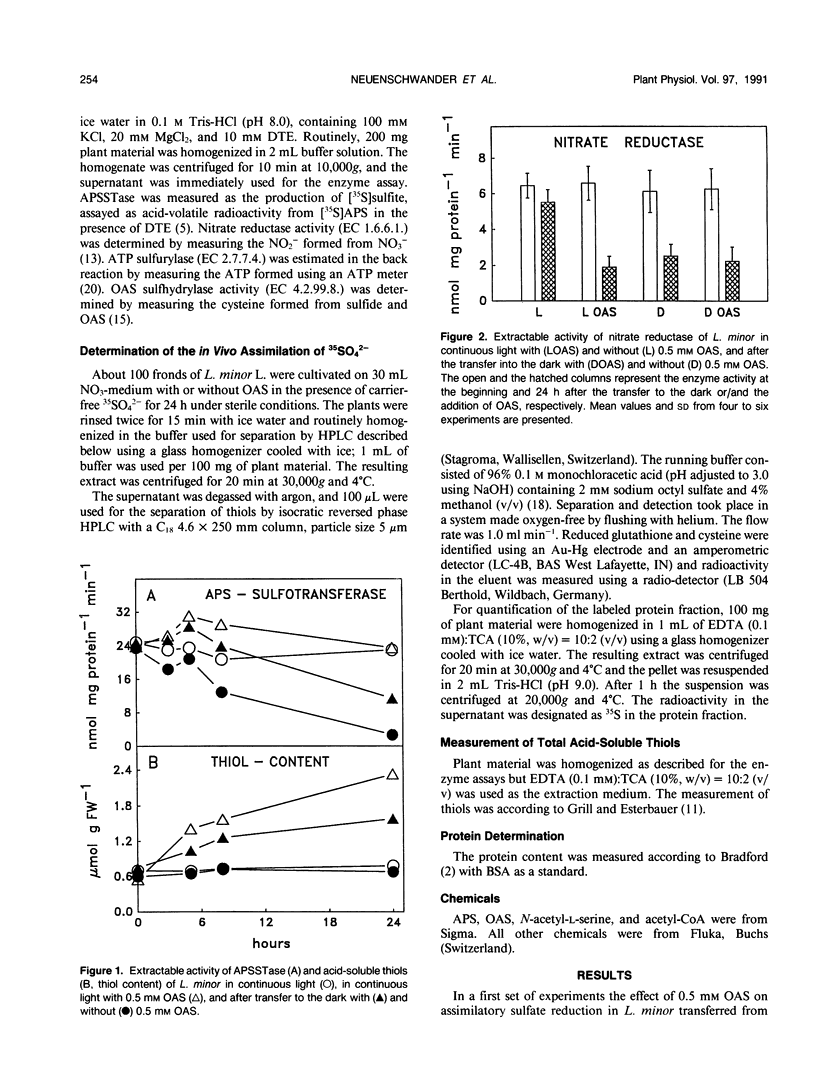
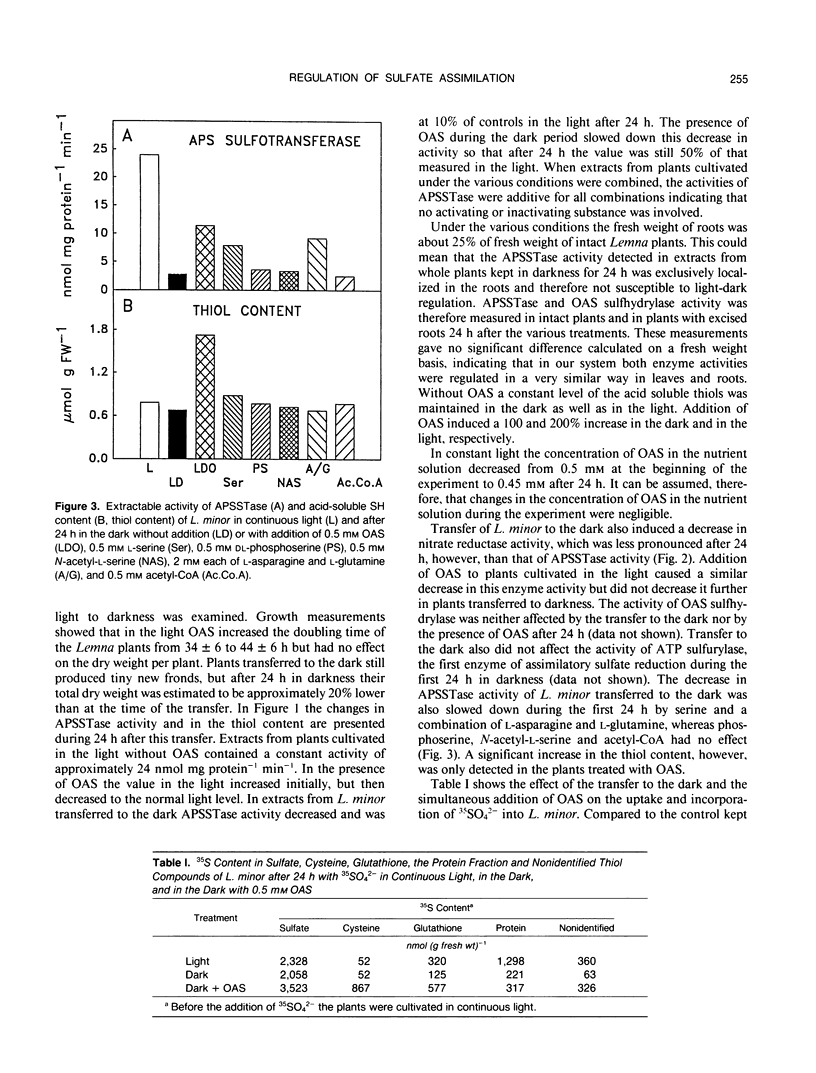
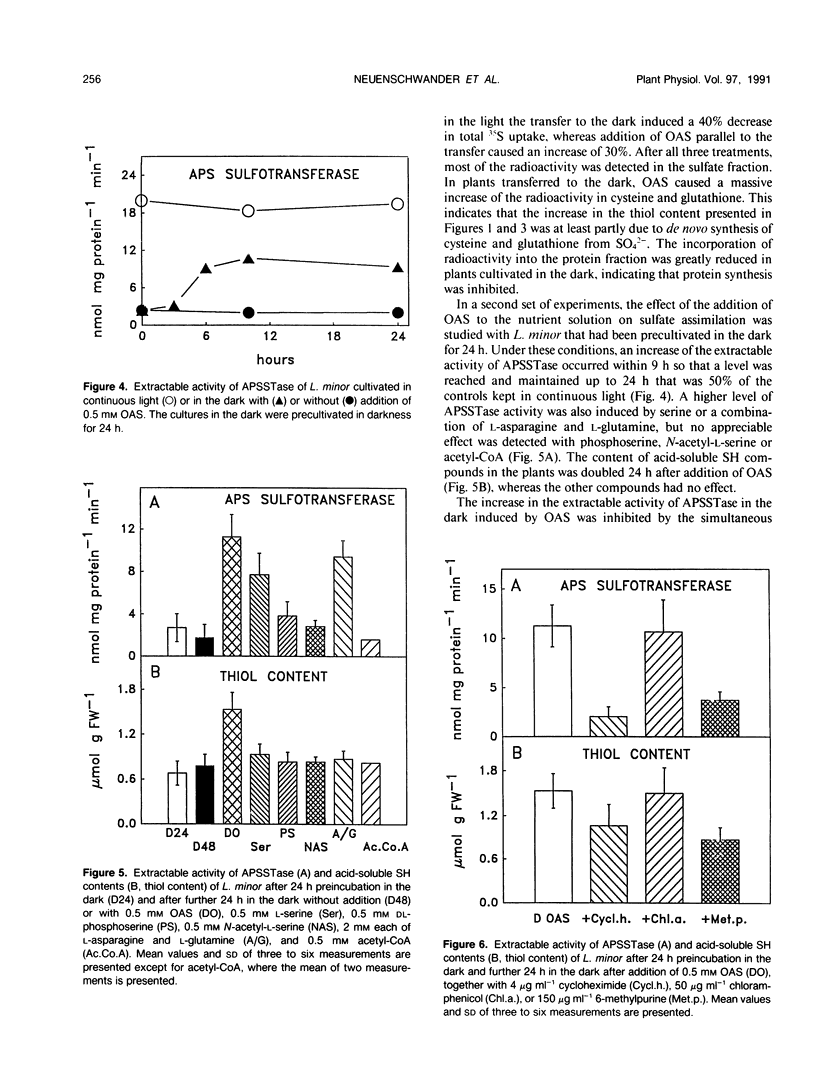
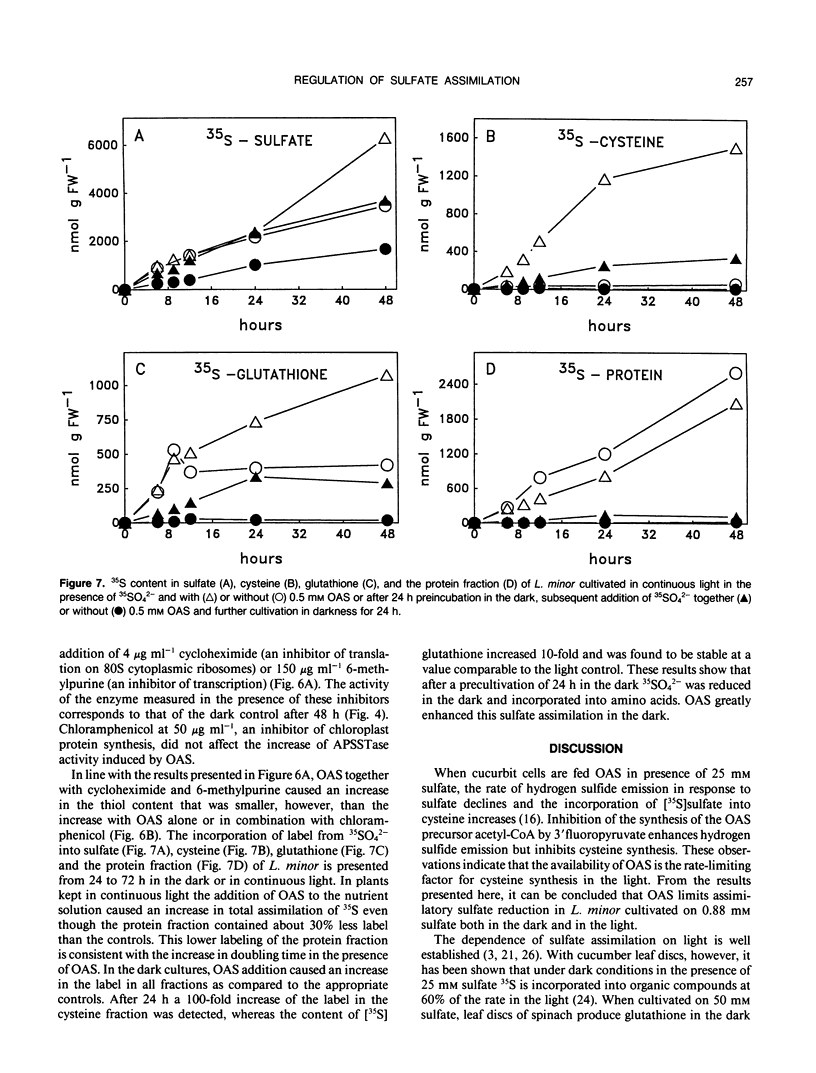
The effect of 0.5 millimolar O-acetyl-l-serine added to the nutrient solution on sulfate assimilation of Lemna minor L., cultivated in the light or in the dark, or transferred from light to the dark, was examined. During 24 hours after transfer from light to the dark the extractable activity of adenosine 5′-phosphosulfate sulfotransferase, a key enzyme of sulfate assimilation, decreased to 10% of the light control. Nitrate reductase (EC 1.7.7.1.) activity, measured for comparison, decreased to 40%. Adenosine 5′-triphosphate (ATP) sulfurylase (EC 2.7.7.4.) and O-acetyl-l-serine sulfhydrylase (EC 4.2.99.8.) activities were not affected by the transfer. When O-acetyl-l-serine was added to the nutrient solution at the time of transfer to the dark, adenosine 5′-phosphosulfate sulfotransferase activity was still at 50% of the light control after 24 hours, ATP sulfurylase and O-acetyl-l-serine sulfhydrylase activity were again not affected, and nitrate reductase activity decreased as before. Addition of O-acetyl-l-serine at the time of the transfer caused a 100% increase in acid-soluble SH compounds after 24 hours in the dark. In continuous light the corresponding increase was 200%. During 24 hours after transfer to the dark the assimilation of 35SO42− into organic compounds decreased by 80% without O-acetyl-l-serine but was comparable to light controls in its presence. The addition of O-acetyl-l-serine to Lemna minor precultivated in the dark for 24 hours induced an increase in adenosine 5′-phosphosulfate sulfotransferase activity so that a constant level of 50% of the light control was reached after an additional 9 hours. Cycloheximide as well as 6-methyl-purine inhibited this effect. In the same type of experiment O-acetyl-l-serine induced a 100-fold increase in the incorporation of label from 35SO42− into cysteine after additional 24 hours in the dark. Taken together, these results show that exogenous O-acetyl-l-serine has a regulatory effect on assimilatory sulfate reduction of L. minor in light and darkness. They are in agreement with the idea that this compound is a limiting factor for sulfate assimilation and seem to be in contrast to the proposed strict light control of sulfate assimilation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunold C., Suter M. Regulation of Sulfate Assimilation by Nitrogen Nutrition in the Duckweed Lemna minor L. Plant Physiol. 1984 Nov;76(3):579–583. doi: 10.1104/pp.76.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R. B., Davies D. D. Protein degradation in lemna with particular reference to ribulose bisphosphate carboxylase: I. The effect of light and dark. Plant Physiol. 1987 Apr;83(4):869–877. doi: 10.1104/pp.83.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich J. W., Schrader L. E. Sulfur deprivation and nitrogen metabolism in maize seedlings. Plant Physiol. 1978 Jun;61(6):900–903. doi: 10.1104/pp.61.6.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galangau F., Daniel-Vedele F., Moureaux T., Dorbe M. F., Leydecker M. T., Caboche M. Expression of leaf nitrate reductase genes from tomato and tobacco in relation to light-dark regimes and nitrate supply. Plant Physiol. 1988 Oct;88(2):383–388. doi: 10.1104/pp.88.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-L-serine on gene expression. J Biol Chem. 1971 Jun 10;246(11):3474–3484. [PubMed] [Google Scholar]
- Pieniazek N., Stepień P. P., Paszewski A. An Aspergillus nidulans mutant lacking cystathionine -synthase: identity of L-serine sulfhydrylase with cystathionine -synthase and its distinctness from O-acetyl-L-serine sulfhydrylase. Biochim Biophys Acta. 1973 Jan 24;297(1):37–47. doi: 10.1016/0304-4165(73)90047-0. [DOI] [PubMed] [Google Scholar]
- Rennenberg H. Role of o-acetylserine in hydrogen sulfide emission from pumpkin leaves in response to sulfate. Plant Physiol. 1983 Nov;73(3):560–565. doi: 10.1104/pp.73.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny Z., Filner P. Regulation of adenosine triphosphate sulfurylase in cultured tobacco cells. Effects of sulfur and nitrogen sources on the formation and decay of the enzyme. J Biol Chem. 1977 Mar 25;252(6):1858–1864. [PubMed] [Google Scholar]
- Richie J. P., Jr, Lang C. A. The determination of glutathione, cyst(e)ine, and other thiols and disulfides in biological samples using high-performance liquid chromatography with dual electrochemical detection. Anal Biochem. 1987 May 15;163(1):9–15. doi: 10.1016/0003-2697(87)90085-6. [DOI] [PubMed] [Google Scholar]


