Abstract
An attenuated strain of Vibrio cholerae was used as a carrier for the expression of heterologous antigens such as fragment C from tetanus toxin (TetC) and tracheal colonization factor from Bordetella pertussis (Tcf). In vitro, high levels of protein were obtained when the Escherichia coli nirB promoter was used and the bacteria were grown with low aeration. Intranasal immunization of mice with IEM101 expressing TetC elicited serum vibriocidal activity and induced antibodies against tetanus toxin which were protective against lethal challenge with 10 times the 50% lethal dose of tetanus toxin. Bacterial viability was essential for the induction of anti-TetC antibodies. Intranasal administration of IEM101 expressing Tcf induced a significant reduction in bacterial colonization of the tracheas of mice challenged with wild-type B. pertussis. These data are in agreement with the putative role of Tcf in Bordetella tracheal colonization. In conclusion, we have demonstrated that V. cholerae may be used as a live vector to deliver heterologous antigens in vivo and that protection to both systemic and local challenge may be achieved.
Vibrio cholerae is the causative agent of cholera, a human diarrheal disease with high rates of morbidity and mortality in developing countries. Infection occurs after ingestion of contaminated water or food; the bacteria reach the small intestine, where they penetrate the mucous layer, adhere to epithelial cells, multiply, and produce cholera toxin. This powerful toxin induces secretion of chloride ions by enterocytes, leading to a severe watery diarrhea (3).
Naturally acquired infection with V. cholerae efficiently stimulates immunity, leading to long-term protection against the disease (24). In the last years, live attenuated oral vaccines against V. cholerae have been developed (9, 25, 30, 35). A naturally attenuated strain of V. cholerae O1 El Tor, Ogawa, isolated in China (IEM101) has been also described (26). IEM101 has been tested for safety and immunogenicity in a human clinical study, which showed that the strain is safe and able to colonize the intestinal mucosa and to induce a strong immune response, eliciting high levels of vibriocidal and antilipopolysaccharide antibodies in the sera of human volunteers. Due to these characteristics and to the noninvasive nature of V. cholerae infection, attenuated strains of V. cholerae may be good candidates for the delivery of foreign antigens to the host.
Previous work on heterologous expression in V. cholerae has used antigens from other enteropathogens, such as Shigella sonnei lipopolysaccharide, Escherichia coli Shiga toxin B subunit, and enterohemorrhagic E. coli EaeA (1, 4, 6, 13, 36). In this study, we have investigated whether the attenuated strain IEM101 is able to express antigens from nonenteric pathogens and whether these recombinant strains induce protective immunity to systemic and mucosal challenge.
The choice of an animal model to evaluate immune responses induced by recombinant V. cholerae strains is a critical step, since natural infection with V. cholerae does not occur in animals. Various animal models have been used in cholera research (33). Infant mice or rabbits are susceptible to infection for a short time after birth; however, given the immature immune system of neonatal animals, they do not constitute a good model for immunogenicity studies. Colonization by V. cholerae has been accomplished in adult rabbits, with tincture of opium used to induce paralysis of intestinal motility, but this model presents some limitations in terms of animal handling. It has been recently described that germfree mice are readily colonized by V. cholerae after oral inoculation (5); however, these animals are usually more expensive than nongermfree ones.
The intranasal route has been shown to be highly efficient for the induction of immune responses, at the systemic and mucosal levels, with a variety of antigens delivered, either with mucosal adjuvants (10, 11, 37) or microcapsules (21). This route has also been used to deliver live Salmonella typhimurium (19), Bordetella pertussis (32), Mycobacterium bovis BCG (22), and even Salmonella typhi, which also lacks a practical small-animal model (2, 16).
In this study we have used intranasal immunization as an alternative mucosal route. Fragment C (TetC) from tetanus toxin (TT) and tracheal colonization factor (Tcf) from B. pertussis were used as model antigens. TetC is the nontoxic 50-kDa C-terminal portion of TT (18); it is immunogenic and able to protect against challenge with the toxin when either administered as purified immunogen (12) or delivered by attenuated Salmonella (8). Tcf is a virulence-associated factor secreted by B. pertussis, and tcfA mutants are impaired in their ability to colonize the mouse trachea after aerosol infection (15). Tcf is produced as a cell-associated precursor form, with an apparent molecular mass of 90 kDa, which is processed to release the 60-kDa form.
MATERIALS AND METHODS
Strains, media, and growth conditions.
E. coli DH5α was used for cloning purposes. V. cholerae IEM101 was grown in Luria broth or Luria broth with ampicillin (100 μg/ml) when required. B. pertussis BP18323 Smr was grown in Stainer-Scholte (SS) modified medium (34) or on Bordet-Gengou (BG) agar (Difco) with 20% defibrinated sheep blood and streptomycin (40 μg/ml). Transformation of IEM101 was performed by electroporation as previously described (17). Growth of bacterial cultures under low-aeration conditions was achieved by inoculation of 2 ml from an overnight culture into a completely filled, tight-capped 50-ml Falcon tube and subsequent incubation at 37°C for 3.5 to 4 h. To recover IEM101 from organ homogenates, TCBS agar (Difco) was used.
Plasmids and constructions.
Plasmid pTETnir15 (obtained from G. Dougan, Imperial College, London, United Kingdom) contains the coding sequence for TetC under the control of the E. coli nirB modified promoter, which is induced under anaerobic conditions (29).
tcfA was amplified from the B. pertussis BP18323 Smr chromosome by using Taq DNA polymerase (Boehringer Mannheim). The forward primer was oligonucleotide Tcf1 (5′-ACTAGTGATCATATGCACAATTTACGGAAATA-3′), which contains the initial codon of tcfA, comprised within the NdeI site, and an upstream BclI site; the reverse primer was oligonucleotide Tcf2 (5′-GTCTAGAATTCTACCAGGCGTAGCGATACC-3′), containing the stop codon of the tcfA gene and an EcoRI site downstream. The amplification product was digested with BclI and EcoRI and cloned into pBlueScript/KS+ (Stratagene) between EcoRI and BamHI sites, giving origin to pBS-tcf. The cloned fragment was completely sequenced, and one mutation, leading to a conservative amino acid substitution (Met to Thr) in position 494, probably due to Taq DNA polymerase inaccuracy, was found.
tcfA was placed under the control of the cholera toxin gene promoter (pctx) as follows: a 190-bp sequence located immediately upstream to the ctx structural region was amplified from plasmid pJM17, which contains the virulence cassette region from V. cholerae classical strain 569B (31). Oligonucleotides Pct1 (5′-TAGATCTAGATACCTTTGCAGCGCAAGG-3′) and Pct2 (5′-TATCTTTACCATATGATGCTCCC-3′) were used as forward and reverse primers, respectively; Pct1 contains an XbaI site at its 5′ extremity, whereas Pct2 corresponds to the reverse sequence of the ctx gene, with the initiation codon (reverse) within an NdeI site. The amplified fragment was digested with XbaI and NdeI and cloned into pBS-tcf digested with the same enzymes, generating pCT-tcf.
Subcloning tcfA downstream of the nirB promoter from pTETnir15 was achieved in two steps. First, pTETnir15 was digested with BglII and BamHI, liberating a fragment containing the coding region for TetC and ca. 30 bp of the 5′ untranslated region, including the putative ribosomal binding site sequence. In order to reconstitute this region, which could be important for RNA stability and/or efficient translation initiation, the fragment containing the vector sequence was ligated to oligonucleotides PnirUTR1 (5′-GATCTTAATCATCCACAGGAGACTTTCATATGATATCTAGATGCATC-3′) and PnirUTR2 (5′-G ATCGATGCATCTAGATATCATATGAAAGTCTCCTGTGGATGATTAA- 3′), corresponding to the 5′ untranslated region, with an NdeI site comprising the initiation codon, and an XbaI site immediately downstream. This plasmid was called pNIR100; it was digested with XbaI, filled in with Klenow fragment from E. coli DNA polymerase I, digested with NdeI, and ligated to the NdeI-EcoRV fragment from pBS-tcf, which contains the tcfA gene, generating pNIR-tcf.
Analysis of protein expression and localization.
Expression of TetC was analyzed by sodium dodecyl sulfate–12.5% polyacrylamide gel electrophoresis (SDS–12.5% PAGE) and immunoblotting of whole-cell lysates, using mouse anti-TT antiserum at a dilution of 1:2,000 (obtained from S. Peppoloni, IRIS, Siena, Italy).
Tcf expression was probed by immunoblotting, using mouse antiserum raised against the N-terminal portion of Tcf fused to MalE (15) at a dilution of 1:8,000. Culture supernatant (1 ml) from B. pertussis or V. cholerae was trichloroacetic acid precipitated and loaded on an SDS-polyacrylamide gel. B. pertussis whole-cell lysate was prepared by pelleting bacteria from a liquid culture with an optical density at 590 nm (OD590) of 1.0 and resuspension in 1× sample boiling buffer in order to have approximately 5 × 109 cells/ml. B. pertussis outer membrane-associated proteins (OMAP) were prepared by resuspending cells in 50 mM Tris-HCl (pH 8.0)–150 mM NaCl to a final concentration of 20 OD units/ml; the suspension was incubated at 60°C for 1 h with gentle shaking; intact cells were pelleted by centrifugation, and the supernatant, containing the OMAP, was used for SDS-PAGE. Whole-cell lysates of IEM101 were prepared by pelleting cells from liquid cultures and resuspending them in sample boiling buffer to a final concentration of approximately 1010 cells/ml; the V. cholerae outer membrane fraction was prepared from 109 cells, as previously described (14).
FACScan analysis was used to probe Tcf exposure on the bacterial cell surface. Approximately 106 cells were pelleted and kept on ice through the procedure. Cells were washed with phosphate-buffered saline (PBS)–2% bovine serum albumin (BSA) and incubated for 1 h with serial dilutions of anti-MalE-Tcf mouse antisera in 200 μl of PBS–2% BSA; mouse preimmune serum was used as a negative control. Bacterial cells were washed twice with PBS–2% BSA and incubated for 30 min with the appropriate dilution of R-phycoerythrin-labeled F(ab′)2 goat anti-mouse immunoglobulin G (IgG) antiserum. Cells were subsequently washed twice with PBS–2% BSA and resuspended in PBS, and 104 bacterial cells were analyzed for cell-bound fluorescence with a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.), using the Lysis II software program from Becton Dickinson. The threshold of positivity was set for each experiment by flow cytometric analysis of IEM101 previously incubated with antisera to MalE-Tcf and with the R-phycoerythrin-labeled secondary antibody.
Immunizations.
Female BALB/c mice (6 to 8 weeks old; Charles River, Calco, Italy) each received 30 μl of a bacterial suspension in PBS by the intranasal route, either without anesthesia or anesthetized with 0.2 ml of a mixture of 15% xylazine hydrochloride (Rompun) and 10% ketamine hydrochloride (Ketavet), on days 0, 28, 42, and 56. Animals were bled on days 27, 35, 49, and 63 and challenged at day 70. Nasal lavages were performed at day 70 by repeated flushing and aspiration with 1 ml of PBS containing 0.1% BSA (Sigma).
Titration of antigen-specific serum antibodies.
Vibriocidal activity was assayed by incubating 107 CFU of IEM101 with 20% rabbit serum as the complement source and serial dilutions of immunized animal sera in 100 μl of PBS in 96-well tissue culture plates (Costar), for 1 h at 37°C; 100 μl of brain heart infusion (BHI) (Difco) was then added to each well, and plates were further incubated for 1 h at 37°C; absorbance at 570 nm was then measured. Titers were calculated as the dilution of the serum that gave 50% growth inhibition compared to preimmune serum diluted 1:25.
Titers of anti-TetC antibodies were determined by enzyme-linked immunosorbent assay (ELISA). Plates were coated overnight at 4°C with 200 ng of tetanus toxoid (formaldehyde-inactivated TT) in 100 μl of PBS/well. After three washes with PBS–0.05% Tween 20 (PBS-T), plates were blocked with 1% BSA in PBS-T (200 μl/well) for 2 h at 37°C, washed three times with PBS-T, and incubated with serial dilutions of sera from immunized animals in PBS-T–0.5% BSA for 1.5 h at 37°C; plates were then incubated with PBS-T–0.5% BSA containing alkaline phosphatase-conjugated anti-mouse IgG antibody (Sigma) for 1.5 h at 37°C. Bound antibodies were revealed by using p-nitrophenylphosphate as a substrate (Sigma). Titers were calculated as the dilution that gave 2.5 times the absorbance (OD405) of preimmune serum diluted 1:100.
Mucosal IgA titers were measured by using biotin-conjugated goat anti-mouse IgA (α-chain specific; Sigma) followed by streptavidin-horseradish peroxidate conjugate (Dako). Bound antibodies were revealed by using o-phenylendiamine as a substrate. Titers were determined as the dilution that gave 2.5 times the absorbance (OD490) of nasal washes of nonimmunized animals.
TT challenge.
Mice were challenged subcutaneously with 10 times the 50% lethal dose (10 × LD50) of TT. Paralysis and death were recorded for 7 days after the challenge.
Bordetella intranasal challenge.
B. pertussis 18323 Smr was grown for 2 days on a BG plate and then inoculated into 100 ml of SS modified medium; the culture was grown until it reached an OD590 of 0.5. Bacteria were then diluted in PBS to a concentration of 3.3 × 108 CFU/ml; the concentration was confirmed by serial dilution and plating. Animals received 30 μl of the bacterial suspension (corresponding to approximately 106 CFU) intranasally, under light anesthesia. Colonization of the trachea and lungs was monitored by counting CFU recovered from organ homogenates.
RESULTS
Expression of TetC in IEM101.
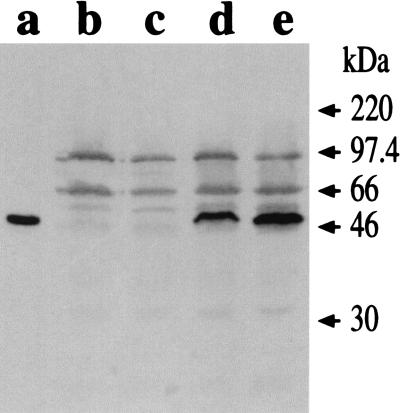
The plasmid pTETnir15, which contains the gene coding for TetC under the control of the modified E. coli nirB promoter, was electroporated into V. cholerae IEM101. Total cell extracts were prepared from both IEM101 and IEM101(pTETnir15) strains and analyzed by Western blotting. As shown in Fig. 1, transformants produced TetC, and expression was induced when bacteria were grown with low aeration, indicating that the nirB promoter was functional in IEM101.
FIG. 1.
TetC expression by IEM101. Whole-cell lysates (corresponding to approximately 2.5 × 108 CFU of bacteria) were fractionated by SDS-PAGE and probed with anti-TT mouse polyclonal antiserum. Purified TetC (100 ng) (lane a) was used as a standard. IEM101 was grown under aerobic conditions (lane b) or low-aeration conditions (lane c); the same conditions were used for growing IEM101(pTETnir15) (lane d, aerobic; lane e, low aeration).
Expression and localization of tcfA in IEM101.
tcfA was subcloned under the control of the ctx promoter (pCT-tcf) and nirB promoter (pNIR-tcf); the recombinant plasmids were electroporated into IEM101. Total cell extracts were prepared from each of the recombinant strains, and the levels of Tcf were visualized by immunoblotting. The results are shown in Fig. 2.
FIG. 2.
Tcf expression by IEM101 under control of different promoters. Whole-cell lysates (corresponding to approximately 5 × 108 CFU of IEM101 or 5 × 107 CFU of BP18323) were fractionated by SDS-PAGE and probed with anti-Tcf antiserum. Samples shown are BP18323 (lane a), IEM101 (lane b), IEM101(pCT-tcf) (lane c), IEM101(pNIR-tcf) grown under aerobic conditions (lane d), and IEM101(pNIR-tcf) grown with low aeration (lane e).
Tcf production was observed when either the ctx or nirB promoters were used (Fig. 2, lanes c and d, respectively). The highest level of expression was achieved when the pnirB construct was induced by growth with low aeration (Fig. 2, lane e).
In order to analyze Tcf localization in the bacteria, we performed immunoblotting experiments using different cellular fractions, and the results are reported in Fig. 3. In B. pertussis, Tcf could be found in the culture supernatant (Fig. 3, lane b), confirming previously reported data (15), and in the OMAP preparation (Fig. 3, lane a), which indicates that it is loosely associated with the outer membrane. In V. cholerae, Tcf localized in the outer membrane fraction (Fig. 3, lane d), although small amounts could be detected in the culture supernatant (Fig. 3, lane e). Tcf appears to be processed at a different site in V. cholerae, since the molecular mass of the protein appears to be less than that of the 60-kDa form detected in Bordetella extracts and supernatant (Fig. 3, lanes a and b, respectively).
FIG. 3.
Tcf localization in IEM101. OMAP preparations from B. pertussis 18323 (from approximately 2 × 108 CFU), outer membrane protein fractions from IEM101 (from approximately 109 CFU), and trichloroacetic acid-precipitated culture supernatant (corresponding to 1 ml) were loaded on SDS-polyacrylamide gels and probed with anti-Tcf antiserum. Samples shown are B. pertussis 18323 OMAP (lane a), B. pertussis 18323 culture supernatant (lane b), IEM101 outer membrane fraction (lane c), IEM101(pNIR-tcf) outer membrane fraction (lane d), and IEM101(pNIR-tcf) culture supernatant (lane e).
Flow cytometry analysis was performed to verify whether Tcf was exposed on the V. cholerae surface. The percentage of cells emitting fluorescence was 31% in the case of B. pertussis and 14% in the case of IEM101(pNIR-tcf), suggesting that the protein is less exposed in V. cholerae than in B. pertussis (data not shown).
Intranasal administration of V. cholerae.
The intranasal route was chosen to deliver V. cholerae to mice since it has been reported to be more efficient than the oral route in inducing an immune response against foreign antigens expressed by live recombinant bacterial strains (16, 19).
Bacterial administration conditions were defined. Animals under anesthesia could receive intranasally up to 5 × 107 CFU without mortality. A higher bacterial dose (5 × 108 CFU) could be delivered either by inoculation of nonanesthetized animals or by using heat-inactivated bacteria. Both treatments resulted in similar serum vibriocidal activity in both groups, as presented in Fig. 4. The titers in mice inoculated with live bacteria were slightly higher than those in mice inoculated with heat-inactivated bacteria, but the difference was not statistically significant.
FIG. 4.
Serum vibriocidal activities. Serum vibriocidal activities in BALB/c mice after receiving one to four doses of 5 × 108 bacteria, either heat inactivated (A) or live (B), with six animals per group, are presented. Results are shown as individual values of log10 vibriocidal activity titers.
After one intranasal administration of 5 × 108 CFU IEM101(pTETnir15) to nonanesthetized animals, bacterial persistence in trachea, lung, small intestine, and stool was analyzed. The results, reported in Fig. 5, show that viable bacteria could be recovered from mouse tracheas 24 h after inoculation, and furthermore, most of the recovered bacteria could be cultured on ampicillin plates, showing that bacteria had not lost the plasmid. However, only some of the animals presented bacteria in their lungs (not shown). Bacteria could also be recovered from small intestine and stool, possibly due to the animals’ swallowing inoculum during administration. No bacteria were detected in any organ after 72 h.
FIG. 5.
IEM101(pTETnir15) persistence in vivo. BALB/c mice received intranasally 108 CFU of IEM101(pTETnir15) (dose indicated by arrow on y axis); the presence of bacteria in the mouse trachea was assessed after 2, 24, and 72 h, with three animals sacrificed per time point. Bacterial counts were performed on TCBS agar plates, with (TCBS-Ap) or without (TCBS) ampicillin. Results are shown as means + standard deviations (error bars) of log10 CFU/organ.
Induction of anti-TT antibody response and in vivo protection after immunization with IEM101-delivered TetC.
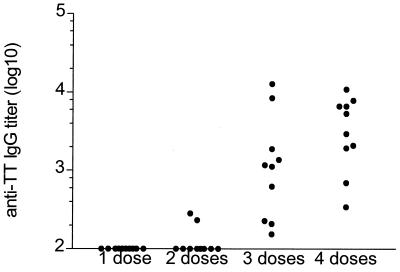
Nonanesthetized BALB/c mice received four doses of ca. 5 × 108 CFU of either IEM101 or IEM101(pTETnir15), grown without aeration, by the intranasal route. A third group of mice received the same dose of heat-inactivated IEM101(pTETnir15). Levels of humoral anti-TT IgG were analyzed by ELISA. When killed bacteria were used for immunization, no anti-TT IgG response was detected in the sera of the immunized mice. After three immunizations, mice immunized with live bacteria mounted an anti-TT humoral response, which was boosted following the fourth immunization (Fig. 6). An anti-TT IgA response could be also detected in nasal washes, indicating that stimulation of local responses occurred (Fig. 7).
FIG. 6.
Anti-TT serum antibody responses. Anti-TT serum antibody responses in 10 BALB/c mice after receiving one to four doses of 5 × 108 CFU of IEM101(pTETnir15) are presented. Results are shown as individual values of log10 anti-TT IgG.
FIG. 7.
Anti-TT mucosal IgA response. Anti-TT IgA antibody responses in nasal washes of five BALB/c mice after receiving four doses of 5 × 108 CFU of either IEM101 or IEM101(pTETnir15) are presented. Results are shown as mean titers, and the error bar indicates the standard deviation from the mean titer.
To verify whether the induced anti-TT antibodies were able to protect immunized mice against a lethal challenge with tetanus toxin, mice were challenged with 10 × LD50 of tetanus toxin on day 70 and observed for mortality for 7 days after the challenge. The results, reported in Fig. 8, show that all mice immunized with IEM101(pTETnir15) survived the challenge, whereas control animals, immunized with IEM101, died within 36 h, demonstrating that the immune response raised by Vibrio-delivered TetC was able to neutralize the toxin in vivo and confer protection.
FIG. 8.
Mouse survival after challenge with TT. Eight mice were immunized with four doses of 5 × 108 CFU of either IEM101 or IEM101(pTETnir15). At day 70, mice were challenged with 10 × LD50 of tetanus toxin and observed for mortality for 7 days after challenge.
In vivo protection following immunization with IEM101-delivered Tcf.
BALB/c animals received four doses of ca. 5 × 108 CFU of either IEM101 or IEM101(pNIR-tcf) by the intranasal route. We were unable to detect specific anti-Tcf responses in the sera and mucosal washes of immunized mice, either by ELISA or Western blotting. Purified recombinant MalE-Tcf and partially purified Tcf from B. pertussis were used as antigens for the assays, but sera of mice immunized with IEM101 also recognized such preparations. These background signals could be due to a response to endogenous MalE from IEM101 or cross-reaction with proteins copurified with Tcf.
Although it was impossible to detect a specific anti-Tcf antibody response, we asked whether mice immunized with IEM101-delivered Tcf were protected from tracheal colonization by B. pertussis, since it has been described that a tcf mutant B. pertussis strain showed decreased tracheal colonization, implying a possible role for this protein in tracheal colonization (15). On day 70, immunized animals were challenged via the intranasal route with BP18323 Smr. Mice were sacrificed 14 days after the challenge, and the bacterial colonization of their tracheas and lungs was evaluated. The results, shown in Fig. 9, indicate that mice immunized with IEM101(pNIR-tcf) had significantly less bacteria in their tracheas than did the control animals immunized with IEM101. No significant difference between the numbers of bacteria isolated from the lungs of the two groups of mice was observed.
FIG. 9.
B. pertussis colonization in trachea and lung following intranasal infection. Six mice were immunized with four doses of 5 × 108 CFU of either IEM101 or IEM101(pNIR-tcf). At day 70, mice were challenged intranasally with 106 CFU of BP18323. Fourteen days after challenge, the mice were sacrificed and colonization in lungs and tracheas was determined. Results are expressed as geometrical means of bacterial counts. Values are significantly different, with a P of <0.03. NS, values not significantly different.
DISCUSSION
The construction of attenuated V. cholerae strains as live oral vaccines for cholera raises the possibility of using such strains to deliver heterologous antigens and to develop multivalent vaccines. We have thus expressed two antigens from nonenteropathogens in a naturally attenuated strain of V. cholerae, IEM101, and investigated its potential as a delivery system.
TetC was expressed in IEM101 by using pTETnir15 (29). Bacteria administered intranasally to mice induced a serum vibriocidal response and anti-TetC antibodies and stimulated local responses to the foreign protein. An anti-TT response able to confer protection against lethal challenge with TT was achieved.
Interestingly, immunization with heat-inactivated bacteria did not raise antibodies against TetC, although there was induction of vibriocidal activity, at levels comparable to those induced by live bacteria. Bacterial viability was shown to be necessary for V. cholerae interaction with M cells in rabbit intestine (28). It is probable that bacterial viability is also needed for interaction with M cells from respiratory mucosa; therefore, live bacteria would interact with M cells more efficiently than inactivated bacteria and consequently induce a stronger immune response. Persistence of IEM101 in the respiratory tract after intranasal inoculation (for at least 24 h) could also provide a more effective stimulation of the immune system. Plasmid retention was considerably high; thus, in vivo expression of the heterologous antigen could potentially occur. However, no significant differences in vibriocidal antibody titers in sera from animals receiving live and heat-inactivated Vibrio bacteria were observed; it is possible that the assay used to measure vibriocidal activity was not sensitive enough to detect such differences.
The nirB promoter was also able to drive the expression of tcfA from B. pertussis in IEM101. Tcf was processed and exposed on the IEM101 surface, although only a low proportion of the population was highly positive in the FACScan assay. This could be due to the low level of Tcf production; alternatively, Tcf could have a different conformation or exposure on the V. cholerae surface, by interaction with and/or sterical hindrance by outer membrane structures. However, it should be noted that only 31% of the B. pertussis population was recognized by the anti-MalE-Tcf antiserum used to probe Tcf. This could mean that the antibodies directed against the MalE-Tcf fusion protein do not bind efficiently to the Tcf protein in its native form.
Mouse immunization with Vibrio bacteria expressing Tcf resulted in a protective effect against Bordetella infection at the tracheal level, with approximately 10-fold reduction in colonization. These results corroborate previous observations that tcf mutants are less able to colonize the mouse trachea after aerosol infection than is the wild-type strain (15). The nature of the protective immunity induced by Vibrio-delivered Tcf is not known, as we were not able to detect a specific anti-Tcf immune response in the sera of immunized mice. Tcf shares an RGD motif with filamentous hemagglutinin and pertactin. The RGD motifs from both filamentous hemagglutinin (20) and pertactin (23) have been shown to be involved in adherence to host cells. Therefore, antibodies against Tcf could provide protection, since they could bind to Tcf on the Bordetella surface, preventing efficient bacterial adhesion to trachea mucosa; in addition, they could have bactericidal or opsonic activity. On the other hand, since cell-mediated responses play an important role in protection against Bordetella infection in the murine model (27, 32), the possibility that V. cholerae-delivered Tcf induced cellular responses should also be considered.
We did not observe protection at the lung level, and this result agrees with the observation that tcf mutants present the same pattern of lung colonization after aerosol infection as does wild-type Bordetella (15). Both observations could be due to lack of Tcf expression at that particular environment by Bordetella and/or the expression of other adhesion factors with more relevant roles in lung colonization.
In conclusion, we demonstrated the potential of V. cholerae as a delivery system for heterologous antigens, since two model antigens from nonenteropathogens were delivered by live Vibrio, and protective responses were elicited in both cases. We also showed that mouse intranasal inoculation with V. cholerae can be used for preliminary evaluation of immunogenicity. In order to enhance the immunogenicity of heterologous product, improvements in expression level and stability should be achieved by investigating different promoters; a promising alternative is the use of promoters from in vivo-induced (ivi) genes (7).
ACKNOWLEDGMENTS
This work was supported in part by EC grant TS3*-CT93-0255.
We thank Sandra Nuti and Domenico Rosa for FACScan analysis; Franco Giovannoni for the TT challenge; Fabrizio Zappalorto for animal handling; Samuele Peppoloni, Gill Douce, Duan Guancai, and Vincenzo Scarlato for sera, protein, and bacterial strains; Roberto Manetti for technical advice; Giorgio Corsi for artwork; and Giuseppe Del Giudice for helpful discussion.
REFERENCES
- 1.Acheson D W, Levine M M, Kaper J B, Keusch G T. Protective immunity to Shiga-like toxin I following immunization with Shiga-like toxin I B-subunit-producing Vibrio cholerae CVD 103-HgR. Infect Immun. 1996;64:355–357. doi: 10.1128/iai.64.1.355-357.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry E M, Gomez-Duarte O, Chatfield S, Rappuoli R, Pizza M, Losonsky G, Galen J, Levine M M. Expression and immunogenicity of pertussis toxin S1 subunit-tetanus toxin fragment C fusions in Salmonella typhi vaccine strain CVD908. Infect Immun. 1996;64:4172–4181. doi: 10.1128/iai.64.10.4172-4181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betley M J, Miller V L, Mekalanos J J. Genetics of bacterial enterotoxins. Annu Rev Microbiol. 1986;40:577–605. doi: 10.1146/annurev.mi.40.100186.003045. [DOI] [PubMed] [Google Scholar]
- 4.Butterton J R, Beattie D T, Gardel C L, Carroll P A, Hyman T, Killeen K P, Mekalanos J J, Calderwood S B. Heterologous antigen expression in Vibrio cholerae vector strains. Infect Immun. 1995;63:2689–2696. doi: 10.1128/iai.63.7.2689-2696.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butterton J R, Ryan E T, Shahin R A, Calderwood S B. Development of a germfree mouse model of Vibrio cholerae infection. Infect Immun. 1996;64:4373–4377. doi: 10.1128/iai.64.10.4373-4377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butterton J R, Ryan E T, Acheson D W K, Calderwood S B. Coexpression of the B subunit of shiga toxin 1 and EaeA from enterohemorrhagic Escherichia coli in Vibrio cholerae vaccine strains. Infect Immun. 1997;65:2127–2135. doi: 10.1128/iai.65.6.2127-2135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilli A, Mekalanos J. Use of recombinase gene fusions to identify Vibrio cholerae genes induced during infection. Mol Microbiol. 1995;18:671–683. doi: 10.1111/j.1365-2958.1995.mmi_18040671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatfield S N, Charles I G, Makoff A J, Oxer M D, Dougan G, Pickard D, Slater D, Fairweather N F. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains; development of a single-dose oral tetanus vaccine. Bio/Technology. 1992;10:888–892. doi: 10.1038/nbt0892-888. [DOI] [PubMed] [Google Scholar]
- 9.Coster T S, Killeen K P, Waldor M K, Beattie D T, Spriggs D R, Kenner J R, Trofa A, Sadoff J C, Mekanalos J J, Taylor D N. Safety, immunogenicity and efficacy of live attenuated Vibrio cholerae O139 vaccine prototype. Lancet. 1995;345:949–952. doi: 10.1016/s0140-6736(95)90698-3. [DOI] [PubMed] [Google Scholar]
- 10.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domeneghini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douce G, Fontana M R, Pizza M, Rappuoli R, Dougan G. Intranasal immunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin. Infect Immun. 1997;65:2821–2828. doi: 10.1128/iai.65.7.2821-2828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairweather N F, Lyness V A, Pickard D J, Allen G, Thompson R O. Immunization of mice against tetanus with fragments of tetanus toxin synthesized in Escherichia coli. Infect Immun. 1986;55:2541–2545. doi: 10.1128/iai.55.11.2541-2545.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Favre D, Cryz S J, Jr, Viret J-F. Development of Shigella sonnei live oral vaccines based on defined rfbInaba deletion mutants of Vibrio cholerae expressing the Shigella serotype D O polysaccharide. Infect Immun. 1996;64:576–584. doi: 10.1128/iai.64.2.576-584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filip C, Fletcher G, Wulff J L, Earhart C F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finn T M, Stevens L A. Tracheal colonization factor: a Bordetella pertussis secreted virulence determinant. Mol Microbiol. 1995;16:625–634. doi: 10.1111/j.1365-2958.1995.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 16.Galen J E, Gomez-Duarte G, Losonsky G A, Halpern J L, Lauderbaugh C S, Kaintuck X, Reymann M K, Levine M M. A murine model of intransal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine. 1997;15:700–708. doi: 10.1016/s0264-410x(96)00227-7. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg M B, Bokyo S A, Calderwood S B. Positive transcriptional regulation of an iron-regulated virulence gene in Vibrio cholerae. Proc Natl Acad Sci USA. 1991;88:1125–1129. doi: 10.1073/pnas.88.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helting T B, Zwisler O. Structure of tetanus toxin. I. Breakdown of the toxin molecule and discrimination between polypeptide fragments. J Biol Chem. 1977;252:194–198. [PubMed] [Google Scholar]
- 19.Hopkins S, Kraehenbuhl J-P, Schödel F, Potts A, Peterson D, De Grandi P, Nardelli-Haefliger D. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect Immun. 1995;63:3279–3286. doi: 10.1128/iai.63.9.3279-3286.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishibashi Y, Claus S, Relman D A. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18) J Exp Med. 1994;180:1225–1233. doi: 10.1084/jem.180.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones D H, McBride B W, Thornton C, O’Hagan D T, Robinson A, Farrar G H. Orally administered microencapsulated Bordetella pertussis fimbriae protect mice from B. pertussis respiratory infection. Infect Immun. 1996;64:489–494. doi: 10.1128/iai.64.2.489-494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langermann S, Palaszynski S, Sadziene A, Stover C K, Koenig S. Systemic and mucosal immunity induced by BCG vector expressing outer-surface protein A of Borrelia burgdorferi. Nature. 1994;372:552–555. doi: 10.1038/372552a0. [DOI] [PubMed] [Google Scholar]
- 23.Leiniger E, Ewanovich C A, Bhargava A, Peppler M S, Kenimer J G, Brennan M J. Comparative roles of the Arg-Gly-Asp sequence present in the Bordetella pertussis adhesins pertactin and filamentous hemagglutinin. Infect Immun. 1992;60:2380–2385. doi: 10.1128/iai.60.6.2380-2385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine M M, Black R E, Clements M L, Cisneros L, Nalin D R, Young C R. Duration of infection-derived immunity to cholera. J Infect Dis. 1981;143:818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- 25.Levine M M, Kaper J B, Herrington D, Ketley J, Losonsky G, Tacket C O, Tall B, Cryz S. Safety, immunogenicity and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988;ii:467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y Q, Qi G M, Xang S X, Yu Y M, Duan G C, Zhang L J, Gao S Y. A natural vaccine candidate strain against cholera. Biomed Environ Sci. 1995;8:350–358. [PubMed] [Google Scholar]
- 27.Mills K H G, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen R L, Pierce N F, Apple R T, Cray W C., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer’s patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986;153:1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- 29.Oxer M D, Bentley C M, Doyle J G, Charles I G, Makoff A J. High level heterologous expression in E. coli using the anaerobically-activated nirB promoter. Nucleic Acids Res. 1991;19:2889–2892. doi: 10.1093/nar/19.11.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearson G D, DiRita V J, Goldberg M B, Bokyo S A, Calderwood S B, Mekalanos J J. New attenuated derivatives of Vibrio cholerae. Res Microbiol. 1990;141:893–899. doi: 10.1016/0923-2508(90)90127-c. [DOI] [PubMed] [Google Scholar]
- 31.Pearson G D N, Mekalanos J J. Molecular cloning of Vibrio cholerae enterotoxin genes in Escherichia coli K12. Proc Natl Acad Sci USA. 1982;79:2976–2980. doi: 10.1073/pnas.79.9.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redhead K, Watkins J, Barnard A, Mills K H G. Effective immunity against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect Immun. 1993;61:3190–3198. doi: 10.1128/iai.61.8.3190-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson S H. Animal models in cholera research. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: ASM Press; 1994. pp. 203–206. [Google Scholar]
- 34.Stainer D W, Scholte M J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1971;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 35.Tacket C O, Losonsky G, Nataro J P, Comstock L, Michalski J, Edelman R, Kaper J B, Levine M M. Initial clinical studies of CVD112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J Infect Dis. 1995;172:883–886. doi: 10.1093/infdis/172.3.883. [DOI] [PubMed] [Google Scholar]
- 36.Viret J F, Cryz S J, Favre D. Expression of Shigella sonnei lipopolysaccharide in Vibrio cholerae. Mol Microbiol. 1996;19:949–963. doi: 10.1046/j.1365-2958.1996.435967.x. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Yamamoto M, Fujihashi K, van Ginkel F W, Noda M, Takeda Y, McGhee J. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]