Abstract
With the increasing global use of medical and adult recreational use of cannabis and cannabinoids, this chapter provides overview of evidence from animal and human studies on psychiatric disorders and cannabinoid receptors. We review and present evaluation of the relationship between changes in the ECS and psychiatric disorders. Evidence suggests the existence of a relationship between changes in components of the ECS, and some of the symptoms present in psychiatric disorders. Both CB1Rs and CB2Rs are components of the endocannabinoid system with different cellular and tissue localization patterns that are differentially expressed in the CNS and PNS and are emerging targets for the treatment of number psychiatric disorders. As cannabis preparations are widely used for recreation globally, it is predictable that cannabis use disorders (CUDs) will increase and there is currently no available treatment for CUDs. Although major advances have been reported from cannabinoid and ECS research, there are gaps in scientific knowledge on long-term consequences of cannabis use. Adolescent and cannabis use during pregnancy presents further challenges, and more research will uncover the signaling pathways that couple the gut microbiota with the host ECS. Development of cannabis and cannabinoid nanomedicine for nanotherapy will certainly overcome some of the shortcomings and challenges in medicinal and recreational use of cannabis and cannabinoids. Thus, nanotechnology will allow targeted delivery of cannabinoid formulations with the potential to elevate their use to scientifically validated nanotherapeutic applications as the field of cannabis nanoscience matures.
Keywords: Cannabinoid receptors, Endocannabinoids, Endocannabinoid system, Cannabis, Cannabinoids, Psychiatric disorders
9.1. Introduction
The shifting landscape on cannabis medicalization, legalization, and recreational use is due in part to advances in cannabis and cannabinoid molecular genetics, and the discovery of the endocannabinoid system (ECS) in human body and brain (Onaivi et al. 2012). Review of the accumulating scientific evidence from cannabis plant constituents, animal, and human studies reveals a previously unknown but ubiquitous and complex cannabinoid and ECS that is involved in almost all aspects of mammalian physiology and pathology (Joshi and Onaivi 2019). In this chapter, we review the growing awareness of the pharmacotherapeutic potential and limitation of targeting cannabinoid receptors (CBRs) and other components of the ECS in psychiatry. The components of the ECS are emerging as multifaceted therapeutic targets for cannabis constituents in a number of psychiatric and neurological disorders. The accruing evidence for the therapeutic efficacy of phytocannabinoids is promising for a number of psychiatric disorders. Although cannabis has been used for millennia, it is not benign, and there are side effects associated with use and exposure during pregnancy and in adolescents with psychiatric vulnerability. Furthermore, the type 1 cannabinoid receptors (CB1Rs) are now regarded as one of the most abundant G protein-coupled receptors (GPCRs) in the mammalian brain and have been extensively studied for their role in the biology of depression, pain, behavior, anxiety, neurodegenerative diseases, nausea, and substance abuse disorders. However, the neuronal function of type 2 cannabinoid receptors (CB2Rs) has been less investigated for central nervous system (CNS) function, because they were thought to be predominantly found in immune cells in the periphery and were called peripheral CB2Rs. With the increasing global use of cannabis and the risk of cannabis use disorders (CUDs), there are still lingering doubts, controversy, and debate about the functional neuronal localization and role of CB2Rs (Ghose 2009). The drawback from some previous studies has been the unsuccessful attempts by some groups to generate mice with selective deletion of CB2Rs from neurons and the generation of CB2R-GFP and CB2R-EGFP with peripheral CB2R promoter-driven transgenic reporter mouse line or other flaws in designs that detected microglial but not neuronal expression of CB2Rs (Ghose 2009; Lopez et al. 2018; Schmöle et al. 2015). Indeed, our studies provided the first evidence for neuronal CNS effects of CB2Rs, and its possible role in drug addiction, eating disorders, psychosis, depression, and autism spectrum disorders (ASDs), (Onaivi et al. 2006a; Onaivi et al. 2015; Onaivi et al. 2013; Onaivi et al. 2008; Ishiguro et al. 2007; Ishiguro et al. 2010a; Ishiguro et al. 2010b; Onaivi et al. 2011). Cell type-specific mechanisms of CB2Rs are unclear because the existing CB2R gene knockout mice are constitutive gene knock, with partial/incomplete deletion of CB2Rs, and are not suitable for tissue- and cell type-specific studies at molecular, pharmacological, and behavioral levels. Therefore, using Cre/Lox P technology, we created Cnr2-floxed mice to produce CB2R cKO, DAT-Cnr2, and Cx3cr1-Cnr2 mice with deletion of CB2Rs in dopamine neurons and microglia, respectively (Liu et al. 2017). Characterization of these mice provides further evidence for the involvement of CB2Rs in models of psychiatric function and disorders (Liu et al. 2017; Onaivi et al. 2018; Canseco-Alba et al. 2018a; Canseco-Alba et al. 2019; Canseco-Alba et al. 2018b).
With increasing global decriminalization and legalization of adult cannabis use, there are growing concerns for CUDs (Acheson and Fantegrossi 2019; Melis et al. 2017), especially as cannabis and cannabinoids including cannabidiol (Premoli et al. 2019) are thought to be safe in comparison with the effects of alcohol, tobacco products, and opioids. In light of the opioid epidemic in USA, there is distinct CNS localization of mu-opioid receptors—the target of opioids and CBRs—the target of cannabis and cannabinoids. CBRs both CB1Rs and CB2Rs are distributed in different areas of the brain; they are not in the pons and medulla oblongata, areas controlling breathing and respiration. This is why there are no cannabis overdosing resulting in respiratory depression or cardiovascular failure that is associated with the current opioid epidemic. This is because opioid receptors are abundant in the pons and medulla oblongata—brain areas involved in the control of respiration, and overdosing on opioids are associated with respiratory depression from opioid addiction. Thus, there is an increasing focus on the therapeutic effects (Premoli et al. 2019), of cannabis and cannabinoids, and targeting CBRs and components of the ECS in psychiatric disorders as reviewed in this chapter.
9.2. Endocannabinoid Signaling in Psychiatric Disorders
Disruption of the endocannabinoid system is associated with psychopathologies involved in psychiatric disturbances and neurological disorders (Onaivi et al. 2015). The ECS consists of CBRs that are activated by cannabinoids, endocannabinoids (eCBs), and their metabolic enzymes (Onaivi et al. 2012; Joshi and Onaivi 2019; Zou and Kumar 2019). Cannabinoids modulate signal transduction pathways associated with GPCRs, ionotropic, and nuclear receptors to exert their biological and therapeutic effects in psychiatry (Zou and Kumar 2019). G protein-coupled receptor, GPCR-CBR signaling activities in neuronal, glia cells, and ion channels have been demonstrated in vitro and in vivo models. A number of these studies provide CBR signaling network and pathways associated with mitogen-activated protein kinase (MAKP) signaling pathways including extracellular signal-regulated kinase 1/2 (ERK1/2), c-jun N-terminal kinase (JNK), p38, β-arrestins, and phosphatidy-linositol-3-kinases (PI3K) / protein kinase B (Akt) pathways. For example, CBR-mediated β-arrestin 1 and 2 translocation is a key mediator of GPCR desensitization and internalization that is species, subtype of CBRs, and agonist dependent (Zou and Kumar 2019; Ibsen et al. 2019). eCBs exert modulatory action on retrograde signaling and act as retrograde messengers at many synapses in the CNS. Some investigators have suggested GPR55 as a putative CB3R as it triggers distinct signaling pathways in response to inflammatory mediators. However, we and others have suggested that TRPV1 or VR1 be classified as CB3R, as anandamide is a CBR partial agonist, but a full agonist at the transient receptor potential cation channel subfamily V member 1, (TRPV1) also called vanilloid receptor 1 (VR1) (Joshi and Onaivi 2019). It turns out that 2-AG is associated with the effects of eCB-mediated retrograde signaling by cannabinoids as the level of 2-arachidonyl glycerol (2-AG) is about 1000 times more than anandamide (AEA) (Zou and Kumar 2019). Therefore, 2-AG serves as retrograde messenger at various types of synapses throughout the brain and is a high efficacy ligand for the translocation of β-arrestins (Ibsen et al. 2019). However, AEA has also been shown to contribute to eCB-mediated synaptic transmission with evidence supporting a tonic role of AEA (Figs. 9.1 and 9.2). The modulatory action of eCB-induced retrograde signaling on GABA-ergic and glutamatergic systems indicates that the main excitatory and inhibitory systems are in part under the influence of the ECS. The discovery that eCBs are principal mediators of retrograde synaptic communication demonstrates the pivotal role that eCBs play as retrograde messengers in GABA-ergic and glutamatergic synapses. One of the major advantages for the physiological actions of CBRs may be associated with the quick response needed for the sequestration of G-proteins and the retrograde signaling of eCBs on presynaptic CB1Rs to inhibit neurotransmitter release. Such retrograde action of eCBs on the inhibition of neurotransmitter release like GABA, glutamate, serotonin, dopamine is modified by CBRs and other components of the ECS that are molecular targets in psychiatric disorders. Depolarization-induced suppression of inhibition (DSI) / excitation (DSE) and in long-term depression (LTD) at both excitatory and inhibitory synapses provided evidence supporting retrograde eCB signaling system in the brain (Castillo et al. 2012; Ohno-Shosaku and Kano 2014).
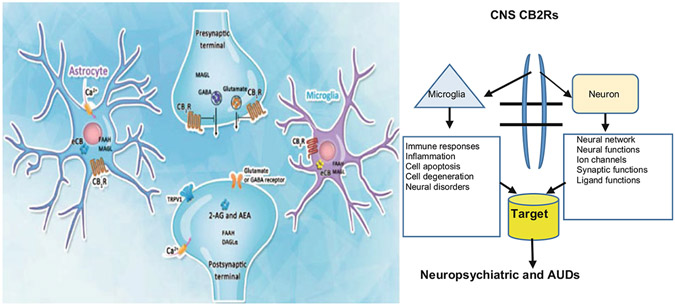
Fig. 9.1.
Schematic of ECS neuro-immune signaling in neuropsychiatric disorders. In our studies, we used both IBA1 and CD11b antibodies, as IBA1 is a good marker that does not cross-react with neurons and astrocytes, while CD11b is a good marker for changes in microglia morphology
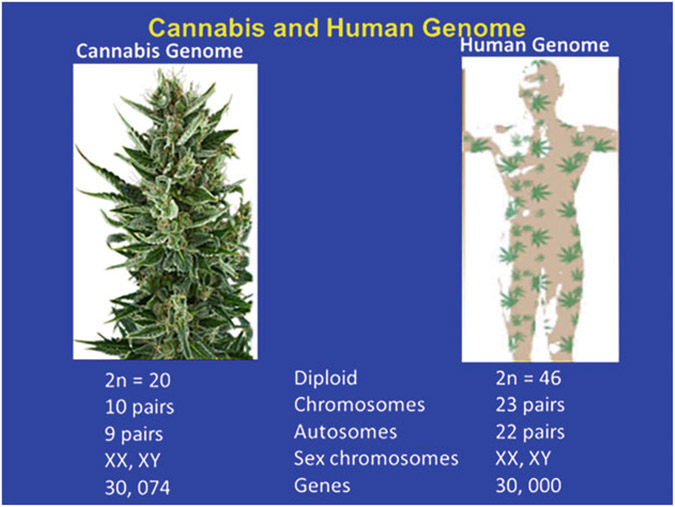
Fig. 9.2.
Cannabis and Human genomes. The decoding of the cannabis and human genomes with 10 and 23 chromosomal pairs with 9 and 22 autosomes, respectively, have similar sex chromosomes. There are 30, 074 genes in the decoded cannabis genome and 30, 000 human genes
Thus, eCBs are critically involved in the suppression of synaptic transmission and eCB-mediated communication between neurons and microglia. This is one of the current focuses of study with the identification of CB2R neuro-immune cross talk following conditional deletion of CB2Rs in microglia and dopamine neurons (Liu et al. 2017; Onaivi et al. 2018; Canseco-Alba et al. 2018a; Canseco-Alba et al. 2019; Canseco-Alba et al. 2018b). Therefore, existing evidence suggests that alterations in endocannabinoid signaling are present in a range of psychiatric disorders. Targeting components of ECS components provides therapeutic potential of cannabinoid medicines as CBRs and other components of the ECS that are involved in diverse neural, immune, function, and dysfunction (Robson 2014), in psychiatric disorders.
9.3. ECS Modulation of Neuro-Immune–Microbiome Cross Talk in Psychiatric Disorders
ECS components participate in numerous physiological processes that include immune and metabolic regulatory functions contributing to the maintenance of an organism’s homeostasis (Onaivi et al. 2012; Magid et al. 2019; Lin et al. 2013). Increased eCB levels and signaling have been linked with inflammation in animal models and human inflammatory disturbances and CBRs providing protective effects in the inflamed gut (Lin et al. 2013). New and improved knowledge has revealed gut-brain neural communication (Kaelberer et al. 2018), and the gut microbiome has been found to signal in part through the ECS network (Di Marzo 2018). Interactions between gut microorganisms and the ECS highlight a role in the gut microbiota-ECS axis (Lin et al. 2013; Kaelberer et al. 2018; Di Marzo 2018; Cani et al. 2016). Thus, the gut microbiome and endocannabinoidome relationship seems to be bidirectional and their alterations have been linked with dysbiosis, increased microglial neuroinflammation in mental disorders, such as psychosis, depression, anxiety, and neurological disturbances (Di Marzo 2018; Cani et al. 2016; DiPatrizio 2016). CBRs and associated orphan and putative cannabinoid GPCRs are expressed at high levels in the immune and/or central nervous systems (CNS) and regulate a number of neurophysiological processes, including key events involved in neuroinflammation. As such, these receptors have been identified as emerging therapeutic targets for a number of brain disorders in which neuroinflammation is a key feature, including multiple sclerosis (MS) and Alzheimer’s disease (AD). There is increasing attention on the role of the ECS components in mediating immune function with a focus on the immune processes that contribute to neuroinflammatory conditions. The gut microbiota plays a critical role in immune system function and regulation of gastrointestinal activities (Kaelberer et al. 2018). Blockade of the CB1R was reported to alter gut microbiota and attenuates inflammation and diet-induced obesity (Mehrpouya-Bahrami et al. 2017).
Microglia cells are the brain’s innate immune cells and primary contributors of CNS neuroinflammatory responses. Microglia-mediated neuroinflammation has been implicated in the progression of neuropsychiatric disorders. Neuro-immune signaling is emerging as a contributor to a number of neuropsychiatric disorders and stress increase in brain levels of known innate immune signaling molecules (Crews et al. 2017). Neuroinflammation is also emerging as a key component in the effects of CB2Rs, expressed in macrophages, microglia, and neurons (Fig. 9.1) that are also key regulators of the immune response (Onaivi et al. 2012). eCB-mediated communication between neuron and neuroglia shows that microglial cells and astrocytes are able to produce eCBs (Zou and Kumar 2019). After conducting the initial pioneering work that culminated in the discovery of functional neuronal CB2Rs (Onaivi et al. 2006b), we have now created and generated mice with cell type-specific deletion of CB2Rs to continue to move the field forward. This will allow us to investigate the cell type-specific functional roles of CB2Rs using DAT-Cnr2 and Cx3Cr1-Cnr2 cKO mice to determine the neuro-immune basis of CB2R activity in alcohol preference and consumption. With accumulating evidence of an interaction between the immune system, the gut microbiota, and the ECS, we have initiated studies to determine the role of neuro-immune-microbiome endocannabinoid axis in mouse CNS models to identify pro- and antiinflammatory effects of CBRs. Others have successfully generated Syn-Cnr2 cKO mice in which synaptic deletion of CB2Rs was shown to mediate a cell type-specific plasticity in the hippocampus (Stempel et al. 2016), and from our recent reports (Liu et al. 2017; Canseco-Alba et al. 2019; Onaivi et al. 2006b).
9.4. Cannabinoid Receptors in Psychiatric and Neurological Disorders
The medical use of cannabis and cannabinoids targeting the CBRs and other components of the ECS had been controversial because evidence of the efficacy to manage many disease conditions was often lacking. There is however increasing evidence-based research to support CBRs in psychiatric, neurological, and other medical indications and Food and Drug Administration (FDA-approved indications) (Kill 2019; Rubin 2018). Furthermore, there are now approved FDA indications supported by high-quality emerging evidence, and current claims and uses for which there is inadequate evidence (Kill 2019). The conclusions of National Academies of Sciences, Engineering and Medicine on the current state of evidence on the health effects of cannabis and cannabinoids provide support for the legitimate study, regulation, and prescription of therapeutic cannabinoids (National Academies of Sciences, Engineering, and Medicine 2017). Not surprisingly, based on positive randomized clinical trials, dronabinol and nabilone have FDA approval for chemotherapy-induced nausea and vomiting and appetite stimulation in conditions that cause weight loss such as in HIV-AIDS. FDA also recently approved cannabidiol for the management of Dravet syndrome and Lennox-Gastaut syndrome that are pediatric epilepsies (Devinsky et al. 2017; Thielle et al. 2018). Thus, disruption of CBRs and endocannabinoid signaling is implicated in an array of psychopathologies ranging from autoimmune diseases associated with neuropsychiatric mental disturbances like anxiety, Autism spectrum disorders (ASDs), depression, insomnia, psychosis, addiction, and neurological disorders such as Alzheimer’s, epilepsy, multiple sclerosis, Parkinsonism, stroke, traumatic brain injury. However, acute or chronic activation of CBRs can produce neurologic adverse effects including impaired learning, memory, attention, and motor coordination, while chronic use can lead to cannabis use disorders (CUDs) in vulnerable individuals (Kill 2019). It is also important to consider the effects of cannabis use in those with mental illness and individuals predisposed to developing addictive disorders (Lowe et al. 2019).
9.4.1. Role of CBRs in Anxiety-Related Disorders
Anxiety disorders are a common global mental illness characterized by feelings of fear and anxiogenesis, and the ECS system is involved in the bidirectional regulation of neural anxiety circuits and behavior (Yin et al. 2018). Anecdotally, different subjective effects have been reported in individual who have smoked marijuana, and in some instances, the opposite have been reported. This is not surprising as numerous constituents of the cannabis plant, not only induce biphasic dose-response profile, with low and high doses producing anxiolysis and anxiogenesis, but also have antagonistic effects (Yin et al. 2018; Onaivi et al. 1990). Therefore, systematic research investigating the interactions between CBR activity and which cannabis constituent or mixtures mediates anxiolysis remains an area of study with the changing legal status of medicinal and legal use of cannabis. However, advances and progress in ECS and cannabinoid research using transgenic mouse models and proxy methods of accessing states are providing the underlying mechanisms and brain circuits associated with expression of the different CBR subtypes and ECS machinery involved in the mediation of anxiety. For example, CB1Rs are densely expressed pre-synaptically and involved in retrograde signally associated with the inhibition of neurotransmitter releases, whereas our recent studies with mice with selected deletion of CB2Rs from dopamine neurons showed a reduced anxiety-like behavior in the plus maze and two-compartment black and white models of anxiety (Liu et al. 2017). Impaired 2-AG signaling in hippocampal glutamatergic neurons has been reported to affect anxiety-like behavior (Guggenhuber et al. 2015). CBRs have been identified in the insular cortex (IC) of rodents and humans. IC hyperactivity and its connectivity to amygdala have been linked with affective and anxiety disorders (Andrade et al. 2019). Studies in humans have investigated THC and cannabidiol (CBD) and have reported opposing effects, with higher doses of THC producing anxiogenic and CBD producing anxiolysis and counteracting the effects of THC (Andrade et al. 2019; Papagianni and Stevenson 2019). Anxiety- and other trauma-related disorders are common psychiatric disturbances with inadequate therapeutic options. These anxiety disorders include generalized anxiety, panic, social anxiety, phobias, and separation anxiety, with post-traumatic stress disorder (PTSD) and obsessive-compulsive disorder. The current treatment approaches with benzodiazepines and selective serotonin reuptake inhibitors (SSRIs) have limitations and side effects. Preclinical and ongoing clinical studies suggest that anxiety and related disorders are associated with decreased eCB tone and that CBRs in the brain are involved in the anxiolytic effects of cannabinoids (Papagianni and Stevenson 2019; Sloan et al. 2018; Korem et al. 2016). Therefore, more research is needed to elucidate the constituents of cannabis and what ECS components can be targeted for the therapeutic potential for the treatment of anxiety disorders. Limited positron emission tomography (PET) studies using available radiotracers in humans have revealed dysregulation of CB1Rs. The development of radiotracers for other components of the ECS may reveal novel targets in a number of psychiatric and neurological disorders in anxiety (Papagianni and Stevenson 2019; Sloan et al. 2018). There has also been recent focus on CBD for a number of neuropsychiatric disorders with some success in pediatric epilepsies, and many trials have begun for the treatment of other neuropsychiatric diseases. CBD appears to be a multi-target drug, and the molecular mechanism (s) of action of CBD in many of the psychiatric disorders are of major research efforts (Premoli et al. 2019). In vivo imaging is a powerful way to quantify CB1R radioligand binding in patients. A study looked at the CB1R radioligand, [11C] OMAR, in patients with PTSD using PET. In the PTSD group, [11C]OMAR volume of distribution was increased compared to healthy controls and trauma-exposed controls. Interestingly, but also consistent with the fact that women are more prone to anxiety disorders, this finding was more pronounced in women. Peripheral anandamide concentrations were lower in the PTSD group compared to healthy and trauma controls, which may be involved in why there was receptor upregulation in the PTSD group. Peripheral cortisol levels in the PTSD group and trauma control groups were lower compared to the healthy control. These findings may pave way for understanding and determining if medical marijuana is efficacious for PTSD. In some states, medical marijuana is approved for PTSD and some studies indicate symptomatic improvement from this treatment. Such data would support the notion that marijuana can provide symptomatic relief due to decreased peripheral anandamide concentrations along with CB1R upregulation.
9.4.2. Role of CBRs in Depression
Neuroanatomical distribution of CBRs and components of the ECS in neural circuits associated with processing and regulation of human emotion have been linked to the formation and development of depression (Zhou et al. 2017; Arjmand et al. 2019; Ishiguro et al. 1836; Ibarra-Lecue et al. 2018). Evidence from preclinical and clinical studies indicates an impairment of the ECS pathway in animal models and in patients with depression (Poleszak et al. 2018). Pathway analysis following a bipolar disorder genomewide association study identifies and implicates ECS gene sets, indicating that bipolar disorder is part of a spectrum of highly correlated psychiatric and mood disorders. Further evidence from patients with bipolar disorders indicates a putative role of CB2Rs (Zhou et al. 2017; Arjmand et al. 2019; Ishiguro et al. 1836; Ibarra-Lecue et al. 2018). Although most studies have focused on CB1Rs, our studies have implicated the involvement of CB2Rs in rodent models of CNS disorders and in human subjects with psychiatric disorders like substance abuse, depression, and psychosis (Onaivi et al. 2008; Ishiguro et al. 2007; Ishiguro et al. 2010a). There are other findings and reports showing alteration in eCB levels, metabolizing enzymes, and CBRs in patients with psychiatric disorders. Table 9.1 shows polymorphisms in the CB1R and CB2R genes that are associated with psychiatric disorders. CBRs and components of the ECS influence the activity and function of neural circuits associated with hypothalamic-pituitaryadrenal (HPA) axis, involved in affective behaviors. The use of CB1R antagonist as anti-obesity medication was withdrawn due to its anxiety-, depression-, and suicide-inducing effects in some patients. This revealed a role of CB1Rs in depression (Ibarra-Lecue et al. 2018; Onaivi 2010). Polymorphisms in eCB metabolizing enzymes along with alterations in eCB levels have also been implicated in depression. Furthermore, the ECS has been shown to modulate multiple monoaminergic systems, which can also influence mood and cognition. The raphe nuclei, locus coeruleus, and ventral tegmental area are targets of many classes of pharmacological agents such as selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs). All of these areas and many of their inputs and outputs are modulated by the ECS. Therefore, it is no surprise that the ECS may have potential therapeutic effects in depression and other mood disorders. CB1R density and their downstream signaling were increased in the dorsolateral prefrontal cortex (DLPFC) in postmortem subjects with depression-related suicides. The increased levels of CB1R-mediated signaling using a [35S]GTPγS binding assay in suicide subjects were comparable to controls (Hungund et al. 2004). These results may point to a potential therapeutic treatment in depression and suicidal patients. Although many studies have looked at CB1Rs, exciting studies are now emerging looking at central CB2Rs in depression and anxiety. A recent study investigated a Cnr2 knockout mouse using assays for depressive behavior, anxiety, and motor activity. Using an immune stressor, poly I:C, heterozygous Cnr2-KO mice experienced significantly decreased amount of time spent in the open arms of the zero maze compared to saline-treated controls, indicating an increase in anxiety-like behavior. Furthermore, anxiety-like behavior was further bolstered in the rotated pulley model, which was induced by poly I:C, showed that the Cnr2-KO mice had a higher number of pulley rotations compared to controls (Ishiguro et al. 2018). ECS involvement in depression presents components of the ECS as therapeutic targets for the development antidepressants (Poleszak et al. 2018).
Table 9.1.
Endocannabinoid system and CNR gene polymorphisms in psychiatric disorders
| CNR Genes Polymorphism (Onaivi et al. 2013) | Linkage or Association |
|---|---|
| CB2R | Associate with mouse model of impulsivity behavior |
| CNR2 (Q63R) but not (H316Y) | Associated with alcoholism and depression |
| CNR2 (rs41311993) | Associated with bipolar disorder |
| CNR2 (FAAH C385A) | Associated with childhood trauma, anxiety, and depression |
| CNR2 (R63Q) | Associated with childhood trauma, anxiety, and depression |
| CNR1/ FAAH gene | Associated impulsivity and marijuana use |
| CNR1 rs806375, rs806371, rs806368 | Associated with drug addiction |
| CNR1 rs806380, rs806368, rs754387 | Associated with cannabis dependence |
| 1359 G/A CNR1 variant | Associated with alcohol dependence |
| 1359 G/A CNR1 variant | Not associated with Tourette syndrome |
| 1359 G/A CNR1 variant | Not associated with alcohol withdrawal tremens |
| CNR1, FAAH, DRD2 gene | Associated with comorbidity of alcoholism & antisocial |
| CNR1 SNPs | No association with anorexia nervosa |
| CNR1 (AAT)n repeats | Associated with restricting and binging/purging anorexia nervosa |
| CNR1 (AAT)n repeats | Associated with depression in Parkinson’s disease |
| CNR1 SNPs | Associated to striatal responses to facial expression |
| (AAT)n repeats | Association with attention deficit hyperactivity disorder (ADHD) in alcoholics |
| CNR1 SNP haplotype | Risk factor for ADHD and post-traumatic stress disorder (PTSD) |
| 1359 G/A CNR1 variant | Associated with schizophrenia |
| (AAT)n repeats | Not associated with schizophrenia and mood disorders |
| (AAT)n repeats | Associated with schizophrenia |
| (AAT)n repeats | Associated with hebephrenic schizophrenia |
| CNR1 rs6454674 | Associated with schizophrenia severity |
| CNR1 variants | Associated with depression and anxiety |
| CNR1 variants and (AAT)n repeats | Associated with impulsivity |
| 1359 G/A CNR1 tag SNP | Associated with antipsychotic response but not schizophrenia |
| CNR1 SNPs | No association with cognitive impairment in MS |
| CNR1rs12720071 | Associated with cognitive performance in schizophrenia |
| CNR1 rs1049353 | Associated with reduction in caudate volume in psychosis |
| CNR1 rs2023239 | Associated with a protective effect against lifetime MDD |
The above includes gene polymorphisms catalog indicating inconsistencies in variation of the ECB system CB1R and CB2R genes in some neuropsychiatric disturbances. Further studies with more number of participants from different ethnic backgrounds are required, with consideration of epigenetic and exposomic factors (Onaivi et al. 2013)
9.4.2.1. Role of CBRs in the Antidepressant Action of Ketamine
The recent approval by the FDA for a nasal ketamine spray (esketamine; Spravato) for treatment-resistant depression has made the inquiry into its mechanism even more pressing. A recent study probed into the possibility of the ECS and its interaction with ketamine in relation to depression in mice. First, they injected 5 and 10 mg/kg of ketamine, which reduced immobility time in the forced swim test (FST), compared to control, which shows an antidepressant effect. A CB1R agonist, ACPA, and an antagonist, AM251, were also used. Administration of ACPA showed decreased immobility time compared to control in the FST, indicating an antidepressant effect. Interestingly, when administering combined non-effective doses of AM251 or a CB2 inverse agonist, AM630 with non-effective doses of ketamine resulted in antidepressant effects compared to control (Khakpai et al. 2019). The link with the ECS might partially explain the mechanism behind ketamine’s antidepressive effects. Upon activation of the CBRs along with the retrograde signaling associated with the modulation of other neurotransmitters, the hypothalamic-pituitary-adrenal axis is associated with providing therapeutic benefits (Onaivi et al. 2015; Onaivi et al. 2013). This is the bases for the modulation of the ECS as therapeutic targets, or as adjunctive treatment in generalized depression. While the role of the ECS in depression is incompletely understood, a number of anecdotal reports and individual variation in antidepressant and depression-like symptoms after consumption of cannabis have been reported (Onaivi et al. 2015; Onaivi et al. 2013).
9.4.3. Role of CBRs in Schizophrenia
Schizophrenia also known as psychosis is a devastating psychiatric syndrome affecting 1% of the world population without a cure, and antipsychotic medications are not effective in all patients. Despite the efforts to elucidate the causes of schizophrenia, the etiopathogenesis remains elusive (Ibarra-Lecue et al. 2018). In vulnerable individuals, the use of cannabis has been known to precipitate psychotic episodes. Alterations in the ECS components in the brains of patients with schizophrenia have been reported (Castillo et al. 2012), and CB1Rs have been implicated in many psychiatric disorders and recent advances have sparked more interest into the ECS role in schizophrenia. As discussed above, ECS system components are expressed in the immune and/or central nervous systems (CNS) and regulate a number of neurophysiological processes, including key events involved in neuroinflammation. ECS interaction with neuroglia changes has been implicated in the pathobiology of schizophrenia. Results on the changes and alterations in CBRs from imaging and postmortem studies of brains from schizophrenia patients compared to controls have yielded inconsistent data with either increase or decrease in CB1R and gene expression in brain areas involved in schizophrenia (Ibarra-Lecue et al. 2018). Many factors have been considered for the inconsistent results when studying CBRs and other components of the ECS in brains of schizophrenics, including age, sex, and the subtypes of schizophrenia. The anterior cingulate gyrus in patients with schizophrenia, bipolar disorder, and major depression were evaluated in postmortem brains, and CB1R immunohistochemical stain was used. In the major depression group, the intake of SSRIs reduced the density of cortical CB1R immunoreactive neurons compared to control. In bipolar disorder, patients taking first-generation antipsychotics had reduced glial CB1R immunoreactive density (Koethe et al. 2007). The results from CB1R changes in different schizophrenic brain regions, provided an indication for the involvement of CB1Rs in this pathology (Ibarra-Lecue et al. 2018). The role of CB2Rs in schizophrenia has not been well investigated when compared to CB1Rs. This was because many investigators were not able to detect the presence of neuronal CB2Rs in healthy brains, and therefore, their role in neuropsychiatric disorders has been much less well characterized. We and others, and many recent studies have reported the discovery and functional characterization of neuronal brain CB2Rs. Indeed, our studies provided the first evidence for neuronal CNS effects of CB2Rs and its possible role in schizophrenia and neuropsychiatric disorders (Onaivi et al. 2012; Onaivi et al. 2015; Ishiguro et al. 2010a; Ishiguro et al. 1836). It must be noted and acknowledged as discussed below that the role of CB2Rs in CNS disturbances involving neurodegenerative diseases associated with neuroinflammation and neuropathic pain has been extensively reported (Hill et al. 2012; Russo 2018; Basavarajappa et al. 2017). CB2Rs in glial cells are modulators during inflammatory conditions. Therefore, modulation of eCB signaling in glia cells may provide pharmacological targets to treat and prevent neuroinflammatory response (Fig. 9.1, and Fig. 9.2), white matter deficits, and pathological mechanisms seen in schizophrenia (de Aleida and Martins-de-Souza 2018).
In our ongoing studies, many features of CBR gene structures, SNPs, copy number variations (CNVs), CPG island, microRNA regulation, and the impact in neuropsychiatry and where possible in rodent models are evaluated. Accumulating evidence suggests the importance of CNVs in the etiology of neuropsychiatric disorders. The clinical consequences of CNV in the coding and non-coding CNR gene sequences associated with human phenotypes and disorders are mostly unknown and under investigation. With advances in genomic technologies and the analysis and identification of CNR gene CNVs may uncover the relationship (if any), between CNR gene CNVs to phenotype and disease. While CNR1 and CNR2 SNPs have been associated with a number of neuropsychiatric disorders (see Table 9.1), it is unclear to what extent CNR gene CNVs are involved in psychiatric disorders. Therefore, more studies are needed to determine the role and contribution of CNR gene CNV to conditions of endocannabinoid dysregulation in psychological and psychiatric disorders. However, a number of focused studies on the polymorphisms in components of ECS have shown that CNR1 and limited studies for CNR2 genes (Table 9.1) contribute to the pathogenesis of specific subtypes of schizophrenia (Onaivi et al. 2013; Ishiguro et al. 2010a; Ibarra-Lecue et al. 2018). We investigated genetic associations between CNR2 gene polymorphisms and schizophrenia in a selected population, and the results from our studies identified that polymorphism in CNR2 gene (Table 9.1) with low CB2R expression and function indicates an increased risk of schizophrenia (Ishiguro et al. 2010a) when combined with other risk factors. Other studies have evaluated eCB levels and eCB enzymes in schizophrenic patients with reports of alterations in 2-AG metabolizing enzyme and in eCB levels in patients with schizophrenia. These findings warrant further investigation into ECS changes and functional implications of ECS in schizophrenia subtypes. Identification of the roles of ECS components may be valuable in designing new medication targets in the treatment of schizophrenia.
9.4.4. Role of CBRs Addictive Disorders
Addictive disorders are now not only limited to drugs of abuse, with the emergence of digital technology, but also to cell phone, various gaming platforms, and food and gambling. Generally, therefore addictive disorders are chronic relapsing disorders characterized by impulsivity and compulsive disorders that are known to be present in psychiatric (De Luca and Fattore 2015; Onaivi 2008; Malloy-Diniz et al. 2007) and addictive disorders. There is accumulating evidence indicating a central role for this previously unknown but ubiquitous ECS in the regulation of the rewarding effects of abused substances. Thus, an endocannabinoid hypothesis of drug reward and addiction was postulated (Onaivi 2008). eCBs mediate retrograde signaling in neuronal tissues and are involved in the regulation of synaptic transmission to suppress neurotransmitter release by the presynaptic CBRs. This powerful modulatory action on synaptic transmission has significant functional implications and interactions with the effects of abused substances (De Luca and Fattore 2015; Onaivi 2008). In humans, recent studies have revealed diverse responses by the ECS to long-term exposure to several drugs of abuse, and cannabis, ethanol, opioids, nicotine, and cocaine were found to alter the ECS regardless of their diverse pharmacological mechanism of action. Our data, along with those from other investigators, provide strong new evidence for a role for ECS modulation in the effects of drugs of abuse, and specifically for involvement of CBRs in the neural basis of addiction (De Luca and Fattore 2015; Onaivi 2008; Malloy-Diniz et al. 2007). We suggested that cannabinoids and eCBs appear to be involved in adding to the rewarding effects of addictive substances, including, nicotine, opiates, alcohol, cocaine, and BDZs. The results suggest that the ECS may be an important natural regulatory mechanism for drug reward and a target for the treatment of addictive disorders. In animal models, the ECS appears to be also involved in the ability of drugs and drug-associated cues to reinstate drug-seeking behavior in animal models of relapse (De Luca and Fattore 2015). As shown in Table 9.1, polymorphisms in the CB1R and CB2R genes have been associated with substance dependence and drug-related behaviors (De Luca and Fattore 2015; Onaivi 2008). Consequently, the ECS is involved in reward mechanisms that facilitate the hedonic value of natural and drug rewards (De Luca and Fattore 2015; Onaivi 2008). This system participates in the primary rewarding effects of cannabinoids, nicotine, alcohol, and opioids and in the common mechanisms, underlying drug addiction and relapse to drug-seeking behavior. In turn, many drugs of abuse, including cannabinoids, opioids, and alcohol, and nicotine, can alter differently the levels of eCBs in selected brain regions (De Luca and Fattore 2015). Therefore, substantial data now point to a role for the ECS in triggering and/or preventing reinstatement of drug-seeking behavior. It appears that the effects of perturbation of the ECS by drugs of abuse can be ameliorated by restoring the perturbed system using cannabinoid ligands. It is not surprising that CB1R antagonists were briefly approved as an anti-obesity medication in Europe and its potential promise in the reduction in drug use, in smoking cessation, and reduction in alcohol consumption. Nevertheless, due to serious side effects of depression and suicide, rimonabant was withdrawn from use (Onaivi 2010). The promiscuous action and distribution of CBRs in most relevant biological systems provide the EPCS with limitless signaling capabilities for cross talk within, and possibly between, receptor families, which may explain the myriad behavioral effects associated with smoking marijuana. The ECS therefore appears to play a central role in regulating the neural substrate underlying many aspects of drug addiction, including craving and relapse. The findings that the ECS is involved in the reinstatement model provided evidence of the ECS in the neural machinery-underlying relapse. In summary, there is a lot more to learn and research to be done to better understand the nature and neurobiology of the eCB physiological role in addictive- and feeding-related disorders.
9.4.5. Role of CBRs in Other Psychiatric Disorders: Autism Spectrum Disorders (ASDs) and Attention Deficit Hyperactivity Disorders (ADHD)
Several studies highlight a key involvement of ECS in ASD pathophysiology (Krebs et al. 2019; Crume et al. 2018; Gunn et al. 2016; Chakrabarti et al. 2015; Maccarrone et al. 2010; Zhang and Alger 2010; Zamberletti et al. 2017; Zou et al. 2019; Brigida et al. 2017; Crespi et al. 2010). In addition to autism, the ECS is also involved in several other psychiatric disorders (ADHD, anxiety, major depression, bipolar disorder, and schizophrenia). ECS is a key regulator of metabolic and cellular pathways involved in autism, such as food intake, energy metabolism, and immune system control. ASD is also characterized by immune system dysregulation. The mRNA and protein for CB2R and ECS enzymes were significantly dysregulated, further indicating the involvement of the ECS in ASD-associated immunological disruptions. Alterations in eCB signaling in neurodevelopmental disorders may be associated with exposure to cannabis and cannabinoids in utero or during adolescence (Krebs et al. 2019; Crume et al. 2018; Gunn et al. 2016). The use of cannabis during pregnancy may increase adverse outcomes for women and their neonates. As the use of cannabis continues to gain social acceptance, pregnant women and their medical providers could benefit from health education on potential adverse effects of use of cannabis during pregnancy. Babies exposed to cannabis while in the womb may suffer significant and permanent changes in neuroplasticity that could alter brain maturation and cause long-lasting changes that persist in the adult brain (Krebs et al. 2019; Crume et al. 2018; Gunn et al. 2016). This is because exogenous cannabinoids interfere with eCB regulation of brain maturation during adolescence. The role of ECS in ASD is a relatively understudied topic. Some studies and hypotheses may lead us to think the ECS plays a role in the multiple aspects of ASD such as social reward responsivity, circadian rhythm, anxiety-related symptoms, and neuronal development (Chakrabarti et al. 2015). The existing evidence shows potential ECS involvement in fragile X syndrome, in which 10–30% of patients are also diagnosed with ASD. In the highly characterized fragile X mental retardation (Fmr1) knockout mice, some studies showed that ECS-mediated responses of GABAergic synapses are increased in the dorsal striatum and hippocampus in the Fmr1-KO mouse model. In the hippocampus, this effect was indirect via group I mGluR activation which can upregulate the levels of eCBs (Maccarrone et al. 2010; Zhang and Alger 2010). Other studies report the presence of alterations in the ECS as well as the effects of its pharmacological manipulations in animal models of ASD-like behaviors (Onaivi et al. 2018; Zamberletti et al. 2017; Zou et al. 2019). In our studies, we used the BTBR T + tf/J mice that have been shown to exhibit autism-like behavioral phenotypes. The data indicated the BTBR mice have an abnormal regulation of dopamine functioning with an upregulated CBR2A gene expression in naïve BTBR mouse model of ASD. These results along with our findings indicating an increased risk of schizophrenia in patients with low CB2R function (Ishiguro et al. 2010a), which is in agreement with the hypothesis that autism and schizophrenia represent diametric conditions (Crespi et al. 2010). Moreover, more research needs to be done to understand the nature of the neurochemical changes recorded in our preliminary study in the hippocampus, striatum, and frontal cortex, where the levels of dopamine and serotonin and their metabolites were differentially altered in the BTBR and C57BL/6 J mice. Thus, our data provide a basis for further studies in evaluating the role of ECS and monoaminergic systems in the etiology of ASDs. Collectively, the findings to date indicate that the ECS plays a key role in the pathophysiology of ASD and may provide new insights into potential interventions and could represent a novel target and strategy for ASD pharmacotherapy.
9.5. CBRs and Comorbidity between Psychiatric Disorders and Neurological Disturbances
Several lines of evidence suggest a primary function and involvement of components of the ECS in the degenerative process (Basavarajappa et al. 2017). Psychiatric disorders are common in many neurological disorders, including epilepsy, migraine, Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntingtin disease (HD), Multiple sclerosis (MS), Brain tumors (BT), essential tremor (ET), and cerebral palsy (CP), Tics and Tourette’s syndrome (TS), epilepsy, migraine, and stroke (Hesdorffer 2016). Traumatic brain injury (TBI) is a common injury characterized by a change in brain function after an external blow to the head and is highly comorbid with psychiatric disorders. TBI is associated with substance abuse, problem gambling, psychological distress, risk-taking, and impulsivity and especially antisocial both violent and non-violent (Vaughn et al. 2019; Turner et al. 2019). Our current knowledge on the emerging role of the ubiquitous ECS in neuro-immune-microbiome cross talk provides targets to study comorbidity between psychiatric disorders and neurological illness. However, these comorbidities increase disease burden and may complicate the treatment of the combined disorders. Initial studies of the comorbidity of psychiatric and neurological disorders were cross-sectional, and time order of the associations was impossible to elucidate. Work that is more recent has clarified time associations between psychiatric disorders and neurological disorders, particularly in epilepsy and stroke where epidemiological evidence suggests that there is a bidirectional relationship. Although these relationships are understood in many neurological disorders, routine screening for psychiatric disorders in neurological disorders is infrequent. The brief summary below capitulates the contribution of the ECS to the development of neurodegenerative generative and other neurological disorders in a number of animal models and human studies. With the ubiquitous distribution of all components of the ECS in most cells and tissues in man and mouse, changes in the ECS machinery have been reported in brain areas associated with the symptoms of AD. Studies indicating alterations of specific component (s) in ECS cellular, and molecular machinery in brain circuits underlying the symptoms in HD, PD, MS, TBI, BT, ET, CP, TS, epilepsy, migraine, and stroke have been reported (Hill et al. 2012; Russo 2018; Basavarajappa et al. 2017). For example, there is evidence to suggest that synthetic and natural cannabinoids may help patients with Alzheimer’s disease-related aggression and agitation. Since AD is a disease of the elderly, many medications are limited to control certain symptoms of AD because of their side effect profile and have not been overly beneficial. Cannabinoids have been of great interest in many neurodegenerative diseases since they pose relatively less risk for abuse and adverse effects. In a study that investigated CB1R and social interaction and aggression, they showed that grouped housed CB1R KO mice spent more time in threat behavior compared to grouped wild type mice. Along with this notion, the grouped CB1R KO had an increased time engaged in attack behavior with increased time in each attack compared to WT mice (Rodriguez-Arias et al. 2013). There is evidence to suggest that synthetic and natural cannabinoids may help patients with Alzheimer’s disease-related aggression and agitation (Liu et al. 2015). Since AD is a disease of the elderly, many medications are limited to control certain symptoms of AD because of their side effect profile and have not been overly beneficial. Cannabinoids have been of great interest in many neurodegenerative diseases since they pose relatively less risk for abuse and adverse effects.
There is growing awareness of the involvement of gut microbiome and inflammation in a number of psychiatric and neurological disorders. The ECS system interaction with the microbiome and neuroglia cells in the brain is providing new therapeutic targets but also in understanding underlying mechanism (s) associated with psychiatric and neurological disorders. With the increasing use of cannabis and cannabinoids, an ECS pharmacogenomic test on individualized basis could be used for diagnosis and whether or not medical cannabis can be of therapeutic benefit. Much more mechanistic studies using in vitro and in vivo techniques, need to be done to improve the detection and treatment of patients affected by neurological and psychiatric disorders when cannabis and cannabinoids are indicated. Cannabinoids and CBRs have the ability to control both anti-inflammatory, anti-oxidant, neuroprotective, and neuromodulatory functions. In addition, the ECS modulates other biochemical pathways that could complement their effects on other receptors, ion channels, and enzymes. Thus, the use of cannabinoids provides interesting, unique, and potential therapeutic investigations in psychiatric and neurological disorders. Therefore, an overwhelming number of studies now document CB2R expression in neuronal, endothelial, and glial cells. Mounting evidence also shows that CB2Rs and its gene variants may play possible roles in psychiatric and neurological disorders with associated inflammatory reactions.
9.6. CBR Polymorphisms and Epigenetic Mechanisms in Psychiatric Disorders
Table 9.1 presents a brief summary of genetic polymorphisms of cannabinoid receptor genes. Genetic polymorphisms of the endocannabinoid system, including CB1R, CB2R, and FAAH genes, have been linked or associated with a number of psychiatric and neurological disorders (Onaivi et al. 2015; Onaivi et al. 2013; Onaivi et al. 2008; Ishiguro et al. 2007; Ishiguro et al. 2010a). Studies show that single nucleotide polymorphisms (SNPs) of CNR1 and FAAH may contribute to drug addiction and other neuropsychiatric disorders. CBR gene variants may provide a deeper insight and novel targets for the effects of cannabinoids in drug addiction and other neuropsychiatric disorders. Variations in genes encoding cannabinoid receptors that are involved in drug addiction, and obesity, for example, provide therapeutic targets in endocannabinoid insufficiency syndrome (Onaivi 2010). Previously, we demonstrated the characteristic features in CB1R gene, and such features and variations in CB2R gene have not been fully characterized (Zhang et al. 2004). Many studies have reported that variations in CB1R genes are linked to drug addiction vulnerability in different ethnic groups (Zhang et al. 2004), and some of these variations are associated with mental and neurological disorders (Onaivi et al. 2015; Onaivi et al. 2013; Zhang et al. 2004). Rare genetic variants in CNR1 and DAGLA genes in neurological phenotypes were reported. In this study, variations in CNR1, CNR2, DAGLA, FAAH, and MGLL genes were studied for any associations with neurological phenotypes. They concluded from their study that mainly CNR1 and DAGLA were associated with neurological phenotypes, but not the other aforementioned genes. Of these genes, CNR1 were associated with migraines, memory disorders, and sleep disorders along with or without anxiety (Smith et al. 2017). These results and other similar studies provide insight into potential ECS treatments into many neurological and psychiatric disorders. Therefore, the study of the CBR genomic structure, and its polymorphic nature, subtype specificity and their variants, and associated regulatory elements that confer vulnerabilities to a number of neuropsychiatric disturbances, may provide a deeper insight into the underlining mechanisms. Thus, understanding the ECS in the human body and brain will contribute to elucidating this natural regulatory mechanism and provide potential therapeutic targets in health and disease. A preliminary study of ECS regulation showing distinct alterations of CNR1 promoter DNA methylation in patients with schizophrenia was reported. This study examined DNA methylation in peripheral blood mononuclear cells (PBMCs) in patients with schizophrenia, bipolar disorder, and major depressive disorder. The goal of the study was to measure the alterations of the promoter site of the CNR1 gene. The only significant changes were found in schizophrenia, but not any of the previously mentioned disorders. In human PBMCs, there was an upregulation of CNR1 and decreased CpG methylation in schizophrenic patients compared to control. These results were confirmed in an animal model of schizophrenia, which was done by administering prenatal methylazoxy-methanol (MAM) acetate that showed an increase in CNR1 expression in the PFC. There was also reduced DNA CpG site methylation in the promoter itself (D’Addario et al. 2017). In rodent studies, prenatal exposure to cannabis triggers epigenetic changes with possible transgenerational immunological consequences (D’Addario et al. 2017).
Epigenetic regulation of ECS components under both physiological and pathological conditions as well as the epigenetic changes induced by eCB signaling is an emerging potential target of ECS ‘epigenetic therapy’ (D’Addario et al. 2017; Parira et al. 2017; Zumbrun et al. 2015). Initial focus is to understand how CB1Rs evoke epigenetic mechanisms, either by directly interacting with the epigenetic machinery or by indirectly. In studies of histone modifications due to cannabinoid signaling, THC-modulated multiple histone modification sites like H3K4me3, H3K9me3, H3K27me3, and H3K36me3 in differentiating mouse lymph node cells showing histone modifications are associated with THC-mediated alterations in antigen-specific T-cell responses (Parira et al. 2017). Alterations in DNA methylation status to the effects of cannabinoids indicated that parental exposure to THC altered DNA methylation status of genes related to synaptic plasticity in rat nucleus accumbens (Parira et al. 2017). Another study done in mice showed that THC administration increased methylation at the promoter region of DNA methyltransferases 3A and 3B in myeloid-derived immune suppressor cells and correspondingly reduced expression of the same DNA methyltransferases (Parira et al. 2017). Furthermore, methylation at the promoter regions of Arg1 and STAT3 was decreased by THC, which led to further increases in levels of Arg1 and STAT3 expression. Arg1, which can metabolize L-arginine, suppresses T-cell function while increasing activation and function of these immunosuppressive cells. Cannabinoid signaling has also been shown to be associated with modulation in certain microRNA-based epigenetic mechanisms. In other studies, data from animal models suggest that in utero exposure to cannabinoids results in profound T-cell dysfunction and a greatly reduced immune response to viral antigens. Furthermore, evidence from animal studies indicates that the immunosuppressive effects of cannabinoids can be mediated through epigenetic mechanisms such as altered microRNA, DNA methylation, and histone modification profiles. Such studies support the hypothesis that that parental or prenatal exposure to cannabis can trigger epigenetic changes that could have significant immunological consequences for offspring as well as long-term transgenerational effects. Epigenetic effects of cannabinoids reveal the ability of cannabinoids to modify neuronal and immune cell functionality either via histone modifications like H3 lysine methylations or by altering DNA methylation (D'Addario et al. 2017; Parira et al. 2017; Zumbrun et al. 2015). A better understanding of the epigenetic regulation of eCB signaling as well as the eCB regulation of epigenetic mechanisms will be of great value for the possible design of more specific eCB epigenetic drugs. Therefore, more studies on the potential maternal and paternal transgenerational and/or epigenetic effects of cannabinoid abuse are needed with global changing landscape in recreational and medical use of cannabis and cannabinoids.
9.7. Emerging Trends in Targeting CBRs in Psychiatric and Neurological Disorders
Advances and new discoveries due to evidence-based in vivo and in vitro studies on molecular and cellular mechanisms of CBRs have implications in psychiatric and neurological disorders. These recent advances in understanding the biological actions of cannabis products are expanding the therapeutic indications and opportunities. Of course, there are continuing trials and tribulations in clinical trials of cannabinoid pharmacotherapy, leading to the withdrawal of CB1R antagonist in obesity and clinical trial tragedy of the FAAH inhibitor that was stopped. The involvement of the ECS in most biological systems shows their vital importance in homeostasis, and disruption in functioning of the ECS leads to catastrophic adverse effects, with suicides and brain-dead patients during clinical trials and use in obesity, respectively. One explanation for the lethal side effects is CBR ligand bias, which is beginning to be accessed (Laprairie et al. 2017a). Ligand-biased binding to its receptor shifts the equilibrium of receptor-dependent signaling toward other possible pathways. Apparently, the limited success of the development of cannabinoid-based therapeutics may be associated with ligand bias (Laprairie et al. 2017a). Progress in understanding of CBR structure and quantification of ligand bias, dimerization, and allosteric modulation of CBRs will optimize drug design, and selection of cannabinoids to match patient indication (Laprairie et al. 2017a; Callén et al. 2012; Alaverdashvili and Lapraire 2018; Tham et al. 2019; Laprairie et al. 2017b; Laprairie et al. 2019). Most cannabinoid drug development and targeting of orthosteric site on CB1R at which eCBs and THC bind. Adverse psychotropic effects limit the clinical utility of CB1R orthosteric agonists (Tham et al. 2019; Laprairie et al. 2017b; Laprairie et al. 2019). This has prompted the search and development of CB1R positive allosteric modulators (PAMs) that enhance orthosteric ligand binding, with improved reduced psychotropic side effects when used in psychiatric and neurological disorders (Tham et al. 2019; Laprairie et al. 2017b; Laprairie et al. 2019). Therefore, allosteric modulation of CB1R holds great therapeutic potential. This is because allosteric modulators do not possess intrinsic efficacy, but instead augment PAM or diminish negative allosteric modulation (NAM), the receptor’s response to endogenous ligand. Consequently, CB1R allosteric modulators have an effect ceiling, which allows for the tempering of CB1R signaling without the desensitization, tolerance, dependence, and psychoactivity associated with orthosteric compounds. Several challenges exist for the development of CB1R allosteric modulators, such as receptor subtype specificity, translation to in vivo systems, and mixed allosteric/agonist/inverse agonist activity. Despite these challenges, elucidation of crystal structures of CB1R and CB2R and compound design based on structure-activity relationships will advance the field. This recent work on the determination of the crystal structures of CB1R and CB2R (Fig. 9.3) reveals a yin-yang relationship and functional profile of CB2R antagonism versus CB1R agonism (Hua et al. 2016; Li et al. 2019). The formation of functional CB1R and CB2R heteromers in neuronal cells in the brain indicates that activation of either receptor leads to negative modulation of the partner receptor via heteromers (Callén et al. 2012). Dimerization of CBRs has therapeutic implication and impact on CNS function and it was suggested that CBR1-CB2R heteromers must be taken into account when designing therapeutic approaches toward alterations involving the ECS (Callén et al. 2012). The direct activation of CBRs results in several beneficial effects; therefore, several CBRs ligands have been synthesized and tested in vitro and in vivo, with disappointing advancement for clinical development due mainly to side effects on the CNS. However, other approaches are being developed for allosteric modulators that might offer a novel therapeutic approach to achieve potential therapeutic benefits avoiding inherent side effects of orthosteric ligands.
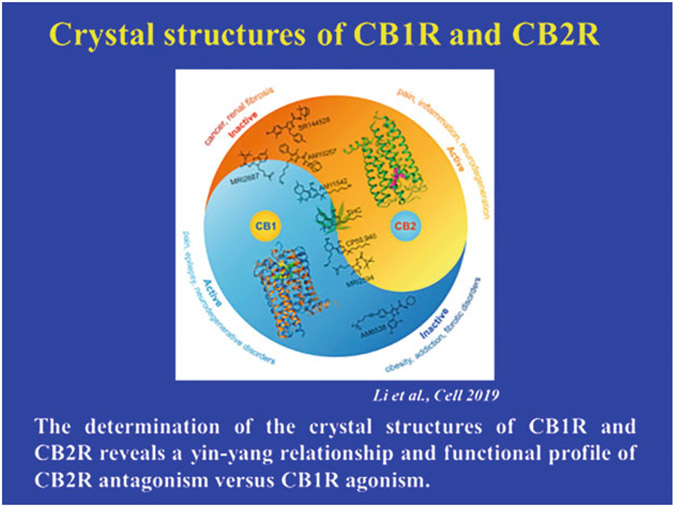
Fig. 9.3.
The determination of the crystal structures of CB1R and CB2R reveals a yin-yang relationship and functional profile of CB2R antagonism versus CB1R agonism (Hua et al. 2016; Li et al. 2019)
The evidence-based scientific knowledge supports novel approaches to cannabinoid-based medication and cannabis use in psychiatric disorders and medicine. With the rapidly expanding nanomedicine formulation of nanoparticulate cannabinoid products presents new opportunities and approaches for cannabis use in health and disease. Development of cannabis and cannabinoid nanomedicine for nanotherapy will certainly overcome some of the shortcomings and challenges in medicinal and recreational use of cannabis and cannabinoids. As cannabinoids are a class of lipophilic compounds, the use of different surfactants and delivery systems to improve cannabinoid solubility and enhance bioavailability must be considered. To overcome these limitations, nanoparticle formulations may offer an attractive alternative. In practice, this means that the cannabinoids will be ‘packed’ in endogenous nanoparticles, offering the opportunity to effectively deliver the cannabis and cannabinoid formulations to the diseased sites, by applying nanotechnological advances to nanomedicine. The application of nanotechnology to medicine involves employing nanoparticles not only to enhance the action of drugs, but also expected to improve diagnosis and therapy of diseases and reduce health care cost (Ngwa et al. 2017). Thus, nanotechnology will allow targeted delivery of cannabinoid formulations with the potential to elevate their use to scientifically validated nanotherapeutic applications as the field of cannabis nanoscience matures. In last decade, the success of new and creative nanosynthetic tools has fashioned new opportunities in drug design, which allows creation of drugs, prodrugs, or diagnostic gears at nanosize regime (Patra et al. 2018). Advances in nanoparticle formulations of cannabinoids as therapeutic molecules for different routes of delivery into peripheral, CNS, and wearable skin patches are being developed for nanomedicine outcomes. A number of ongoing studies are building nanoparticles for nanodelivery of cannabis, cannabinoids, and endocannabinoid system components as nanotherapeutics (Singh et al. 2018). Now it is possible to predictively synthesize nanosized objects with well-defined surface chemistry and predefined size and morphology, which permit certain degree of control over therapeutic outcomes and minimizes side effects of the drugs. Encapsulation strategies for cannabis products and edibles will require greater physical stability, protection against oxidation, and flavor masking using liposomes, micelles, polyplexus, polymersomes, and silica nanoparticles as the industry matures and develops. While research in nanoengineering area is still not sufficiently mature, but it is conceivable that the surface of nano-objects can be tailored to control the solubility of the drugs, ensuring effective circulation time, and limiting the biodistribution. In addition, this strategy allows one to control drug release, which in turn reduces and diminishes immunogenicity. One of the very promising strategies has been to conjugate the nano-object surface with microenvironment-specific and/or receptor-specific biomacromolecules such as peptides, proteins, aptamers. A very elegant review of such studies provides a comprehensive picture of accomplishments and bottlenecks in the research in this area (Spicer et al. 2018).
9.8. Current State of Evidence on the Health Effects of Cannabis and Cannabinoids
The conclusions of the National Academies of Sciences, Engineering and Medicine (National Academies of Sciences, Engineering, and Medicine 2017) provide support for the legitimate study, regulation, and prescription of therapeutic cannabinoids. Evidence supports reform to allow the legitimate study, regulation, and prescription of therapeutic cannabinoids (National Academies of Sciences, Engineering, and Medicine 2017). The following conclusions have invigorated the debate over cannabis use and present opportunities for quality improvement and approaches to bridge the gaps between safe cannabis products, science, and medicine:
a). Conclusive and substantial evidence that cannabis and cannabinoids are effective in nausea and vomiting and in multiple sclerosis spasticity, b). Moderate evidence that cannabis or cannabinoids are effective in sleep apnea, fibromyalgia, and some chronic pain, and acute cognitive impairment, c). Limited evidence that cannabis or cannabinoids are effective in Tourette syndrome, anxiety, dementia, glaucoma, HIV/AIDS, and appetite, and d). There is substantial evidence of a statistical association between cannabis use and increased risk of motor vehicle crashes, lower birth weight of the offspring exposed to cannabis and cannabinoids in-utero, and psychosis in vulnerable individuals.
9.9. Concluding Remarks
Existing evidence suggests that alterations in eCB signaling are present in a range of psychiatric disorders. Targeting components of ECS components provides therapeutic potential of cannabinoid medicines as CBRs and other components of the ECS are involved in diverse neural, immune, function, and dysfunction, in psychiatric disorders. The ECS evidently gives novel ideas and options in the field of antidepressant treatment; however, further studies are needed to determine which group of patients could benefit from this type of therapy. The ECS is also involved in the pathogenesis and treatment of depression, though its role in this psychiatric disorder has not been fully understood. Both the pro- and antidepressant activities have been reported after cannabis consumption, and a number of preclinical studies have demonstrated that both agonist and antagonist of the endocannabinoid receptors act similarly to antidepressants. Identification of the roles of ECS components may be valuable in designing new medications targets in the treatment of schizophrenia. The ECS may be an important natural regulatory mechanism for drug reward and a target for the treatment of addictive disorders. Several studies highlight a key involvement of ECS in ASD pathophysiology. In addition to autism, the ECS is also involved in several other psychiatric disorders (ADHD, anxiety, major depression, bipolar disorder, and schizophrenia). Our findings indicating an increased risk of schizophrenia in patients with low CB2R function, is in agreement with the hypothesis that autism and schizophrenia represent diametric conditions. Collectively, the findings to date indicate that the ECS plays a key role in the pathophysiology of ASD and may provide new insights into potential interventions and could represent a novel target and strategy for ASD pharmacotherapy. Our current knowledge on the emerging role of the ubiquitous ECS in neuro-immune-microbiome cross talk provides targets to study comorbidity between psychiatric disorders and neurological illness. There is also growing awareness of the involvement of gut microbiome and inflammation in a number of psychiatric and neurological disorders. The ECS system interaction with the microbiome and neuroglia cells in the brain is providing new therapeutic targets but also in understanding underlying mechanism (s) associated with psychiatric and neurological disorders. Mounting evidence shows that CB2Rs and its gene variants may play possible roles in psychiatric and neurological disorders with associated inflammatory reactions. With the increasing use of cannabis and cannabinoids, an ECS pharmacogenomic test on individualized basis could be used for diagnosis and whether or not medical cannabis can be of therapeutic benefit. A better understanding of the epigenetic regulation of eCB signaling as well as the eCB regulation of epigenetic mechanisms will be of great value for the possible design of more specific eCB epigenetic drugs. Therefore, more studies on the potential maternal and paternal transgenerational and/or epigenetic effects of cannabinoid abuse are needed with the global changing landscape in recreational and medical use of cannabis and cannabinoids. Development of cannabis and cannabinoid nanomedicine for nanotherapy will certainly overcome some of the shortcomings and challenges in medicinal and recreational use of cannabis and cannabinoids. As cannabinoids are a class of lipophilic compounds, the use of different surfactants and delivery systems to improve cannabinoid solubility and enhance bioavailability must be considered. To overcome these limitations, nanoparticle formulations may offer an attractive alternative. In practice, this means that the cannabinoids will be ‘packed’ in endogenous nanoparticles, offering the opportunity to effectively deliver the cannabis and cannabinoid formulations to the diseased sites, by applying nanotechnological advances to nanomedicine. The application of nanotechnology to medicine involves employing nanoparticles not only to enhance the action of drugs, but also expected to improve diagnosis and therapy of diseases and reduce health care cost. Thus, nanotechnology will allow targeted delivery of cannabinoid formulations with the potential to elevate their use to scientifically validated nanotherapeutic applications as the field of cannabis nanoscience matures.
Acknowledgments
This work was partly supported by William Paterson University and Assigned Release Time to ESO who was funded by NIH grant DA032890 and is currently a Guest researcher at the NIDA intramural research program.
Abbreviations
- 2-AG
2-Arachidonyl glycerol
- ABHD6
alpha beta hydrolase domain
- ABHD12
proteins
- AD
Alzheimer’s disease
- ADHD
Attention hyperactivity disorders
- ASDs
Autism spectrum disorders
- BT
Brain tumors
- CBRs
Cannabinoid receptors
- CNR
cannabinoid receptor gene
- CNS
central nervous system
- CP
cerebral palsy
- CUDs
Cannabis use disorders
- DSI/DSE
Depolarization-induced suppression of inhibition / excitation
- eCB
endocannabinoids
- ECS
endocannabinoid system
- ENS
enteric nervous system
- ERK1/2
Extracellular signal-regulated kinase 1/2
- ET
essential tremor
- FAAH
Fatty acid amide hydrolase
- GABA
Gamma-Aminobutyric acid
- GPCR
G protein-coupled receptor
- HD
Huntingtin disease
- IC
insular cortex
- JNK
c-jun N-terminal kinase
- LTD
long-term depression
- MAGL
Monoacylglycerol lipase
- MAOIs
monoamine oxidase inhibitors
- MS
Multiple sclerosis
- NAc
nucleus accumbens
- PBMCs
peripheral blood mononuclear cells
- p-CREB
phosphor-cAMP response element-binding protein
- PD
Parkinson’s disease
- PET
positron emission tomography
- PI3K/Akt
phosphatidylinositol-3-kinases / protein kinase B
- PNS
Peripheral nervous system
- PPARs
peroxisome proliferator-activated receptors
- SNPs
single nucleotide polymorphisms
- SNRIs
selective norepinephrine reuptake inhibitors
- SSRIs
selective serotonin reuptake inhibitors
- TBI
Traumatic brain injury
- TCAs
tricyclic antidepressants
- THC
tetrahydrocannabinol
- TRPV1
transient receptor potential vanilloid type 1
- TS
Tics and Tourette’s syndrome
Contributor Information
Neal Joshi, Rowan University School of Osteopathic Medicine, Stratford, NJ, USA.
Emmanuel S. Onaivi, Department of Biology, William Paterson University, Wayne, NJ, USA
References
- Acheson A, Fantegrossi E (2019) Introduction to special issue: therapeutic and abuse-related effects of cannabis and cannabinoids. Exp Clin Psychoparmaco 27:299–230 [DOI] [PubMed] [Google Scholar]
- Alaverdashvili M, Lapraire RB (2018) The future of type 1 cannabinoid receptor allosteric ligands. Drug Met Rev 50(1):14–25. 10.1080/03602532.2018.1428341 [DOI] [PubMed] [Google Scholar]
- Andrade AK, Renda B, Murray JE (2019) Cannabinoids, interoception, and anxiety. Pharmacol Biochem Behav 180:60–73 [DOI] [PubMed] [Google Scholar]
- Arjmand S, Behzadi M, Kohlmeier KA, Mazhari S (2019) Bipolar disorder and the endocannabinoid system. Acta Neuropsychiatrica 31:193–201 [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Shivakumar M, Joshi V, Subbanna S (2017) Endocannabinoid system in neurodegenerative disorders. J Neurochem 142:624–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigida AL, Schultz S, Cascone M, Antonuchi N, Siniscalco D. Endocannabinod signal Dysregulation in autism Spectrum disorders: a correlation link between inflammatory state and Neuro-immune alterations. Int J Mol Sci. 18(7). pii: E1425. doi: 10.3390/ijms18071425. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callén L, Moreno E, Barroso-Chinea P, Moreno-Delgado D, Cortés A, Mallol J et al. (2012) Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J Biol Chem 287:20851–20865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Plovier H, Hul MV, Geurts L, Delzenne NM, Druart C et al. (2016) Endocannabinoids- at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinology 12:133–146 [DOI] [PubMed] [Google Scholar]
- Canseco-Alba A, Hammouda M, Schanz N, Liu QR, Onaivi ES (2018a) Neurodevelopmental and behavioral alterations after conditional deletion of type 2 cannabinoid receptors in dopamine neurons. Soc Neurosc Abst, San Diego [Google Scholar]
- Canseco-Alba A, Schanz N, Ishiguro H, Liu QR, Onaivi ES. Behavioral Evaluation of Seeking and Preference of Alcohol in Mice Subjected to Stress. Bio Protoc. 8 (20). pii: e3061. doi: 10.21769/BioProtoc.3061. 2018b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canseco-Alba A, Schanz N, Sanabria B, Zhao J, Lin Z, Liu QR et al. (2019) Behavioral effects of psychostimulants in mutant mice with cell-type specific deletion of CB2 cannabinoid receptors in dopamine neurons. Behav Brain Res 360:286–297. 10.1016/j.bbr.2018.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y (2012) Endocannabinoid signaling and synaptic function. Neuron 76:70–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti B, Persico A, Battista N, Maccarrone M (2015) Endocannabinoid signaling in autism. Neurotherapeutics 12:837–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi B, Stead P, Elliot M (2010) Evolution in health and medicine Sackler colloquium: comparative genomics of autism and schizophrenia. Proc Natl Acad Sci 107 (Suppl 1):1736–1741. 10.1073/pnas.0906080106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, Coleman LG Jr (2017) The role of neuroimmune signaling in alcoholism. Neuropharmacology 122:56–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crume TL, Juhl AL, Brooks-Russel A, Hall KE, Wymore E, Borgelt LM (2018) Cannabis use during the perinatal period in a state with legalized recreational and medical marijuana: the association between maternal characteristics, breastfeeding patterns, and neonatal outcomes. J Pediatr 197:90–96 [DOI] [PubMed] [Google Scholar]
- D’Addario C, Micale V, Di Bartolomeo M, Stark T, Pucci M, Sulcova A et al. (2017) A preliminary study of endocannabinoid system regulation in psychosis: distinct alterations of CNR1 promoter DNA methylation in patients with schizophrenia. Schizophr Res 188:132–140 [DOI] [PubMed] [Google Scholar]
- de Aleida V, Martins-de-Souza D (2018) Cannabinoids and glial cells: possible mechanism to understand schizophrenia. Eur Arch Psych Clin Neurosci 268 (7):727–737. 10.1007/s00406-018-0874-6 [DOI] [PubMed] [Google Scholar]
- De Luca MA, Fattore L (2015) Cannabinoids and drug addiction. In: Fattore L (ed) Cannabinoids in neurologic and mental disease. Elsevier, pp 289–314 [Google Scholar]
- Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R et al. (2017) Cannabidiol in Dravet syndrome study group. Trial of cannabidiol for drug-resistant seizures in Dravet syndrome. N Engl J Med 37:2011–2020. 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- Di Marzo V (2018) New approaches and challenges to targeting the endocannabinoid system. Nat Rev Drug Discov 17:623–639 [DOI] [PubMed] [Google Scholar]
- DiPatrizio NV (2016) Endocannabinoids in the gut. Cannabis Cannabinoid Res 1:1. 10.1089/can.2016.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose H. (2009) Cannabinoid controversy. http://www.the-scientist.com/news/display/55969/) [Google Scholar]
- Guggenhuber S, Romo-Parra H, Bindila L, Leschik J, Lomazzo E, Remmers F et al. (2015) Impaired 2-AG signaling in hippocampal neurons: aggravation of anxiety-like behavior and unaltered seizure susceptibility. Int J Neuropsychopharmacol. 10.1093/ijn/pyv091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn JK, Rosales CB, Center KE, Nunez A, Gibson SJ, Christ C et al. (2016) Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BML Open 6(4):e009986. 10.1136/bmjopen-2015-009986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesdorffer DC (2016) Comorbidity between neurological illness and psychiatric disorders. CNS Spectr 21 (3):230–238. 10.1017/S1092852915000929 [DOI] [PubMed] [Google Scholar]
- Hill A, Williams CM, Whalley BJ, Stephens GJ (2012) Phytocannabinoids as novel therapeutic agents in CNS. Pharmacol Therapeutics 133:79–97 [DOI] [PubMed] [Google Scholar]
- Hua T, Vemuri K, Pu M, Qu L, Han GW, Wu Y et al. (2016) Crystal structure of human cannabinoid receptor 1. Cell 167:750–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungund BL, Vinod KY, Kassir SA, Basavarajappa BS, Yalamanchili R, Cooper TB et al. (2004) Upregulation of CB 1 receptors and agonist-stimulated [35 S] GTPγS binding in the prefrontal cortex of depressed suicide victims. Mol Psychiatry 9(2):184. [DOI] [PubMed] [Google Scholar]
- Ibarra-Lecue I, Pilar-Cuellar F, Muguruza C, Florensa-Zanuy E, Diaz A, Uriguen L et al. (2018) The endocannabinoid system in mental disorders: evidence from human brain studies. Biochem Pharmacol 151:97–107 [DOI] [PubMed] [Google Scholar]
- Ibsen MS, Finlay DB, Patel M, Javitch JA, Glass M, Grimsey NL (2019) Cannabinoid CB1 and CB2 receptor-mediated arrestin translocation: species, subtype, and agonist-dependence. Front Pharmacol 10:350. 10.3389/fphar.2019.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Carpio O, Horiuchi Y, Shu A, Higuchi S, Schanz N, Benno R et al. (2010b) A nonsynonymous polymorphism in cannabinoid CB2 receptor gene is associated with eating disorders in humans and food intake is modified in mice by its ligands. Synapse 64:92–96 [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Horiuchi Y, Ishikawa M, Koga M, Imai K, Suzuki Y et al. (2010a) Brain cannabinoid CB2 receptor in schizophrenia. Biol Psychiatry 67:974–982 [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Horiuchi Y, Tabata K, Liu QR, Arinami T, Onaivi ES (2018) Cannabinoid CB2 receptor gene and environmental interaction in the development of psychiatric disorders. Molecules 23. 10.3390/molecules23081836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Iwasaki S, Teasenfitz L, Higuchi S, Horiuchi Y, Saito T et al. (2007) Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. Pharmacogenomics J 7:380–385 [DOI] [PubMed] [Google Scholar]
- Josh N, Onaivi ES (2019) Endocannabinoid system components: overview and tissue distribution. Adv Exp Med Biol 1162:1–12 [DOI] [PubMed] [Google Scholar]
- Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X (2018) A gut-brain neural circuit for nutrient sensory transduction. Science 361: eaat5236. 10.1126/science.aat5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakpai F, Ebrahimi-Ghiri M, Alijanpour S, Zarrindast MR (2019) Ketamine-induced antidepressant like effects in mice: a possible involvement of cannabinoid system. Biomed Pharmacother 112:108717. [DOI] [PubMed] [Google Scholar]
- Kill KP (2019) Medical use of cannabis in 2019. JAMA. 10.1001/Jama.2019.11868 [DOI] [PubMed] [Google Scholar]
- Koethe D, Llenos IC, Dulay JR, Hoyer C, Torrey EF, Leweke FM et al. (2007) Expression of CB 1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J Neural Transm 114(8):1055. [DOI] [PubMed] [Google Scholar]
- Korem N, Zer-Aviv TM, Ganon-Elazar E, Abush H, Akirav I (2016) Targeting the endocannabinoid system to treat anxiety-related disorders. J Basic Clin Physiol Pharmacol 27:193–202 [DOI] [PubMed] [Google Scholar]
- Krebs MO, Kebir O, Jay TM (2019) Exposure to cannabinoids can lead to persistent cognitive and psychiatric disorders. Eur J Pain 23:1225–1233 [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Denovan-Wright EM (2017a) Cannabinoid receptor ligand bias: implications in the central nervous system. Curr Opin Pharmacol 32:32–43 [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Rourke JL, Zrein A, Cairns EA, Kelly MEM et al. (2019) Positive allosteric modulation of type 1 cannabinoid receptor reduces the signs and symptoms of Huntington’s disease in the R6/2 mouse model. Neuropharmacology 151:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie RB, Kulkarni PM, Deschamps JR, Kelly MEM, Janero DR, Cascio MG et al. (2017b) Enantiospecific allosteric modulation of cannabinoid 1 receptor. ACS Chem Neurosci 8:1188–1203 [DOI] [PubMed] [Google Scholar]
- Li X, Hua T, Vemuri K, Ho JH, Wu Y, Wu L et al. (2019) Crystal structure of the human cannabinoid receptor CB2. Cell 176:459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X-H, Wang Y-Q, Wang H-C, Ren X-Q, Li Y-Y (2013) Role of endogenous cannabinoid system in the gut. Acta Physiologica Sinica 65:451–460 [PubMed] [Google Scholar]
- Liu QR, Canseco-Alba A, Zhang HY, Taglaiferro P, Chung M, Dennis E et al. (2017) Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci Rep 7:17410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CS, Chau SA, Ruthirakuhan M, Lanctôt KL, Herrmann N (2015) Cannabinoids for the treatment of agitation and aggression in Alzheimer’s disease. CNS Drugs 29:615–623 [DOI] [PubMed] [Google Scholar]
- Lopez A, Aparicio N, Pazos MR, Grande MT, Barreda-Manso MA, Benito-Cuesta I et al. (2018) Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease. Neuroinflammation 15:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DJE, Sasiadek JD, Coles AS, George TP (2019) Cannabis and mental illness: a review. Europ Arch Psychiat and Clin Neurosci 269:107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Rapino C, Musella A et al. (2010) Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology 35:1500–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magid L, Heymann S, Elgali M, Avram L, Cohen Y, Liraz-Zaltsman S et al. (2019) Role of CB2 receptor in recovery of mice after traumatic brain injury. J Neurotrauma 36:1836–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A (2007) Impulsive behavior in adults with attention deficit/hyperactivity disorder: characterization of attentional, motor and cognitive impulsiveness. J Int Neuropsychol Soc 13:693–698 [DOI] [PubMed] [Google Scholar]
- Mehrpouya-Bahrami P, Chitrala KN, Ganewatta MS, Tang C, Murphy EA, Enos RT et al. (2017) Blockade of CB1 cannabinoid receptors alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci Rep 7:15645. 10.1038/s41598-017-15154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Frau R, Kalivas PW, Spencer S, Chioma V, Zamberletti E et al. (2017) New visitas on cannabis use disorder. Neuropharmacology 124:62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (2017) The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. National Academies Press, Washington, DC. 10.17226/24625 [DOI] [PubMed] [Google Scholar]
- Ngwa W, Kumar R, Moreau M, Dabney R, Herman A (2017) Nanoparticle drones to target lung cancer with radiosensitizers and cannabinoids. Front Oncol 7:208. 10.3389/fonc.2017.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Kano M (2014) Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr Opin Neurobiol 29:1–8 [DOI] [PubMed] [Google Scholar]
- Onaivi ES (2008) An endocannabinoid hypothesis of drug reward and drug addiction. Ann N Y Acad Sci 1139:412–421 [DOI] [PubMed] [Google Scholar]
- Onaivi ES (2010) Endocannabinoid system, pharmacogenomics and response to therapy. Pharmacogenomics 11(7):907–910 [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Benno R, Halpern T, Mehanovic M, Schanz N, Sanders C et al. (2011) Consequences of cannabinoid and monoaminergic system disruption in a mouse model of autism spectrum disorders. Curr Neuropharmacol 9:209–214. 10.2174/157015911795017047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Canseco-Alba BD Sanabria BD, Zhang HY, Eita M, Rohani T et al. Identification of cannabinoid CB2 receptor neuro-immune crosstalk following conditional deletion of type 2 cannabinoid receptors in microglia and dopamine neurons. Soc Neurosc Abst. San Diego. 2018 [Google Scholar]
- Onaivi ES, Green MR, Martin BR (1990) Pharmacological characterization of cannabinoids in the elevated plus maze. The J Pharmaco Expl Ther 252:1002–1009 [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L et al. (2008) Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS One 3(2):e1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA (2006b) Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci 1074:514–536 [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gu S, Liu QR (2012) CNS effects of CB2 cannabinoid receptors: beyond neuro-immunocannabinoid activity. J Psychopharmacol 26:92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Liu QR (2015) Future perspectives: cannabinoid CB2 receptor ligands and their therapeutic potential in mental diseases. In: Fattore L (ed) Cannabinoids in neurologic and mental disease. Elsevier Inc, pp 425–444 [Google Scholar]
- Onaivi ES, Ishiguro H, Sgro S, Leonard CM (2013) Cannabinoid receptor gene variations in drug addiction and neuropsychiatric disorders. J Drug Alcohol Res. 10.4303/jdar/235714 [DOI] [Google Scholar]
- Onaivi ES, Sugiura T, DiMarzo V (2006a) Eds. Endocannabinoids: the brain and body’s marijuana and beyond. CRC Press, Taylor and Francis Group, Boca Raton [Google Scholar]
- Papagianni EP, Stevenson CW (2019) Cannabidiol regulation of fear and anxiety: an update. Curr Psychiatric Reports 21:38. 10.1007/s11920-019-1026-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parira T, Laverde A, Agudelo M (2017) Epigenetic interactions between alcohol and cannabinergic effects: focus on histone modification and DNA methylation. J Alcohol Depend 5:259. 10.4172/2329-6488.1000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS et al. (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16:1–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleszak E, Wosko S, Salwinska K, Szopa A, Wrobel A, Serefko A (2018) Cannabinoids in depressive disorders. Life Sci 213:18–24 [DOI] [PubMed] [Google Scholar]
- Premoli M, Aria F, Bonini SA, Maccarinelli G, Gianoncelli A, Pina SD (2019) Cannabidiol: recent advances and new insights for neuropsychiatric disorders. Life Sci 224:120–127 [DOI] [PubMed] [Google Scholar]
- Robson PJ (2014) Therapeutic potential of cannabinoid medicines. Drug Test Analysis 6:24–30 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arias M, Navarrete F, Daza-Losada M, Navarro D, Aguilar MA, Berbel P et al. (2013) CB1 cannabinoid receptor-mediated aggressive behavior. Neuropharmacology 75:172–180 [DOI] [PubMed] [Google Scholar]
- Rubin R (2018) The path to the first FDA-approved cannabis-derived treatment and what comes next. JAMA 320:1227–1229. 10.1001/jama.2018.11914 [DOI] [PubMed] [Google Scholar]
- Russo EB (2018) Cannabis therapeutics and the future of neurology. Front Integr Neurosci 12:51. 10.3389/fnint.2018.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmöle AC, Lundt R, Gennequin B, Schrage H, Beins E, Krämer A et al. (2015) Expression analysis of CB2-GFP BAC transgenic mice. PLoS One 10(9): e0138986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Pandit S, Garnaes J, Tunic S, Mokkapati VR, Sultan A et al. (2018) Green synthesis of gold and silver nanoparticles from cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int J Nanomedicine 13:3571–3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan ME, Grant CW, Gowin JL, Ramchandani VJ, Le Foll B (2018) Endocannabinoid signaling in psychiatric disorders: a review of positron emission tomography studies. Act Pharmacologica Sinca 40:342–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Stanley CM, Foss T, Boles RG, McKernan K (2017) Rare genetic variants in the endocannabinoid system genes CNR1 and DAGLA are associated with neurological phenotypes in humans. PLoS One 12(11): e0187926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer CD, Jumeaux C, Gupta B, Stevens MM (2018) Peptide and protein nanoparticle conjugates versatile platforms for biomedical applications. Chem Soc Rev 47:3574–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempel AV, Zhang HY, Ozdogan T, Pannasch U, Theis AK (2016) Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron 90:795–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham M, Yilmaz O, Alaverdasvili M, Kelly MEM, Denovan-Wright EM, Laprairie RB (2019) Allosteric and orthesteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type cannabinoid receptors. Br J Pharmacol 176:1455–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielle EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C et al. (2018) Cannabidiol in patients with seizures associated with Lennos-Gastaut syndrome (GWPCARE4): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet 391:1085–1096. 10.1016/S0140-6736(18)30136-3 [DOI] [PubMed] [Google Scholar]
- Turner NE, McDonald AJ, Lalomiteanu AR, Mann RE, McCready J, Millstone D et al. (2019) Moderate to severe gambling problems and traumatic brain injury: a population-based study. Psychiatry Res 272:692–697. 10.1016/j.psychres.2018.12.170 [DOI] [PubMed] [Google Scholar]
- Vaughn MG, Sala-Wright CP, John R, Holzer KJ, Qian Z, Veeh C (2019) Traumatic brain injury and psychiatric co-morbidity in the United States. Psychiatry Q 90 (1):151–158. 10.1007/s11126-018-9617-0 [DOI] [PubMed] [Google Scholar]
- Yin A, Wang F, Zhang X (2018) Integrating endocannabinoid signaling in the regulation of anxiety and depression. Act Pharmacologica Sinca. 40:336–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamberletti E, Gabaglio M, Parolaro D. The endocannabinoid system and autism spectrum disorders: insights from animal models. Int J Mol Sci, 18(9). Pii: E1916. Doi: 10.3390/ijms18091916. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Alger BE (2010) Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci 30:5724–5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PW, Ishiguro H, Ohtsuki T, Hess J, Carillo F, Walther D et al. (2004) Human cannabinoid receptor 1:5′ exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol Psychiatry 9:916–931 [DOI] [PubMed] [Google Scholar]
- Zhou D, Li Y, Tian T, Quan W, Wang L, Shao Q et al. (2017) Role of the endocannabinoid system in the formation and development of depression. Pharmazie 72:435–439 [DOI] [PubMed] [Google Scholar]
- Zou S, Kumar U (2019) Cannabinoid receptors and endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci 19:833. 10.3390/ijms19030833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou M, Li D, Wu L, Sun C (2019) Role of the endocannabinoid system in neurological disorders. Int J Dev Neurosci 76:95–102 [DOI] [PubMed] [Google Scholar]
- Zumbrun EE, Sido JM, Nagarkatti PS, Nagagarkatti PS (2015) Epigenetic regulation of immunological alterations following prenatal exposure to marijuana cannabinoids and its long-term consequences in offspring. J Neuroimmune Pharmacol 10:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]