Abstract
Purpose
To evaluate whether fibrosis contributes to corneal transplant failure and to determine whether effector CD4+ T cells, the key immune cells in corneal transplant rejection, play a direct role in fibrosis formation.
Methods
Allogeneic corneal transplantation was performed in mice. Graft opacity was evaluated by slit-lamp biomicroscopy, and fibrosis was assessed by in vivo confocal microscopy. Expression of alpha-smooth muscle actin (α-SMA) in both accepted and failed grafts was assessed by real-time PCR and immunohistochemistry. Frequencies of graft-infiltrating CD4+ T cells, neutrophils, and macrophages were assessed using flow cytometry. In vitro, MK/T-1 corneal fibroblasts were co-cultured with activated CD4+CD25– effector T cells isolated from corneal transplant recipient mice, and α-SMA expression was quantified by real-time PCR and ELISA. Neutralizing antibody was used to evaluate the role of interferon gamma (IFN-γ) in promoting α-SMA expression.
Results
The majority of failed grafts demonstrated clinical signs of fibrosis which became most evident at week 6 after corneal transplantation. Failed grafts showed higher expression of α-SMA as compared to accepted grafts. Flow cytometry analysis showed a significant increase in CD4+ T cells in failed grafts compared to accepted grafts. Co-culture of activated CD4+CD25– effector T cells with corneal fibroblasts led to an increase in α-SMA expression by fibroblasts. Inhibition of IFN-γ in culture significantly suppressed this increase in α-SMA expression as compared to immunoglobulin G control.
Conclusions
Fibrosis contributes to graft opacity in corneal transplant failure and is mediated at least in part by effector CD4+ T cells via IFN-γ.
Keywords: T cells, fibrosis, corneal transplantation
Corneal disease is one of the leading causes of blindness worldwide, and corneal transplantation is designed to restore corneal clarity and vision.1 Over the past century, corneal transplantation has become the most common form of solid organ transplantation performed worldwide.2,3 It is estimated that over 100,000 corneal transplant surgeries are performed annually worldwide, with over 30,000 full-thickness corneal transplants performed annually in the United States alone.4 Corneal transplantation restores visual function by restoring corneal clarity, but, when a corneal graft develops opacity and is no longer clear, the transplant is considered to have failed. Corneal graft opacity and failure are most often attributed to corneal edema resulting from immune rejection of the transplanted cornea, specifically immune rejection of the corneal endothelium.5 There are other causes of corneal opacity besides edema, however, and in the non-transplant setting one of the most common causes of corneal opacity is scarring or fibrosis.6–8
Corneal insults and injuries trigger a complex healing response that includes the development of myofibroblasts, the key cells in the development of fibrosis.9 These cells are characterized by their expression of alpha-smooth muscle actin (α-SMA), and, to repair damaged tissue following an inflammatory insult, they produce disorganized extracellular matrix components, leading to corneal fibrosis and opacity.10–12 Although fibrosis represents a physiologic response to injury, it is pathologic in that it often results in decreased organ function. Nowhere is this more evident than in the cornea, where the primary function is the transmission and refraction of light. In the cornea, several groups have elucidated the cytokines, cells, and signaling pathways that coordinate this response following non-specific injury and inflammation.13 Interestingly, although fibrosis is recognized as a component of failed allografts in other organ systems, including liver and kidney, it has not been formally investigated in corneal transplantation.14,15
Immune rejection is the most common cause of graft failure in corneal transplantation. The alloimmune response in corneal transplantation is primarily mediated by CD4+ T cells, specifically interferon-gamma (IFN-γ)–producing T helper 1 (Th1) cells, and we know that corneal transplant rejection is characterized by an influx of multiple infiltrating immune cell types and production of pro-inflammatory cytokines.13,16,17 Thus, immune rejection and subsequent graft failure may create an environment conducive to myofibroblast formation and fibrosis.18 In the present study, we evaluated whether fibrosis contributes to corneal transplant opacity and failure and investigated whether effector CD4+ T cells, the key immune cells in corneal transplant rejection, play a direct role in fibrosis formation.
Methods
Murine Corneal Transplantation
Allogeneic murine corneal transplantation was performed as previously described.19 Briefly, 8- to 10-week-old male BALB/c recipient and C57BL/6 donor mice were purchased from Charles River Laboratories (Wilmington, MA, USA). A 2-mm excised central corneal button was transplanted onto a 1.5-mm host bed and secured with 11-0 nylon sutures (AB-0550S; MANI, Tochigi, Japan) in the right eye. Ethiqa (Fidelis Animal Health, North Brunswick, NJ, USA) was injected postoperatively via a subcutaneous route for analgesia. All procedures were performed under anesthesia with intraperitoneal injection of ketamine (120 mg/kg) and xylazine (20 mg/kg). Tarsorrhaphy sutures were removed 3 days after transplantation. Corneal sutures were removed 7 days after transplantation. Transplanted corneas were assessed every week using slit-lamp biomicroscopy to grade corneal opacity (Supplemental Table S1).20 This study was designed and animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
In Vivo Confocal Microscopy and Fibrosis Evaluation
Corneas of all mice were examined by in vivo confocal microscopy (IVCM) using the Heidelberg Retina Tomograph II with the Rostock Corneal Module, version 1.2 (RCM/HRT II; Heidelberg Engineering, Heidelberg, Germany). At the beginning of the examination, mice were anesthetized by intraperitoneal injection of ketamine (120 mg/kg) and xylazine (20 mg/kg). The right (transplanted) eye was anesthetized with topical anesthetic (proparacaine 0.5%; Bausch + Lomb, Berlin, Germany), and ophthalmic gel (Vidisic Gel; Bausch + Lomb) was applied in a sterile polymethylmethacrylate cap (TomoCap; Heidelberg Engineering), which was placed over the objective lens. Sections were scanned from the epithelium to endothelium for fibrosis analysis. IVCM images of the stroma of the center of the corneal graft were graded according to the area within the field of view occupied by hyperreflective bands as follows: 0, none; 1, minimal (<1/3 area); 2, moderate (1/3–1/2 area); and 3, advanced (>1/2 area).
Real-Time Polymerase Chain Reaction
Total RNA from corneas or MK/T-1 cells was extracted using the RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. Total RNA was quantified with a spectrophotometer (NanoDrop 2000; Thermo Fisher Scientific, Waltham, MA, USA). cDNA was synthesized from RNA using the QIAGEN QuantiTect Reverse Transcription Kit. Real-time polymerase chain reaction (RT-PCR) for α-SMA (Assay ID Mm01546133; Thermo Fisher Scientific) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Assay ID Mm99999915_g1; Thermo Fisher Scientific) was performed using TaqMan Universal PCR Master Mix (Thermo Fisher Scientific) reagents. GAPDH served as the internal reference gene.
Immunohistochemistry
Mice were euthanized and corneas excised. Whole-mount corneas were fixed in acetone and incubated in blocking solution containing 5% BSA and 0.5% Triton X-100 in PBS for 1 hour at room temperature. Corneas were washed in PBS and were incubated in blocking solution containing 10 µg/mL anti-α-SMA antibody (53-6496-82; Thermo Fisher Scientific) or control (53-4714-80; Thermo Fisher Scientific) overnight at 4°C. After they were washed in PBS, the corneas were mounted on glass slides using a 4′,6-diamidino-2-phenylindole (DAPI)–containing mounting medium. Slides were then visualized with a Leica SP8 confocal microscope (Leica, Wetzlar, Germany).
Flow Cytometry
Transplanted corneas were collected and digested with collagenase and Dnase, and single-cell suspensions were prepared and filtered. Cells were incubated with an Fc receptor blocking antibody (R&D Systems, Minneapolis, MN, USA). After they were washed, the cells were incubated with flow cytometry antibodies from BioLegend (San Diego, CA, USA) or Thermo Fisher Scientific for 1 hour: PE-Cy7-anti-mouse CD45; Pacific Blue-anti-mouse CD11b Ab; FITC-anti-mouse CD4 Ab; APC-anti-mouse Ly-6G Ab; and PE-anti-mouse F4/80 Ab. Stained cells were rinsed and analyzed using an LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA), and the results were analyzed using Summit 4.3 (Agilent Dako, Santa Clara, CA, USA). Dead cells were excluded by performing forward-scatter versus side-scatter analysis, and samples were subsequently analyzed by gating on CD45+ cells (Supplementary Fig. S1).
In Vitro Co-Culture of MK/T-1 and T Effector Cells and IFN-γ Neutralization
The cervical draining lymph nodes, located beneath the superficial layer of skin on the neck, near the jugular vein, were identified as two or three yellowish, round nodes and collected. Single-cell suspensions were created from cervical draining lymph nodes to isolate the CD4+CD25– T effector cell fraction by magnetic activated cell sorting (MACS) sorting (130-091-041; Miltenyi Biotec, Bergisch-Gladbach, Germany), a protocol that removes non-CD4+ cells and positively selects CD4+CD25+ cells, allowing collection of CD4+CD25– T effectors. T effector cells from allogeneic transplant recipient (week 2 post-transplantation) or naïve mice were co-cultured with MK/T-1 cells, a mouse corneal fibroblast cell line at 70% confluence in a 24-well plate and activated with soluble anti-CD3 (10 µg/mL, 100359; BioLegend) and anti-CD28 (4 µg/mL, 102116; BioLegend).21 For IFN-γ neutralization, the co-culture system was incubated with IFN-γ neutralizing antibody or isotype control for 24 hours at a concentration of 1 mg/mL (BE0055; Bio X Cell, Lebanon, NH, USA).
Enzyme-Linked Immunosorbent Assay
Protein extraction and enzyme-linked immunosorbent assay (ELISA) analyses were performed according to a previously described protocol.22 In brief, total protein from MK/T-1 cells was extracted with radioimmunoprecipitation assay (RIPA) buffer with protease inhibitor. Protein levels were determined using a Pierce BCA Protein Assay Kit (23227; Thermo Fisher Scientific), and α-SMA expression was measured using a commercially available α-SMA ELISA kit (NBP2-66429; Novus Biologicals, Centennial, CO, USA). Transforming growth factor beta-1 (TGF-β1) levels were also analyzed using a commercially available ELISA kit (BMS608-4; Thermo Fisher Scientific).
Statistical Analysis
All quantitative data are reported as the mean ± standard error of the mean (SEM) from one of at least two independent experiments. The two-sided t-test or Wilcoxon rank-sum test was used for two group comparisons, and ANOVA or the Kruskal–Wallis test was used for comparison of quantitative data among multiple groups, as appropriate. P < 0.05 was considered statistically significant.
Results
Failed Corneal Transplants Demonstrate Clinical Signs of Fibrosis
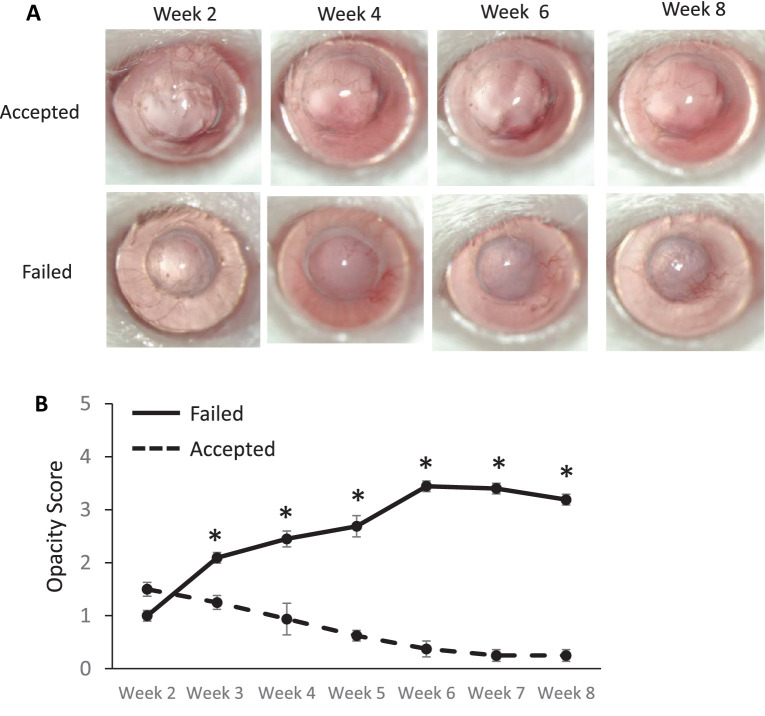
Allogeneic murine corneal transplantation was performed, and graft opacity was monitored through 8 weeks of follow-up to identify two groups of mice: accepted (n = 20) or failed (n = 20) transplant recipients. Accepted grafts remained clear, but failed grafts developed clinically evident opacity (Fig. 1A). Graft opacity was evaluated according to a standardized scoring system, and there was a statistically significant difference in graft opacity between accepted and failed transplants at weeks 3 through 8. At week 8, the opacity score in the failed transplant group was 3.2 ± 0.12 as compared to 0.25 ± 0.16 in the accepted transplant group (P < 0.005). The contralateral (unoperated) eyes in all mice remained clear, with an opacity score of 0 through 8 weeks of follow-up.
Figure 1.
Corneal opacity in accepted or failed allogeneic transplantation. Corneal grafts were harvested from C57BL/6 donors and transplanted to BALB/c recipients, and graft opacity was assessed weekly for 8 weeks. (A) Representative slit-lamp images showing accepted (top row) and failed (bottom row) corneas post-transplantation in vivo. (B) Accepted grafts (dashed line) remained clear, but grafts that failed developed graft opacity (solid line). *P < 0.05 (n = 20/group).
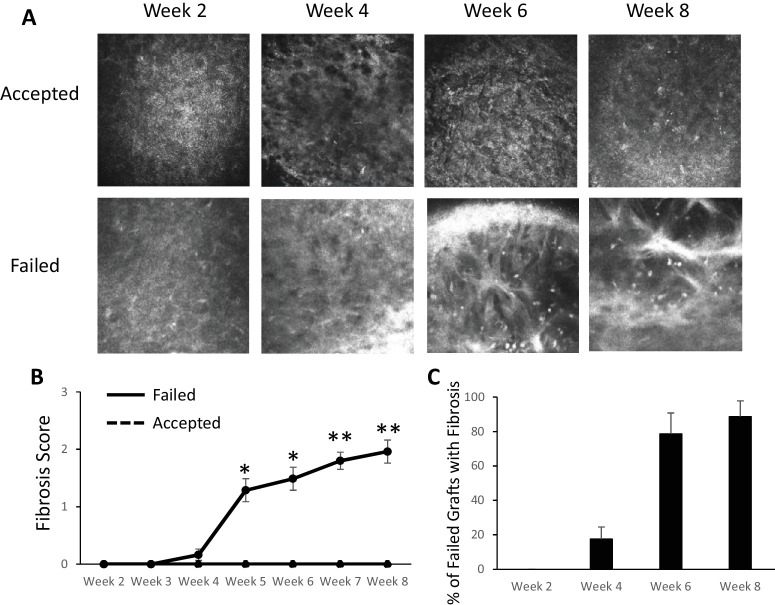
In vivo confocal microscopy showed thickened, hyperreflective bands in the anterior stroma in failed grafts (Fig. 2A). We graded these confocal images according to a novel grading system based on the area occupied by fibrotic bands (see Methods). In the failed graft group, fibrosis increased over time, whereas accepted grafts maintained a score of 0 at all time points. At week 8, the failed transplant group had a fibrosis score of 1.96 ± 0.4 (P < 0.005) (Fig. 2B). We determined the number of failed grafts with evidence of fibrosis on confocal microscopy and found that fibrosis developed in the majority of failed grafts (79%) by week 6. At week 8, 89% of failed grafts demonstrated signs of fibrosis (Fig. 2C). Through 8 weeks of follow-up the contralateral (unoperated) eyes in all mice maintained a fibrosis score of 0. For subsequent experiments, week 6 was selected as the time point for analysis, as this represented the approximate onset of fibrosis.
Figure 2.
In vivo confocal microscopy evaluation of fibrosis in corneal transplantation. (A) Representative in vivo confocal microscopy images showing the development of hyperreflective, thickened bands in the anterior stroma in failed grafts (bottom row). (B) Fibrosis score increased in failed grafts (solid line) over time, but accepted grafts continued to have a corneal opacity score of 0 (dashed line). (C) The majority of failed grafts demonstrated signs of fibrosis on confocal microscopy, and fibrosis became most evident around week 6. *P < 0.05, **P < 0.005 (failed, n = 16; accepted, n = 14).
Increased α-SMA Expression in Failed Corneal Transplants
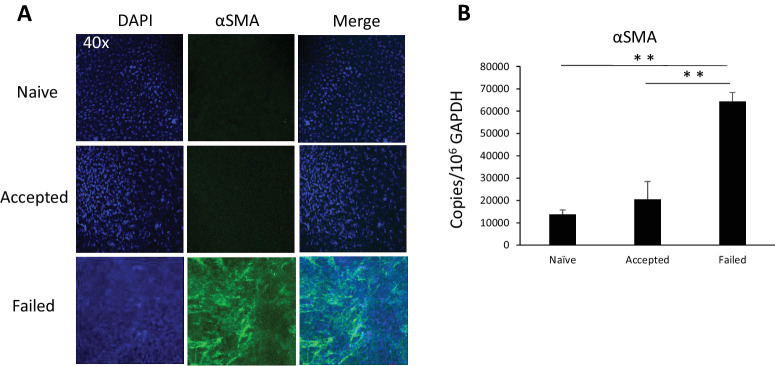
Myofibroblasts are characterized by their production of α-SMA, and to further characterize failed and accepted corneal transplants with respect to fibrosis, we evaluated accepted and failed corneal transplants at 6 weeks post-transplantation for α-SMA expression levels by immunohistochemistry and RT-PCR. On immunohistochemistry, we observed relatively greater expression of α-SMA in failed transplants as compared to accepted transplants (Fig. 3A). Using RT-PCR, we found significantly higher levels of α-SMA mRNA in the failed transplant group as compared to the accepted transplant group (64,349 ± 1060 vs. 20,496 ± 124 copies/106 copies GAPDH; P < 0.005) (Fig. 3B).
Figure 3.
α-SMA expression by grafts 6 weeks after corneal transplantation. (A) Representative images showing immunohistochemistry staining of α-SMA in the graft center. Blue indicates DAPI (nuclei staining), and green indicates α-SMA (magnification, 40×). (B) mRNA levels of α-SMA determined by PCR. **P < 0.01 (n = 3/group; representative data from one of two repeats are shown).
Failed Corneal Transplants Demonstrate Increased Levels of Infiltrating Immune Cells
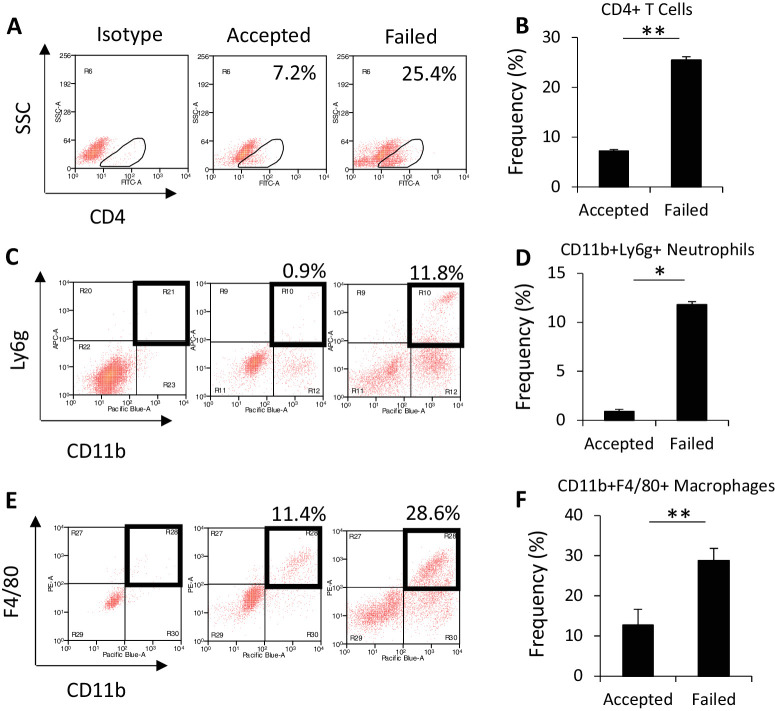
Transplanted corneas were harvested at week 6 after corneal transplantation to evaluate graft-infiltrating immune cell populations present at the same time as the development of fibrosis on IVCM. Using flow cytometry, we evaluated the frequency of CD4+ T cells, given that these cells are the key effector cells in corneal transplantation rejection, as well as CD11b+Ly6g+ neutrophils and CD11b+F4/80+ macrophages.23,24 We found significantly higher frequencies of CD4+ T cells (25.4 ± 0.2 vs. 7.2 ± 0.4; P < 0.005), macrophages (28.83 ± 0.22 vs. 12.7 ± 0.9; P = 0.006), and neutrophils (11.8 ± 0.04 vs. 0.92 ± 0.05; P < 0.005) in failed as compared to accepted transplants (Figs. 4A–4F).
Figure 4.
Immune cell frequency in corneal transplantation. (A, C, E) Corneas were harvested at week 6 after corneal transplantation for flow cytometry. Representative images show CD4+ T cell, neutrophil, and macrophage populations in accepted or failed grafts, gated on CD45+ cells. (B, D, F) Frequency of CD4+ T cells, neutrophils, and macrophages. *P < 0.05, **P < 0.005 (n = 6/group; representative data from one of two repeats are shown).
Effector T Cells Increase Myofibroblast Formation Via IFN-γ
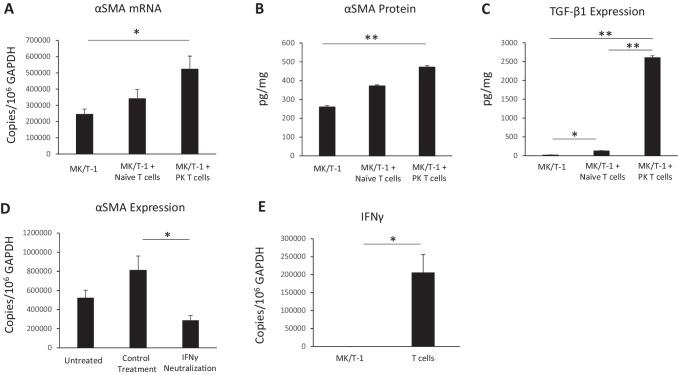
To determine whether effector T cells promote corneal myofibroblast formation, we co-cultured MK/T-1 corneal fibroblasts with activated CD4+CD25– effector T cells isolated from the draining lymph nodes of corneal transplant recipient mice or naïve mice for 24 hours. Effector T cells from both naïve and corneal transplant mice increased α-SMA mRNA expression by corneal fibroblasts, with higher expression seen in the group with effector T cells from corneal transplant mice (340,520 ± 57,744 copies/106 copies GAPDH) as compared to corneal fibroblasts alone (24,4539 ± 32,853 copies/106 copies GAPDH; P = 0.05) (Fig. 5A). We found a similar pattern in terms of α-SMA protein expression with 472.1 ± 7.44 pg/mL in fibroblasts cultured with T cells from transplant mice versus 260.1 ± 7.1 pg/mL in fibroblasts alone (P = 0.006) (Fig. 5B). To further evaluate the effect of T cells on corneal fibroblasts with respect to fibrosis, TGF-β1 protein levels were assessed by ELISA, and we found significantly higher levels of TGF-β1 in culture supernatant in the MK/T-1 with allo-primed T cells from corneal transplant mice (2613.2 ± 40.0 ng/mL) as compared to the MK/T-1 with T cells from unprimed naïve mice (132 ± 3.7 ng/mL; P < 0.005) and MK/T-1–alone groups (23.7 ± 0.85 ng/mL; P < 0.005). To explore the mechanism by which T cells mediate this effect of increased α-SMA, we performed a functional co-culture experiment in which we neutralized IFN-γ, as CD4+ Th1 cells are the key effector cells in corneal transplant rejection23,25 and are characterized by production of their signature cytokine, IFN-γ, which has been well characterized as the predominant effector cytokine in corneal transplantation failure.2,24 MK/T-1 corneal fibroblasts were then co-cultured with effector T cells from corneal transplant mice, and we found that neutralization of IFN-γ significantly decreased α-SMA expression (28,8295 ± 53,529 copies/106 copies GAPDH) as compared to treatment with immunoglobulin G (IgG) control (810,776 ± 174,602 copies/106 copies GAPDH; P = 0.04) (Fig. 5D). Finally, we confirmed that T cells rather than fibroblasts were the source of IFN-γ in our co-culture system by RT-PCR, as we found that fibroblasts express significantly less α-SMA mRNA (59.9 ± 10.7 copies/106 copies GAPDH) as compared to CD4+CD25– T cells isolated from transplant recipients (1,617,187 ± 248,878 copies/106 copies GAPDH; P = 0.02) (Fig. 5E).
Figure 5.
Expression of α-SMA in corneal fibroblasts co-cultured with effector T cells. (A, B) CD4+CD25– effector T cells were sorted from corneal transplant recipient (allo-primed) and naive (unprimed) mice. MK/T-1 corneal fibroblasts demonstrated increased α-SMA mRNA and protein expression when co-cultured with activated CD4+CD25– effector T cells isolated from corneal transplant recipient mice 2 weeks post-transplantation. (C) TGF-β1 protein levels in the culture supernatant were significantly increased in the MK/T-1 with penetrating keratoplasty (corneal transplantation) (PK) effector T-cell group. Anti-CD3 and anti-CD28 antibodies were included in all groups. Naïve T cells, effector T cells from naïve mice; PK T cells, effector T cells from corneal transplant recipient mice. (D) When MK/T-1 corneal fibroblasts were co-cultured with effector T cells from corneal transplant mice, inhibition of IFN-γ with neutralizing antibody suppressed α-SMA expression compared to IgG control. (E) The expression of IFN-γ mRNA by T cells was significantly greater than IFN-γ expression by fibroblasts. *P < 0.05, **P < 0.005 (n = 6/group; representative data from one of two repeats are shown).
Discussion
Fibrosis is an essential process for wound healing and tissue repair that occurs in a variety of situations including infection, inflammation, autoimmune disorders and injury.26 In addition, it represents a final common pathway of graft failure in multiple forms of solid-organ transplantation, including kidney, liver, and lung.27,28 In the setting of corneal transplantation, endothelial immune rejection of the transplanted cornea is the most common cause of transplant failure, with immune rejection leading to endothelial dysfunction and subsequent graft edema; thus, graft failure is most often attributed to graft edema.29 However, because immune rejection is characterized by a significant increase in multiple immune cell types and upregulation of pro-inflammatory cytokines, corneal transplant rejection and failure may create an environment conducive to fibrosis. Here, we evaluated whether fibrosis is present in failed corneal transplants and demonstrated that effector T cells promote myofibroblast formation through IFN-γ.
Using an established murine model of corneal transplantation, we characterized graft failure clinically by slit-lamp biomicroscopy and IVCM. Slit-lamp examination confirmed opacity in failed grafts, and confocal microscopy revealed highly reflective and thickened bands in the anterior stroma suggestive of fibrosis. These confocal changes support the findings of two previous histological studies that showed evidence of fibrosis in the stroma of failed corneal transplants.30,31 We also found that IVCM evidence of fibrosis in failed grafts became most evident around week 6 following transplantation. We further evaluated failed transplants with respect to fibrosis by evaluating grafts for their expression of α-SMA, the key identifier of myofibroblasts, at the mRNA and protein levels. Although some amount of fibrosis at the graft–host junction is expected regardless of graft acceptance or failure, we found a significantly higher level of α-SMA in failed grafts, which is consistent with our clinical findings demonstrating fibrosis in the center of the transplanted cornea.
Immune rejection is the most common cause of corneal graft failure, and effector CD4+ T cells are the key drivers of rejection in corneal transplantation. These cells, particularly CD4+ Th1 cells, produce IFN-γ and facilitate the recruitment and activation of additional immune cells, including macrophages and neutrophils, ultimately leading to graft damage and failure. To investigate the relationship between this alloimmune response and fibrosis, we characterized graft-infiltrating immune cells in failed corneal transplants at 6 weeks post-transplantation, the time of fibrosis onset as demonstrated by our IVCM results. We evaluated the frequency of CD4+ T cells, CD11b+F4/80+ macrophages, and CD11b+Ly6g+ neutrophils and found a significant increase in all cell types in failed grafts as compared to accepted grafts.22,23 Because of the known critical role of CD4+ cells in corneal transplant rejection,32 we turned our attention toward investigating the mechanism by which these cells promote fibrosis.
To do this we utilized an in vitro co-culture system in which we cultured activated CD4+CD25– effector T cells derived from corneal transplant recipient mice with MK/T-1 corneal fibroblasts and evaluated the transformation of fibroblasts to myofibroblasts by quantifying α-SMA levels. Compared to corneal fibroblasts alone, the addition of effector T cells from transplant mice to corneal fibroblasts led to a significant increase in α-SMA. Given that IFN-γ is the signature cytokine of Th1 cells and plays a critical role in corneal transplantation rejection,33 we performed a functional proof-of-concept assay in which we inhibited IFN-γ in our co-culture system. We found that inhibiting IFN-γ with a neutralizing antibody significantly suppressed corneal fibroblast expression of α-SMA compared to IgG control antibody. In the literature, Th1 cells and IFN-γ have been reported to exert both pro- and antifibrotic effects, and which effect predominates may depend on the experimental model, organ system, and disease.34,35 Our results suggest that, in the setting of corneal transplantation, IFN-γ is profibrotic and may be a potential therapeutic target in the management of fibrosis and graft failure. Our in vitro experiments showed a direct profibrotic effect of IFN-γ on corneal fibroblasts, an effect that may be mediated by IFN-γ–dependent induction of TGF-β1 by fibroblasts, as has been shown in the setting of cardiac fibrosis,35 or by activation of the Janus kinase (JAK)/signal transducer of activation (STAT) signaling pathway, which is modulated by IFN-γ and can influence fibrosis in multiple organ systems.36,37 In vivo, IFN-γ may also act to promote fibrosis by upregulating proinflammatory cytokine expression and through recruitment and activation of immune cells, including macrophages, which are a potent source of TGF-β1.38 Future investigations may look more closely at these pathways to further define the mechanisms underlying the relationship between T cells and corneal fibrosis.
In the present study, we investigated the relationship between T cells and fibrosis in corneal transplantation and found that fibrosis is present and contributes to graft opacity in failed corneal transplants. We additionally found that effector T cells derived from corneal transplant recipients promote myofibroblast formation through the cytokine IFN-γ, a novel mechanism that links alloimmune rejection to graft fibrosis and failure in corneal transplantation and may have implications for other forms of solid organ transplantation.
Supplementary Material
Acknowledgments
Supported by grants from the National Eye Institute, National Institutes of Health (K08 EY031759 to THD; R01 EY12963 to RD), by a Core Grant for Vision Research (5P30EY003790), and by New England Corneal Transplant Research Foundation (THD).
Disclosure: S. Wang, None; S.K. Mittal, None; S. Lee, None; A.E. Herrera, None; M. Krauthammer, None; E. Elbasiony, None; T. Blanco, None; H. Alemi, None; H. Nakagawa, None; S.K. Chauhan, None; R. Dana, None; T.H. Dohlman, None
References
- 1. Coco G, Romano V. Corneal disease & transplantation. J Clin Med. 2022; 11: 4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dana MR, Qian Y, Hamrah P. Twenty-five–year panorama of corneal immunology. Cornea. 2000; 19: 625–643. [DOI] [PubMed] [Google Scholar]
- 3. Hamrah P. High-risk penetrating keratoplasty. Arch Soc Esp Oftalmol. 2005; 80: 5–7. [PubMed] [Google Scholar]
- 4. EBAA. 2014 Eye Banking Statistical Report. Washington, DC: Eye Bank Association of America; 2014. [Google Scholar]
- 5. Coster DJ, Williams KA. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am J Ophthalmol. 2005; 140: 1112–1122. [DOI] [PubMed] [Google Scholar]
- 6. Torricelli AAM, Santhanam A, Wu J, Singh V, Wilson SE. The corneal fibrosis response to epithelial–stromal injury. Exp Eye Res. 2016; 142: 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuest M, Yam GH-F, Peh GS-L, Mehta JS. Advances in corneal cell therapy. Regen Med. 2016; 11: 601–615. [DOI] [PubMed] [Google Scholar]
- 8. Stern JH, Tian Y, Funderburgh J, et al.. Regenerating eye tissues to preserve and restore vision. Stem Cell. 2018; 22: 834–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shu DY, Lovicu FJ. Myofibroblast transdifferentiation: the dark force in ocular wound healing and fibrosis. Prog Retin Eye Res. 2017; 60: 44–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jester JV, Moller-Pedersen FJ, Huang J, et al.. The cellular basis of corneal transparency: evidence for ‘corneal crystallins.’ J Cell Sci. 1999; 112: 613–622. [DOI] [PubMed] [Google Scholar]
- 11. Micallef L, Vedrenne N, Billet F, Coulomb B, Darby IA, Desmouliére A. The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis Tissue Repair. 2012; 5: S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mohan RR, Hutcheon AEK, Choi R, et al.. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003; 76: 71–87. [DOI] [PubMed] [Google Scholar]
- 13. Pleyer U, Schlickeiser S. The taming of the shrew? The immunology of corneal transplantation. Acta Ophthalmol. 2009; 87: 488–497. [DOI] [PubMed] [Google Scholar]
- 14. Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017; 127: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rayego-Mateos S, Valdivielso JM. New therapeutic targets in chronic kidney disease progression and renal fibrosis. Expert Opin Ther Targets. 2020; 24: 655–670. [DOI] [PubMed] [Google Scholar]
- 16. Lam H, Dana MR. Corneal graft rejection. Int Ophthalmol Clin. 2009; 49: 31–41. [DOI] [PubMed] [Google Scholar]
- 17. Yamagami S, Miyazaki D, Ono SJ, Dana MR. Differential chemokine gene expression in corneal transplant rejection. Invest Ophthalmol Vis Sci. 1999; 40: 2892–2897. [PubMed] [Google Scholar]
- 18. Schmid F, Mayer C, Büttner-Herold M, von Hörsten S, Amann K, Daniel C. CD161a-positive natural killer (NK) cells and α-smooth muscle actin-positive myofibroblasts were upregulated by extrarenal DPP4 in a rat model of acute renal rejection. Diabetes Res Clin Pract. 2021; 173: 108691. [DOI] [PubMed] [Google Scholar]
- 19. Dohlman TH, Omoto M, Hua J, et al.. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation. 2015; 99: 678–686. [DOI] [PubMed] [Google Scholar]
- 20. Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice–evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992; 54: 694–704. [DOI] [PubMed] [Google Scholar]
- 21. Gendron RL, Liu CY, Paradis H, Adams LC, Kao WW. MK/T-1, an immortalized fibroblast cell line derived using cultures of mouse corneal stroma. Mol Vis. 2001; 7: 107–113. [PubMed] [Google Scholar]
- 22. Nii T, Makino K, Tabata Y. A cancer invasion model of cancer-associated fibroblasts aggregates combined with TGF-β1 release system. Regen Ther. 2020; 14: 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hazlett LD, Hendricks RL. Reviews for immune privilege in the year 2010: immune privilege and infection. Ocul Immunol Inflamm. 2010; 18: 237–243. [DOI] [PubMed] [Google Scholar]
- 24. Amouzegar A, Chauhan SK, Dana R. Alloimmunity and tolerance in corneal transplantation. J Immunol. 2016; 196: 3983–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen Y, Wang S, Alemi H, Dohlman T, Dana R. Immune regulation of the ocular surface. Exp Eye Res. 2022; 218: 109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang M, Zhang ST. Cells in fibrosis and fibrotic diseases. Front Immunol. 2020; 11: 1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shenderov K, Collins SL, Powell JD, Horton MR. Immune dysregulation as a driver of idiopathic pulmonary fibrosis. J Clin Invest. 2021; 131: e143226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marsh JW, Makowka L, Todo S, et al.. Liver transplantation today. Postgrad Med. 1987; 81: 13–23. [DOI] [PubMed] [Google Scholar]
- 29. Lu LM, Boyle AB, Niederer RL, Brookes NH, McGhee CNJ, Patel DV. Repeat corneal transplantation in Auckland, New Zealand: indications, visual outcomes and risk factors for repeat keratoplasty failure. Clin Exp Ophthalmol. 2019; 47: 987–994. [DOI] [PubMed] [Google Scholar]
- 30. Kurz GH, D'Amico RA. Histopathology of corneal graft failures. Am J Ophthalmol. 1968; 66: 184–199. [DOI] [PubMed] [Google Scholar]
- 31. Lahav M, Cadet J-C. Subepithelial fibrous tissue in failed corneal grafts. Albrecht von Graefes Arch Klin Exp Ophthalmol. 1979; 211: 145–154. [DOI] [PubMed] [Google Scholar]
- 32. Hamrah P, Hamrah P. Corneal allograft rejection: immunopathogenesis to therapeutics. J Clin Cell Immunol. 2013; 2013: 006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh RB, Marmalidou A, Amouzegar A, Chen Y, Dana R. Animal models of high-risk corneal transplantation: a comprehensive review. Exp Eye Res. 2020; 198: 108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wynn TA. Fibrotic disease and the TH1/TH2 paradigm. Nat Rev Immunol. 2004; 4: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nevers T, Salvador AM, Velazquez F, et al.. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med. 2017; 214: 3311–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005; 5: 375–386. [DOI] [PubMed] [Google Scholar]
- 37. Liu J, Wang F, Luo F. The role of JAK/STAT pathway in fibrotic diseases: molecular and cellular mechanisms. Biomolecules. 2023; 13: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wynn T, Barron LM. Master regulators of inflammation and fibrosis. Semin Liver Dis. 2010; 30: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.