Abstract
Aims:
Primary skin mucinous carcinoma is a rare sweat gland neoplasm with a high local recurrence rate after conventional excision but a low distant-metastasis rate. The genetic underpinning of skin mucinous carcinoma is presently unknown. Here, we sought to define whether the repertoire of somatic mutations of a primary mucinous carcinoma of the skin would be similar to that of mucinous breast carcinomas, given the histologic similarities between these tumor types.
Methods and results:
The tumor was situated in the dermis and partially involved the subcutaneous fat. Tumor cells were suspended in periodic acid–Schiff diastaseresistant- positive mucin lakes and expressed cytokeratin 7, synaptophysin and estrogen receptor. DNA samples extracted from microdissected tumor and matched normal tissue were subjected to massively parallel sequencing targeting 410 cancer-related genes. The skin mucinous tumor was found to have a low tumor mutation burden, but to harbor a clonal GATA3 frameshift mutation (p. T418Hfs*89) and amplification of FOXA1, genes not uncommonly altered in breast mucinous carcinomas.
Conclusions:
In this primary skin mucinous carcinoma, GATA3 and FOXA1 driver genetic events were identified, consistent with a possible developmental relationship between skin and breast mucinous neoplasms.
Keywords: Primary skin mucinous carcinoma, Targeted sequencing, Immunohistochemistry
1. Introduction
Primary skin mucinous carcinoma is a rare, low-grade cutaneous neoplasm that commonly displays a rather indolent course [1]. It is believed to originate from apocrine sweat glands, showing a predilection for the head and especially the eyelids, but on occasions affecting also other sites of the body. Recurrence rates of skin mucinous carcinoma reach up to 26% of cases but metastases are exceedingly rare, involving primarily regional lymph nodes [1,2].
Skin mucinous carcinomas are composed of islands of cuboidal neoplastic cells suspended in pools of periodic acid–Schiff (PAS) positive mucin, separated by delicate fibrous trabeculae [3]. Lymphocytic infiltrate is often mild, neuroendocrine differentiation, albeit rare, has been reported in a minority of cases [4]. In fact, these tumors bear a greater histologic resemblance to breast mucinous carcinomas than to mucinous carcinomas of other anatomical sites (e.g., colon, pancreas and lung), including the presence of glandular differentiation, cribriform, solid or micropapillary growth patterns. The histologic similarities may stem from a possible common developmental origin [2]. The repertoire of genetic alterations in skin mucinous carcinomas have yet to be reported. On the other hand, studies have demonstrated that mucinous breast carcinomas, less frequently harbor PIK3CA mutations, chromosome 1q gains and 16q losses than common forms of breast cancer, and, conversely, display a higher frequency of GATA3 mutations [5]. In contrast with colorectal, pancreatic and ovarian mucinous carcinomas, breast mucinous carcinomas lack microsatellite instability, KRAS and BRAF mutations [6–8]. In this hypothesis-generating study, we investigated the repertoire of somatic genetic alterations of a skin mucinous carcinoma to define whether its genetic features would more closely recapitulate those of breast mucinous carcinomas rather than mucinous carcinomas from other anatomical sites.
2. Materials and methods
2.1. Immunohistochemical and histochemical analyses
Immunohistochemical studies were performed on 4-μm-thick sections of formalin-fixed paraffin embedded tissue sections with the following antibodies on a DAKO autostainer: Estrogen receptor (ER) (clone SP1; 1:200; Thermo Fisher Scientific); Cytokeratin 7 (CK7) (clone OV-TL 12/30; 1:300; DAKO); Synaptophysin (clone SP11; 1:200; Thermo Fisher Scientific); Cytokeratin 20 (CK20) (clone Ks20.8; 1:200; DAKO), p63 (DAK-p63, RTU; DAKO), GATA3 (L50–823; 1:100; Cell Marque), GCDFP15 (RTU; DAKO), PgR (clone 16; RTU; Leica) and S100 (clone Ab-8; 1:200; Thermo Fisher Scientific). Periodic Acid-Schiff (PAS) Diastase Stain kit (BiogGenex) was employed for histology slide staining, following the manufacturer’s protocol.
2.2. Targeted capture massively parallel sequencing
DNA samples extracted from microdissected tumor and normal tissues were subjected to MSK-IMPACT sequencing targeting 410 cancer-related genes at Memorial Sloan Kettering Cancer Center’s (MSK’s) Integrated Genomics Operation (IGO), as previously described [9]. Bioinformatic analyses for the identification of somatic mutations, their potential functional effect, copy number alterations (CNAs) and cancer cell fractions (CCFs), and for assessing clonal relatedness were performed as previously described [10]. This study was approved by the Patras General Hospital Scientific and Bioethics committee (89/09012018).
3. Case report
A 62-year-old woman, with an otherwise unremarkable medical history, presented with a non-ulcerating soft nodule in the gluteal region, which was surgically excised. Gross analysis revealed a tumor mass of 2.5 cm in its greatest dimension, occupying the whole dermis. The tumor presented as a solid mass near the epidermis but of a more gelatinous consistency at the deeper margin, with no macroscopic evidence of epidermal involvement and sufficient surgical margins (minimum 4 mm). The patient was referred for a thorough medical evaluation, including CT scan and gastroscopy, with no other primary tumors being identified.
Histologic examination revealed a circumscribed tumor mass with large amounts of mucin in the center and a more solid tumor cell conformation at the periphery, classifying the present tumor as a mixed mucinous carcinoma (~50% mucinous element) with an invasive ductal component. Further, mucin pools and islands of scattered floating cells were compartmentalized by fine fibrous septa occupying the dermis at full thickness. The proliferation was composed of mid-sized cells with abundant eosinophilic cytoplasm and round to oval-shaped nuclei, displaying a few prominent nucleoli (Fig. 1A). The mitotic rate was 3 mitosis/10 HPFs (High Power Fields). An in situ component was identified by a partial, disorganized, p63-positive myoepithelial cell layer in some cell-nests in the periphery of the tumor, further supporting the notion that this is a primary lesion (Fig. 1B). Lakes of mucin were positive for PAS diastase-resistant stain while immunohistochemical analysis revealed that tumor cells expressed CK7, synaptophysin, PgR, GATA3 and ER but lacked CK20, GCDFP15 and S100 protein expression (Fig. 1C).
Fig. 1.
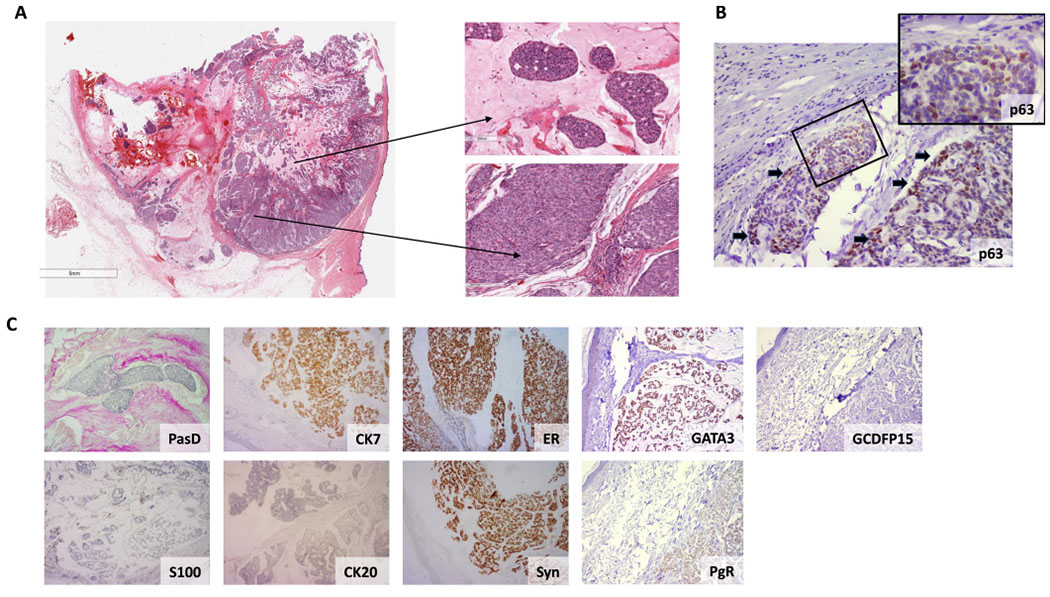
Histopathologic features of a primary mucinous carcinoma of the skin. (A) Low power magnification of the skin nodule extending from the epidermis to the lower sub cutis (Left panel). Magnification of two tumor areas with a variable degree of cellularity and mucin production (Right panel, magnification 20x). Neoplastic cells are predominantly uniform round to cuboidal exhibiting mild to moderate nuclear variability. (B) A partial, disorganized, p63-positive myoepithelial cell layer in some cell nests at the tumor periphery, indicative of an in situ component (magnification 10x). Black arrows indicate p63 positive cells, suggestive of a layer of myoepithelial cells. Insert magnification of the squared area is 40x. (C) Tumors cells produce PasD-positive mucin. Immunohistochemical analysis revealed tumor cells expressing CK7, estrogen receptor (ER), progesterone receptor (PgR), GATA binding protein 3 (GATA3) and Synaptophysin (Syn) but lacking GCDFP15, CK20 and S100 protein expression (magnification 4x).
Targeted sequencing analysis at depth of 1454x for the tumor and 730x for the normal sample revealed a low tumor mutational burden (TMB) with 3 somatic mutations, including 2 non-synonymous mutations. Furthermore, the tumor was microsatellite stable based on MSI-sensor analysis [11]. The tumor harbored a clonal heterozygous frameshift mutation in GATA3 (p.Thr419fs*89), a haploinsufficient transcription factor. Using a combination of mutation function predictors, the GATA3 insertion leads to a GATA3 truncated protein, previously reported in breast cancer (COSMIC, cancer.sanger.ac. uk/cosmic) (Fig. 2A) and inferred to be a driver somatic genetic alteration. In addition, a clonal somatic single nucleotide variant (SNVs) affecting the RAF1 gene (p.Thr324Ile) was identified, which was considered a passenger mutation. Gene copy number analysis revealed a relatively complex genome lacking concurrent chromosome 1q gain and 16q loss, but harboring FOXA1 (14q21.1) gene amplification and chromosome 20q12-q13 and 20q21 amplification/ high level gain, features akin to those observed in ER-positive breast cancer [5,12] (Fig. 2B).
Fig. 2.
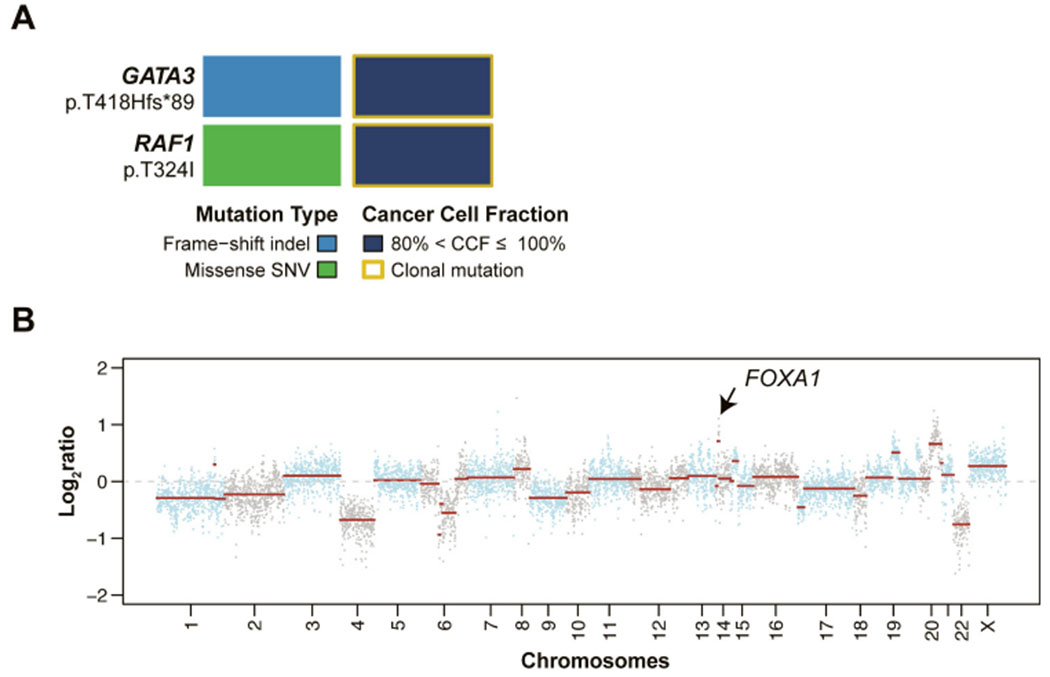
Mutational and gene copy number profile of the primary skin mucinous carcinoma. (A) Non-synonymous somatic mutations and their cancer cell fractions (CCFs) identified in the skin mucinous carcinoma, color-coded according to the legend. SNV, single nucleotide variant. (B) Copy number plot with Log2-ratios on the y-axis and chromosome location on the x-axis.
4. Discussion
Here we demonstrate that consistent with the histologic similarities between primary skin mucinous carcinomas and mucinous breast cancers, these lesions also harbor similar repertoires of somatic genetic alterations, including GATA3 somatic frameshift mutation, lack of concurrent chromosome 1q gain and 16 loss, and amplification of FOXA1, features that are not uncommonly found in breast mucinous carcinomas [5,13,14]. In conjunction with the histologic features of the skin tumor and ER expression, in the absence of a primary breast neoplasm, the findings from this hypothesis-generating analysis further support the notion of skin and breast mucinous carcinomas may be related. Conversely, the skin mucinous carcinoma analyzed here lacked microsatellite instability and mutations in KRAS and BRAF, features present in gastrointestinal and ovarian mucinous carcinomas, respectively [6–8]. Finally, it should be noted that rare lesions arising in the anogenital area (from the so-called anogenital mammary-like glands) have been described, which histologically resemble their mammary counterparts [15].
This study has several limitations. The sequencing analysis performed was limited to 410 cancer-related genes; nevertheless, our results are consistent with the notion that skin mucinous carcinomas may be more closely related to breast mucinous carcinomas rather than other skin lesions. In addition, RNA-sequencing could not be performed due to insufficient RNA quantity/ quality, and thus information on putative driver fusion genes is not available. Further studies analyzing a large series of skin mucinous carcinomas employing more comprehensive sequencing approaches are warranted to delineate the extent of the genomic similarities between mucinous carcinomas from these anatomical sites.
Funding
Research reported in this publication was supported in part by a Cancer Center Support Grant of the NIH/NCI (Grant No. P30CA008748). JSR-F was funded in part by Breast Cancer Research Foundation and Susan G Komen Leadership grants, BW by Breast Cancer Research Foundation and Cycle for Survival grants.
Abbreviations:
- PasD
Periodic acid–Schiff Diastase
- CCF
Cancer cell fraction
- CNA
copy number alteration
- MSK
Memorial Sloan Kettering Cancer Center
- MSK-IMPACT
Integrated Mutation Profiling of Actionable Cancer Targets
Footnotes
Declaration of Competing Interest
JSR-F reports receiving personal/consultancy fees from Goldman Sachs, Bain Capital, REPARE Therapeutics, Saga Diagnostics and Paige. AI, membership of the scientific advisory boards of VolitionRx, REPARE Therapeutics and Paige.AI, membership of the Board of Directors of Grupo Oncoclinicas, and ad hoc membership of the scientific advisory boards of AstraZeneca, Merck, Daiichi Sankyo, Roche Tissue Diagnostics and Personalis, outside the scope of this study. BW reports research funding from Repare Therapeutics, outside the scope of the submitted work. The remaining authors have no conflicts of interest to declare.
CRediT authorship contribution statement
ADP and JSR-F performed the histopathologic review, conceived and designed the study. ADP, MRDF, PS, CS, MR, BW provided tissue samples, carried out experiments and/or analyzed data. ADP, BW and JSR-F wrote the manuscript. All authors read, edited and approved the final manuscript.
References
- [1].Calonje E, Brenn T, Lazar AJ, et al. McKee’s Pathology of the Skin: with Clinical Correlations Fifth Edition, Elsevier, Philadelphia, PA, 2020. [Google Scholar]
- [2].Kazakov DV, Suster S, LeBoit PE, et al. Mucinous carcinoma of the skin, primary, and secondary: a clinicopathologic study of 63 cases with emphasis on the morphologic spectrum of primary cutaneous forms: homologies with mucinous lesions in the breast, Am. J. Surg. Pathol 29 (2005) 764–782, 10.1097/01.pas.0000159104.02985.6b. [DOI] [PubMed] [Google Scholar]
- [3].Moulin AP, Oberic A, Lechneitner Y, Spahn B, Letovanec I, Ikonomidis C, Primary mucinous carcinoma of the skin with orbital invasion, Klin. Monbl Augenheilkd 232 (2015) 506–508, 10.1055/s-0035-1545787. [DOI] [PubMed] [Google Scholar]
- [4].Miquelestorena-Standley E, Dujardin F, Arbion F, et al. Recurrent primary cutaneous mucinous carcinoma with neuroendocrine differentiation: case report and review of the literature, J. Cutan. Pathol 41 (2014) 686–691, 10.1111/cup.12347. [DOI] [PubMed] [Google Scholar]
- [5].Pareja F, Lee JY, Brown DN, Piscuoglio S, et al. The genomic landscape of mucinous breast cancer, J. Natl. Cancer Inst 111 (7) (2019) 737–741, 10.1093/jnci/djy216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kakar S, Deng G, Smyrk TC, et al. Loss of heterozygosity, aberrant methylation, BRAF mutation and KRAS mutation in colorectal signet ring cell carcinoma, Mod. Pathol 25 (2012) 1040–1047, 10.1038/modpathol.2012.44. [DOI] [PubMed] [Google Scholar]
- [7].Gemignani ML, Schlaerth AC, Bogomolniy F, et al. Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma, Gynecol. Oncol 90 (2003) 378–381, 10.1016/s0090-8258(03)00264-6. [DOI] [PubMed] [Google Scholar]
- [8].Hugen N, Simons M, Halilovic A, et al. The molecular background of mucinous carcinoma beyond MUC2, J. Path Clin. Res 1 (2014) 3–17, 10.1002/cjp2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Geyer FC, Li A, Papanastasiou AD, Smith A, et al. Recurrent hotspot mutations in HRAS Q61 and PI3K-AKT pathway genes as drivers of breast adenomyoepitheliomas, Nat. Cornmun 9 (1) (2018), 1816, 10.1038/S41467-018-04128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moukarzel LA, Da Cruz Paula A, Ferrando L, et al. Clonal relationship and directionality of progression of synchronous endometrial and ovarian carcinomas in patients with DNA mismatch repair-deficiency associated syndromes, Mod. Pathol 34 (5) (2021) 994–1007, 10.1038/s41379-020-00721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Niu B, Ye K, Zhang Q, Lu C, Xie M, McLellan MD, Wendl MC, Ding L, MSIsensor: microsatellite instability detection using paired tumor-normal sequence data, Bioinformatics 30 (7) (2014) 1015–1016, 10.1093/bioinformatics/btt755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Augello MA, Hickey TE, Knudsen KE, FOXA1: master of steroid receptor function in cancer, EMBO J. 30 (19) (2011) 3885–3894, 10.1038/emboj.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fujii H, Anbazhagan R, Bornman DM, et al. Mucinous cancers have fewer genomic alterations than more common classes of breast cancer, Breast Cancer Res Treat. 76 (2002) 255–260, 10.1023/a:1020808020873. [DOI] [PubMed] [Google Scholar]
- [14].Weigelt B, Geyer FC, Horlings HM, et al. Mucinous and neuroendocrine breast carcinomas are transcriptionally distinct from invasive ductal carcinomas of no special type, Mod. Pathol 22 (2009) 1401–1414, 10.1038/modpathol.2009.112. [DOI] [PubMed] [Google Scholar]
- [15].Kazakov DV, Spagnolo DV, Kacerovska D, Michal M, Lesions of anogenital mammary-like glands: an update, Adv. Anat. Pathol 18 (1) (2011) 1–28, 10.1097/PAP.0b013e318202eba5. [DOI] [PubMed] [Google Scholar]


