Abstract
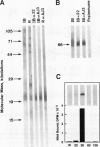
We examined the changes in the levels of indoleacetic acid (IAA), IAA esters, and a 22-kilodalton subunit auxin-binding protein (ABP1) in apical mesocotyl tissue of maize (Zea mays L.) during continuous red light (R) irradiation. These changes were compared with the kinetics of R-induced growth inhibition in the same tissue. Upon the onset of continuous irradiation, growth decreased in a continuous manner following a brief lag period. The decrease in growth continued for 5 hours, then remained constant at 25% of the dark rate. The abundance of ABP1 and the level of free IAA both decreased in the mesocotyl. Only the kinetics of the decrease in IAA within the apical mesocotyl correlated with the initial change in growth, although growth continued to decrease even after IAA content reached its final level, 50% of the dark control. This decrease in IAA within the mesocotyl probably occurs primarily by a change in its transport within the shoot since auxin applied as a pulse moved basipetally in R-irradiated tissue at the same rate but with half the area as dark control tissue. In situ localization of auxin in etiolated maize shoots revealed that R-irradiated shoots contained less auxin in the epidermis than the dark controls. Irradiated mesocotyl grew 50% less than the dark controls even when incubated in an optimal level of auxin. However, irradiated and dark tissue contained essentially the same amount of radioactivity after incubation in [14C]IAA indicating that the light treatment does not affect the uptake into the tissue through the cut end, although it is possible that a small subset of cells within the mesocotyl is affected. These observations support the hypothesis that R causes a decrease in the level of auxin in epidermal cells of the mesocotyl, consequently constraining the growth of the entire mesocotyl.
Full text
PDF
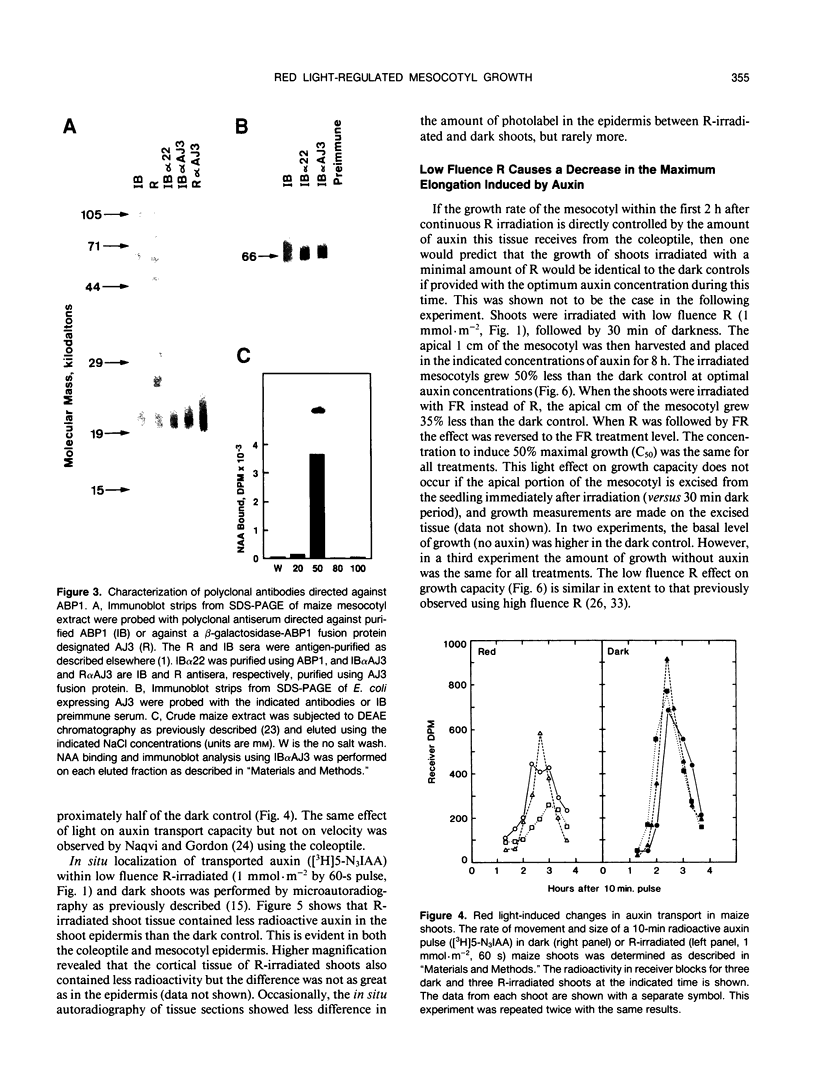
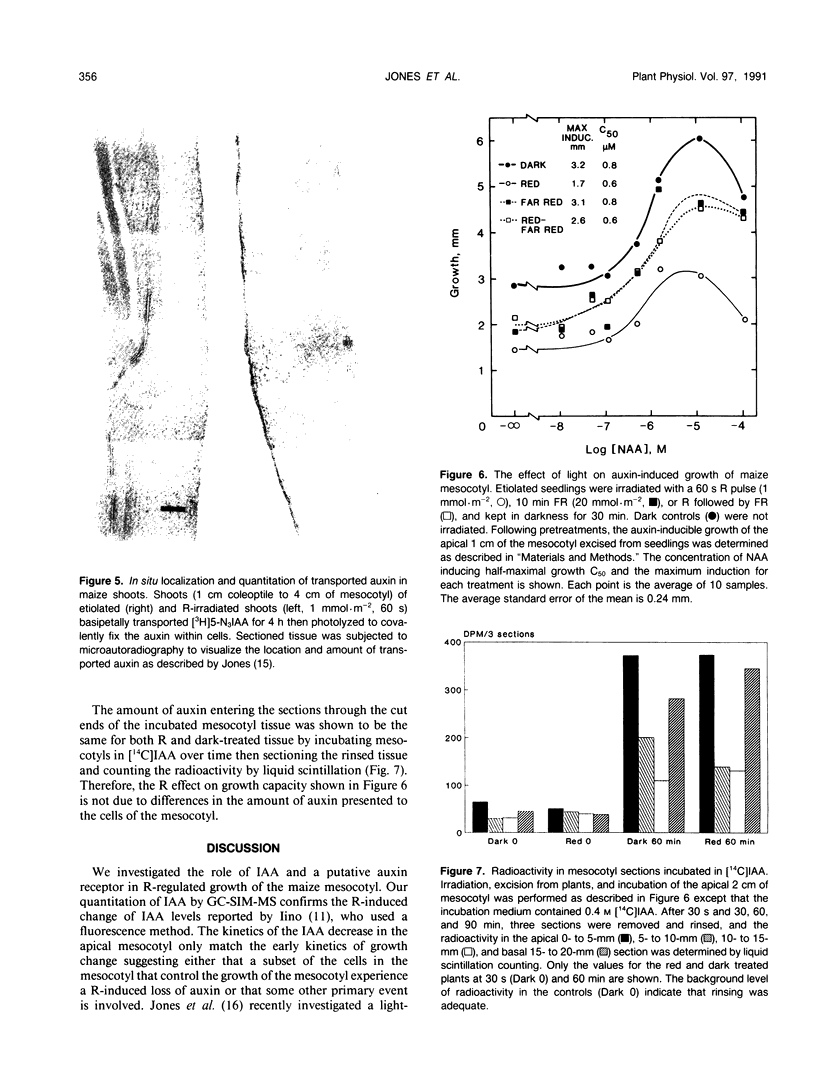


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandurski R. S., Schulze A., Cohen J. D. Photo-regulation of the ratio of ester to free indole-3-acetic acid. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1219–1223. doi: 10.1016/0006-291x(77)91136-6. [DOI] [PubMed] [Google Scholar]
- Barbier-Brygoo H., Ephritikhine G., Klämbt D., Ghislain M., Guern J. Functional evidence for an auxin receptor at the plasmalemma of tobacco mesophyll protoplasts. Proc Natl Acad Sci U S A. 1989 Feb;86(3):891–895. doi: 10.1073/pnas.86.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleiss W., Smith H. Rapid suppression of extension growth in dark-grown wheat seedlings by red light. Plant Physiol. 1985 Mar;77(3):552–555. doi: 10.1104/pp.77.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. H., Miller A. N., Patterson G. W., Cohen J. D. A Rapid and Simple Procedure for Purification of Indole-3-Acetic Acid Prior to GC-SIM-MS Analysis. Plant Physiol. 1988 Mar;86(3):822–825. doi: 10.1104/pp.86.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L. A New Sensitive Root Auxanometer: Preliminary Studies of the Interaction of Auxin and Acid pH in the Regulation of Intact Root Elongation. Plant Physiol. 1976 Oct;58(4):599–601. doi: 10.1104/pp.58.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring C. A., Williams D. A., Cody S. H., Parish R. W. Phototropism and geotropism in maize coleoptiles are spatially correlated with increases in cytosolic free calcium. Nature. 1990 Jun 7;345:528–530. doi: 10.1038/345528a0. [DOI] [PubMed] [Google Scholar]
- Green A. Tracking cobalt project. Nature. 1965 Sep 18;207(5003):1311–1311. doi: 10.1038/2071311a0. [DOI] [PubMed] [Google Scholar]
- Guo L. H., Stepień P. P., Tso J. Y., Brousseau R., Narang S., Thomas D. Y., Wu R. Synthesis of human insulin gene. VIII. Construction of expression vectors for fused proinsulin production in Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):251–254. doi: 10.1016/0378-1119(84)90186-0. [DOI] [PubMed] [Google Scholar]
- Inohara N., Shimomura S., Fukui T., Futai M. Auxin-binding protein located in the endoplasmic reticulum of maize shoots: molecular cloning and complete primary structure. Proc Natl Acad Sci U S A. 1989 May;86(10):3564–3568. doi: 10.1073/pnas.86.10.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Rubery P. H. Naturally occurring auxin transport regulators. Science. 1988 Jul 15;241(4863):346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Jones A. M. Location of transported auxin in etiolated maize shoots using 5-azidoindole-3-acetic Acid. Plant Physiol. 1990 Jul;93(3):1154–1161. doi: 10.1104/pp.93.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. M., Melhado L. L., Ho T. H., Leonard N. J. Azido auxins: quantitative binding data in maize. Plant Physiol. 1984 Feb;74(2):295–301. doi: 10.1104/pp.74.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. M., Venis M. A. Photoaffinity labeling of indole-3-acetic acid-binding proteins in maize. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6153–6156. doi: 10.1073/pnas.86.16.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhado L. L., Jones A. M., Leonard N. J., Vanderhoef L. N. Azido auxins: synthesis and biological activity of fluorescent photoaffinity labeling agents. Plant Physiol. 1981 Aug;68(2):469–475. doi: 10.1104/pp.68.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi S. M., Gordon S. A. Auxin Transport in Zea mays Coleoptiles II. Influence of Light on the Transport of Indoleacetic Acid-2-C. Plant Physiol. 1967 Jan;42(1):138–143. doi: 10.1104/pp.42.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer J. A., Mandoli D. F., Briggs W. R. Phytochrome-mediated cellular photomorphogenesis. Plant Physiol. 1983 Jul;72(3):706–712. doi: 10.1104/pp.72.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann U., Viola G., Kayser B., Siemeister G., Hesse T., Palme K., Löbler M., Klämbt D. cDNA clones of the auxin-binding protein from corn coleoptiles (Zea mays L.): isolation and characterization by immunological methods. EMBO J. 1989 Sep;8(9):2463–2467. doi: 10.1002/j.1460-2075.1989.tb08381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Quail P. H., Briggs W. R. Red Light-inhibited Mesocotyl Elongation in Maize Seedlings: II. Kinetic and Spectral Studies. Plant Physiol. 1979 Jun;63(6):1062–1067. doi: 10.1104/pp.63.6.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J. D., Ray P. M. Evidence for Receptor Function of Auxin Binding Sites in Maize : RED LIGHT INHIBITION OF MESOCOTYL ELONGATION AND AUXIN BINDING. Plant Physiol. 1981 Dec;68(6):1334–1338. doi: 10.1104/pp.68.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner T. J., Ross J. D. Phytochrome control of maize coleoptile section elongation: the role of cell wall extensibility. Plant Physiol. 1981 Nov;68(5):1024–1026. doi: 10.1104/pp.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]