Abstract
Context:
SARS-CoV-2 virus causes COVID-19 by infecting nasal and oral cavities primarily by attaching its spike proteins to ACE 2 receptors expressed in epithelial cells.
Aim:
This study was done to evaluate the micronucleated cell count, metanuclear abnormalities, and genotoxic factor in exfoliated buccal mucosal cell among the COVID-19 suspected patients.
Settings and Design:
This cross-sectional study was conducted at AIIMS, Mangalagiri, between August and October 2022.
Methods:
One hundred COVID-19 suspected patients were recruited for this study after obtaining informed and written consent; buccal smear was obtained and stained for papanicolaou test (PAP). The PAP-stained slides were analyzed for micronuclei (MN), pyknotic, karyolytic, and karyorrhexic cell count, respectively. Based on their reverse transcription-polymerase chain reaction (RT-PCR) report, the patients were grouped into COVID-19 positive and negative groups.
Statistical Analysis:
The genotoxicity factor was calculated using the micronucleated cell count from both the groups using mean and standard deviation.
Results:
The MN, micronucleated cell, pyknotic, karyolitic, and karyorrhexic cell count in COVID-19 positive patients were 24.12, 15.24, 3.08, 2.88 and 4.40, respectively, than COVID-19 negative patients 5.69, 8.17, 1.08, 1.00 and 2.43, respectively. The genotoxicity factor for SARS-CoV-2 was 2.68 which is a positive genotoxic effect on buccal mucosal cells.
Conclusion:
SARS-CoV-2 increases the expression of micronucleated cells, pyknotic cells, karyolytic cells, and karyorhexic cells and concludes SARS-CoV-2 is having cytogenotoxic effect on the buccal mucosal cells. This can be used as a reliable marker in identifying the early carcinogenic effects of virus causing COVID-19.
Keywords: Buccal mucosal cells, COVID 19, micronuclei
INTRODUCTION
The novel corona virus outbreak started in December 2019 and is still a persisting pandemic which has infected over 620 million people and caused the death of more than 6.8 million people worldwide.[1] The outbreak started in Wuhan, Hubei province in China caused by a novel corona virus named SARS-CoV-2, the disease was named as COVID-19.[2] SARS-CoV-2 virus spike proteins bind exclusively to angiotensin converting enzyme II receptors (ACE 2) present in human respiratory tract and gastrointestinal tract like alveolus, throat, nasal and oral mucosa leading to fever, dry cough, fatigue, loss of taste, dyspnea, progressive alveolar damage, and death.[2,3] Most of the viral infections in a cell cause cellular and nuclear damage.[4] Recurrent insult through infection and inflammation can result eventually in more evident nuclear damage that can be visualized as micronuclear and metanuclear abnormalities.
Micronuclear and metanuclear cells
The microscopically visible round or oval cytoplasmic chromatin within the cell due to aberrant mitosis consisting of chromatin fragments that have failed to incorporate into daughter nuclei is known as micronuclei (MN).[5] Micronucleus has been identified as a useful marker of cell damage in various exposures conditions caused by tobacco chewing,[6] smoking,[7] alcohol consumption[8] and even in infectious diseases.[9] Micronucleus assay usually done in buccal mucosal smear is one of the sensitive markers of DNA damage, used for genotoxicity studies[5] and is a parameter to identify high-risk individuals susceptible to oral lesions.[10] The metanuclear alterations are nuclear changes like pyknosis, karyorrehexis, and karyolysis which are considered as indicators of cytogenic toxicity.[11] Pyknosis refers to the shrinkage of the cell nucleus, karyorrhexis is the disintegration of the nuclear membrane, fragmentation, and distribution of the basophilic chromatin materials throughout the cytoplasm of the cell, karyolysis is the dissolution of the cellular nucleus due to the lysosomal enzymes released by the damaged or dead cell [Figure 1].[12] COVID-19 has been infecting humans for the past few years and it may even persist longer; there is an urgent need to understand the exact pathogenesis of the disease as there are no studies done to evaluate the micronuclear and metanuclear cells. Since SARS-CoV-2 virus outbreak started in 2019 and still ongoing, many of us were infected several times either diagnosed or undiagnosed; this study was done aiming to get more insight into the cytogenic and genotoxic changes pertaining to the pathogenesis of this virus in detail.
Figure 1.
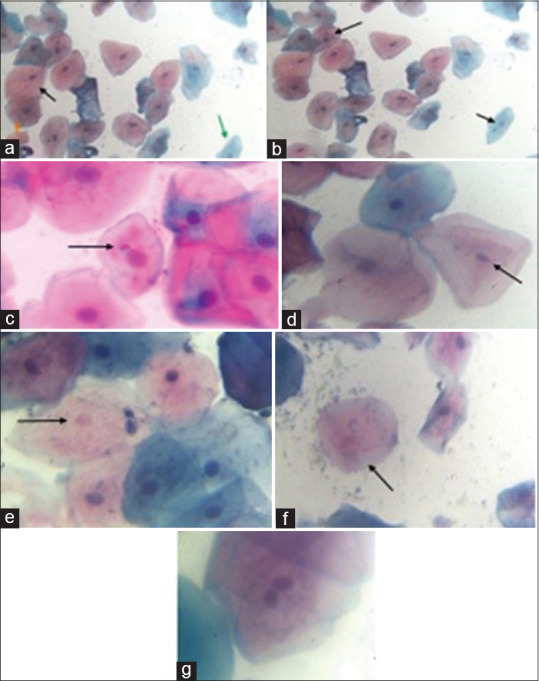
Exfoliated buccal mucosal cells stained with PAP stain (a) – superficial cells (Black arrow); (a) – keratinized squamous cell (orange arrow); (a) – intermediate cells (blue arrow); (b and c) – Micronucleus (black arrow); (d)- pyknotic nucleus; (e) – karyolitic nucleus; (f) – karyorrhexic nucleus; (g) – binucleated cell
MATERIAL AND METHODS
This cross-sectional study was conducted in symptomatic COVID-19 suspected patients between age group 18 and 45 years attending outpatient screening area for RT PCR – SARS-CoV-2 testing between August 2022 and October 2022 after obtaining clearance from Institute Ethics Committee (AIIMS/MG/IEC/2022-23/177) from the institute. A previous study[7] done for MN count among the control Indian population showed 10% of prevalence of micronucleated cell. Expecting the prevalence of micronucleated cell in buccal mucosa, more than 50% of COVID-19 positive patients with 5% alpha error and 80% power, the sample size was calculated to 25 in each group using Open Epi Version 3.01 Software by Bill and Melinda Gates Foundation, United States, 2013. Based on current COVID-19 positivity rates, 100 samples were required to achieve the expected sample size. [Group 1: RT-PCR positive patients (n = 25); Group 2: RT-PCR negative patients (n = 75)]. Asymptomatic patients, tobacco/pan chewing, smoking and alcoholism, pregnant women and lactating mothers and H/O any oral lesions and previous oral surgeries were excluded from the study. The buccal swab was taken using wooden spatula and smeared in marked glass slides and stained for PAP stain (RAPID-PAP). Appropriate COVID-19 precautionary measures were followed in all steps and all standard COVID-19 laboratory precautionary guidelines as per ICMR were followed.
Micronuclei count and metanuclear abnormalities count
The buccal smear-stained slides were focused in 100x objective (oil immersion method) and the zigzag method of counting MN and metanuclear abnormalities were followed until 100 intact exfoliated cells were counted. Only cells with complete and intact cytoplasm and nucleus with no overlapping were considered for counting. The MN with round, smooth periphery, staining intensity similar to that of nucleus was counted for the frequency. The metanuclear changes like number of pyknotic (shrinkage of nucleus), karyorhexic (nuclear fragmentation), and karyolytic (nuclear dissolution) cell count per 100 cells were counted and correlated between the two groups. Categorical variables like study group were summarized as frequency and proportion. Continuous variables like micronucleated cell count were summarized as mean and standard deviation. Association between categorical and continuous variable was analyzed using student’s “t” test. Association between categorical variables will be analyzed using Chi-square test. P-value less than 0.05 was considered as statistically significant.
Genotoxic factor
Genotoxicity factor was calculated to evaluate the level of genotoxicity expressed by the COVID-19 positive samples. It was calculated by

If the GF is more than 1.4, it will be taken as positive [Table 1].[13,14]
Table 1.
Ranges of Genotoxic factor (GF) with negative and positive value
| Genotoxic Factor Range | Outcome |
|---|---|
| GF ≤1.4 | Negative |
| 1.5 ≤ GF ≤2.9 | Slightly positive |
| 3.0≤ GF ≤14.9 | Positive |
| GF ≥15.0 | Strongly positive |
RESULTS
The exfoliated buccal cells stained with PAP smear showed varying shades of buccal squamous cells; eosin-stained cells are superficial squamous cells [Figure 1a black arrow]; orangish-stained cells are keratinized squamous cells [Figure 1a orange arrow]; and hematoxylin-stained cells are intermediate squamous cells [Figure 1a green arrow]. The buccal mucosal cells showed extra nuclear material with round, smooth periphery, and same staining intensity of nucleus which are MN [Figure 1b and c]. The metanuclear changes like pyknotic nucleus [Figure 1d], karyolytic nucleus [Figure 1e], and karyorrhexic nucleus [Figure 1f] were counted under higher magnification and tabulated. There was one binucleated cell found in one COVID-19 positive sample [Figure 1g].
Nuclear abnormalities
The mean of total micronucleated cell count and MN count in COVID-19 positive was 15.24 ± 5.897 and 24.12 ± 12.245, respectively, [Table 2] which was significantly higher than the COVID-19 negative micronucleated cell count and total MN count which were 5.69 ± 2.691 and 8.17 ± 4.078, respectively. The mean metanuclear count analysis [Table 3] showed pyknotic nucleus was 3.08 ± 2.197, karyorhexic nucleus was 2.88 ± 1.740, and karyolytic nucleus was 4.40 ± 2.614 in COVID-19 positive patients which was significantly higher than COVID-19 negative patients that showed pyknotic nucleus 1.08 ± 1.271, karyorhexic nucleus 1.00 ± 1.36, and karyolytic nucleus 2.43 ± 2.243.
Table 2.
Comparison of micronucleated cell count and total micronuclei count between COVID 19 positive and negative patients
| Items | COVID 19-Status | Mean | Std. Deviation | p value |
|---|---|---|---|---|
| Micro nucleated Cell count | Positive | 15.24 | 5.897 | <0.001* |
| Negative | 5.69 | 2.691 | ||
| Total Micronuclei Count | Positive | 24.12 | 12.245 | <0.001* |
| Negative | 8.17 | 4.078 |
p<0.05 is considered statistically significant
Table 3.
Comparison of Metanuclear abnormalities between the COVID 19 positive and negative patients
| Items | COVID 19-Status | Mean | Std. Deviation | p Value |
|---|---|---|---|---|
| Pyknotic Cell count | Positive | 3.08 | 2.197 | <0.001* |
| Negative | 1.08 | 1.271 | ||
| Karyorhexic Cell count | Positive | 2.88 | 1.740 | <0.001* |
| Negative | 1.00 | 1.336 | ||
| Karyolytic Cell count | Positive | 4.40 | 2.614 | <0.001* |
| Negative | 2.43 | 2.243 |
p<0.05 is considered statistically significant
Genotoxicity factor
Genotoxicity factor was calculated by the frequency of micronucleated cell in COVID-19/frequency of micronucleated cell in COVID-19 negative samples which is 15.24/5.69 = 2.68. A GF of 2.68 shows a positive genotoxicity effect of COVID-19 infection on buccal mucosal cells.
DISCUSSION
The buccal mucosal permeability is an important aspect while analyzing the effects of SARS-CoV-2 and its mutagenic effects. The buccal mucosa is lined by nonkeratinized stratified squamous cells which are more permeable than keratinized cells lining palate and gingiva.[15] The multiple layers show different stages of differentiation between deepest layer and intermediate layer appear as blue color and superficial cells stained by eosin appear pink and keratinized cells from gingiva appear orangish in PAP stain [Figure 1]. The complete turnover of buccal mucosa epithelial cells is very rapid ranging from 5 to 6 days; however, the mature cells and nonviable cells remain on the surface.[16] Due to this high turnover rate, there will be continuous cell renewal produced by mitosis in the basal layer and migrate towards the surface to replace and maintain epithelial homeostasis.[17] Factors such as infection and inflammation can affect the mitotic figures leading to nuclear abnormalities like MN, binuclei, pyknosis, karyolysis, karyorhexic, and finally apoptosis. Any factor affecting balance of rate of mitosis and rate of programmed cell death (apoptosis) may lead to premalignant and malignant conditions.[18]
Micronuclei count
The direct ways of transmission of SARS-CoV-2 virus are through salivary and respiratory droplets as well as it may transmit through blood, urine, feces, and tears.[19] The oropharyngeal, nasopharyngeal, nasal and oral cavities were the potential sites of SARS-CoV-2 viruses’ replication.[20] SARS-CoV-2 entry factor like ACE 2 receptor had been identified in oral mucosa, tongue, and salivary gland.[21] In this study, there was a significant number of MN in buccal cells in COVID-19 positive patients than in COVID-19 negative patients. As MN are induced by chromosomal breakage, significant increase of this cytogenetic parameter in COVID-19 patients indicates mutagenicity of buccal epithelial cells due to genomic instability. There are various studies conducted on exfoliated buccal cells to study the cytogenetic changes following tobacco chewers,[6] smokers,[7] alcoholics,[8] painters,[22] and patients with breast carcinoma.[23] All these studies conclude there is a significant increase in frequencies of MN occurrences compared to healthy individuals predicting high risk for development of oral carcinomas. The quantitative evaluation of frequencies of MN in the buccal mucosa can be used as a biomarker in oral cancer susceptibility and its progression to the advanced stages, and this had been confirmed by a study done on oral mucosa of healthy, epithelial dysplasia, and oral squamous cell carcinoma.[24]
Micronuclei count in viral infections
The frequency of MN in chronic viral infections like human immunodeficiency virus (HIV),[25] hepatitis B, and hepatitis C[26] has been studied in buccal cells and lymphocytes proving there is significant increase in the quantity of micronucleated cells and it can be used as a screening method to identify early premalignant lesions. One study was done on the MN frequency in acute SARS-CoV-2 viral infections as a pilot study between COVID-19 infected patients and healthy subjects concluding a significant increase in the number of MN in COVID-19 infected patients and thus proving SARS-CoV-2 is causing cytotoxicity to epithelial cells.[27] Even to be more specific, the present study was done in symptomatic patients (symptomatic COVID-19 positive and symptomatic COVID-19 negative) and there was a significant increase in MN count thus confirming the cytogenotoxicity of SARS-CoV-2 virus than any other microorganisms causing flu-like illness.
Mechanism of formation of Micronuclei
The mechanism of MN formation is through malsegregation of sister chromatids due to inappropriate attachment of spindle microtubules to kinetochores.[28] The chromosomal malsegregation occurs due to hypomethylation of centromeric and paracentromeric regions called satellite repeats [Figure 2]. Usually these satellite repeats are hypermethylated and when this methylation is lost it leads to formation of decreased tension of kinetochores creating wrong connections between spindles and chromosomes during anaphase and lag behind at the telophase[29] [Figure 2]. This MN generates from acentric chromosomal fragments, acentric chromatid fragments, or whole chromosome which fail to be included in daughter nuclei during the completion of telophase of mitosis. The separated fragments are enclosed by nuclear membrane and they are morphologically similar to nuclear staining.[30]
Figure 2.
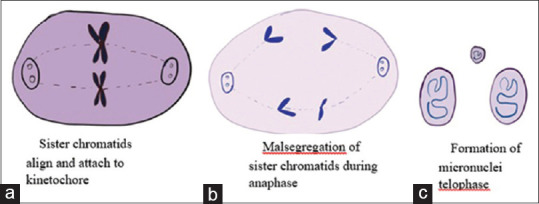
Mitotic division of a cell showing malsegregation of sister chromatids resulting in formation of micronuclei. (a) sister chromatids align and attach to kinetochore; (b) malsegregation of sister chromatids during anaphase; (c) Formation of micronuclei telophase
Metanuclear abnormalities in COVID-19 patients
There was significantly a higher number of pyknotic cells, karyolytic cells, and karyorrhexic cells in COVID-19 positive patients’ buccal smear than in symptomatic COVID-19 negative patients’ buccal smear. Pinto et al. 2021[27] showed only karyolytic and karyorrhexic cells were significantly higher in COVID-19 positive patients than in healthy controls. The karyolysis and karyorrhexis, which are induced by SARS-CoV-2, are closely associated with necrosis and apoptosis due to very high insult.[31] The pro-inflammatory cytokines like TNF alpha produced as an immune response from mononuclear cells[32] following SARS-CoV-2 is responsible for a diverse range of signaling pathways by activating nuclear factor kappa[33] [Figure 3] which results in karyolysis and karyorrhexis leading to necrosis or apoptosis. Early apoptosis is characterized by cell shrinkage condensation of cytoplasm, chromatin, and other organelles leading to pyknosis, followed by extensive plasma membrane blebbing, nuclear fragmentation causing karyorhexis, and finally cell fragments into apoptotic bodies.[34] In karyolytic cells, the nucleus is completely devoid of DNA and appears anucleated and this may happen due to an adaptive response to cellular trauma in pre-keratinization process that is evident in necrotic cytotoxic cells.
Figure 3.
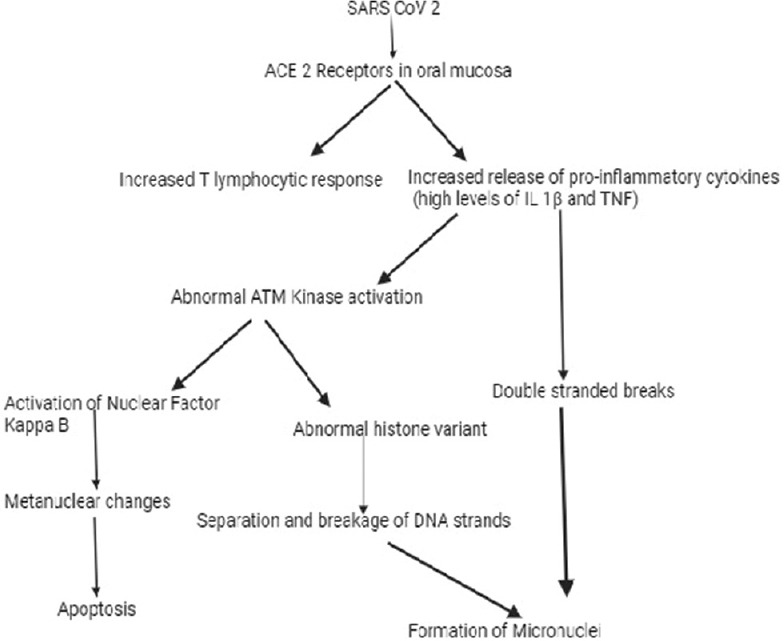
Possible molecular mechanism in formation of nuclear changes in COVID 19 infection
Possible molecular mechanisms involved in nuclear abnormalities in COVID-19 infection
The SARS-CoV-2 replicate by triggering inflammation through local immune cells activation thus induces mutagenesis and cytotoxicity in the oral and nasal mucosal cells. Previous studies in oral mucosal cells had proven increased local immune response with predominance of T lymphocytic response and increased local pro-inflammatory cytokines like interleukin 1 beta (IL-1β), tumor necrosis factor alpha (TNF–α),[35] and tumor growth factor beta (TGF β)[36] following SARS-CoV-2 infection. This excess activation and elevated immune response especially through interleukin-1 beta and TNF–α can alter the DNA damage response (DDR) through ataxia telangiectasia mutated (ATM) kinase which is responsible for regulating cellular response to double stranded breaks (DSB) leading to double-stranded breakage of DNA. This further leads to nuclear breakage forming number of MN depending upon the severity or apoptosis[33] through activation of nuclear factor kappa B based on severity of DNA damage [Figure 3].[31] The excess release of cytokines like tumor growth factor beta through ATM kinase activation can alter histone variants leading to genomic instability and separation of cleaved DNA strands.[36] Thus the broken strands of DNA will be included as MN in daughter cells and if there is extensive breakage it will form other metanuclear abnormalities and finally to apoptosis.
Quantification of MN in exfoliated cells is a promising tool in the study of epithelial carcinogens. This procedure is minimally invasive, low cost, easy to store, easy for slide preparation and staining, identifying MN and its frequency, low cost, well tolerated by the patients, and highly reliable in identifying genomic damage. This makes it a first choice for early detection of carcinogenic effects of various factors including infections.
Genotoxicity factor
The genotoxicity factor of SARS-CoV-2 in COVID-19 patients was 2.68 which shows positive genotoxicity effect of the virus on the buccal mucosal cells. This genotoxicity factor shows the level of genotoxicity expressed by the samples and compares it with the cutoff. The cutoff of more than 1.4 is considered as positive genotoxicity and in this study, it was 2.68 showing that SARS-CoV-2 is having a positive genotoxic effect on buccal mucosa.[13] According to the classification done by Regional Agency Environmental Protection, Emilia Romagna, Italy, ARPA ER, 1997–2001.[13] GFs less than 1.4 is considered as negative; range between 1.5 and 2.9 is slightly or weakly positive; range between 3.0 and 14.9 is positive and more than 15 is strongly positive. A similar study has been conducted on smokers and nonsmokers showing that a frequency of micronucleated cell was 17.57 and 3.53, respectively, and reported a GF of 4.9 in which the cigarettes are having high positiveGF.[14] As far as viral GF is concerned, this is the first study to report the GF and that too for acute highly contagious SARS-CoV-2 virus.
Limitations
This study only focused on exfoliated buccal cells and did not examine other biological samples or tissues that might provide more insight into the pathogenesis of COVID-19. At the time of small sample collection, there were very low positivity rates due to which the present sample size was calculated.
CONCLUSION
This study concludes that the SARS-CoV-2 causing COVID-19 is having positive genotoxicity effect and expresses its carcinogenic effect on buccal mucosal cells by increasing the expression of micronucleated cells, pyknotic cells, karyolytic cells, and karyorhexic cells and thus concluding it is having a cytogenotoxic effect on the buccal mucosal cells. These MN and metanuclear changes can be used as a reliable marker to identify the early genomic damage caused by SARS-CoV-2 virus expressing on buccal mucosal cells.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
This research work was selected among the ICMR-STS projects with reference number 2022-04105 and we are grateful to the ICMR-STS team. We would like to express our sincere gratitude to the co-guides, nursing officers, and supporting staff from the Department of Anatomy and ENT for help in obtaining samples and staining. VB likes to thank his guide (Dr PKS), for the constant motivation and never-ending support, without whom the project would have never happened.
REFERENCES
- 1.WHO Coronavirus (COVID-19) Dashboard. 2022. [[Last accessed on 2022 Oct 24]]. Available from: https://covid19.who.int .
- 2.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura T, Hasegawa-Nakamura K, Sakoda K, Matsuyama T, Noguchi K. Involvement of angiotensin II type 1 receptors in interleukin-1b-induced interleukin-6 production in human gingival fibroblasts. Eur J Oral Sci. 2011;119:345–51. doi: 10.1111/j.1600-0722.2011.00850.x. [DOI] [PubMed] [Google Scholar]
- 4.Drummond RL, Rhoden CR, Lubicana Neto JF, Fleck ADS, Padoin RCPK, Amantéa SL. Micronucleus count in nasal epithelial cells from patients with chronic rhinosinusitis and polyps. Braz J Otorhinolaryngol. 2020;86:743–7. doi: 10.1016/j.bjorl.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh S, Saini M, Yadav AS. Elevated frequencies of micronuclei and other nuclear anomalies in alcoholic subjects. J Entomol Zool Stud. 2015;3:243–5. [Google Scholar]
- 6.Sharma S, Rai S, Misra A, Sharma A, Misra D, Dhanpal R. Quantitative and qualitative analysis of micronuclei in the buccal mucosal cells of individuals associated with tobacco. MAMC J Med Sci. 2018;4:12–7. [Google Scholar]
- 7.Naderi NJ, Farhadi S, Sarshar S. Micronucleus assay of buccal mucosa cells in smokers with the history of smoking less and more than 10 years. Indian J Pathol Microbiol. 2012;55:433–8. doi: 10.4103/0377-4929.107774. [DOI] [PubMed] [Google Scholar]
- 8.Jeeva PT, Vidyasankar, Sankaran PK, Kishor KC. Effects of alcohol on micronucleus in human exfoliated buccal cells. IAIM. 2015;2:55–8. [Google Scholar]
- 9.Karlsson AC, Humbert M, Buggert M. The known unknowns of T cell immunity to COVID-19. Sci Immunol. 2020;5 eab:e8063. doi: 10.1126/sciimmunol.abe8063. [DOI] [PubMed] [Google Scholar]
- 10.Fenech M. The cytokinesis-block micronucleus technique and its application to genotoxicity studies in human populations. Environ Health Perspect. 1993;101((Suppl 3)):101–7. doi: 10.1289/ehp.93101s3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belien JA, Cooper MP, Braakhuis BJ, Snow GB, Baak JP. Standardization of counting micronuclei:Definition of a protocol to measure genotoxic damage in human exfoliated cells. Carcinogenesis. 1995;16:2395–400. doi: 10.1093/carcin/16.10.2395. [DOI] [PubMed] [Google Scholar]
- 12.Kumar V, Abbas AK, Aster AC. “Cell injury, cell death and adaptations”. 10th ed. Philadepa: Elsevier Saunders; 2020. Pathologic basis of diseases, Robbins &Cotran. Editors; pp. 10–40. [Google Scholar]
- 13.ARPAER (Agenzia Regionale Prevenzione e Ambiente dell'Emilia Romagna) Sezione Regionale di Parma, 1997- (Rete di Monitoraggio della Genotossicita del particolato atmosferico urbano) 2001 [Google Scholar]
- 14.Leonardi S, Poma AM, Colafarina S, D'Aloisio F, Scatigna M, Zarivi O, et al. Early genotoxic damage through micronucleus test in exfoliated buccal cells and occupational dust exposure in construction workers:A cross-sectional study in L'Aquila, Italy. Ecotoxicol Environ Saf. 2020;203:110989. doi: 10.1016/j.ecoenv.2020.110989. [DOI] [PubMed] [Google Scholar]
- 15.Elesawy BH, El-hafez AA, Mohamed AS, Salih MM. Application of Bethesda terminology to categorize buccal epithelial smears among petroleum station workers in Taif city, KSA. Int J Basic Appl Sci. 2016;5:172–6. [Google Scholar]
- 16.Borthakur G, Butryee C, Stacewicz-Sapuntzakis M, Bowen PE. Exfoliated buccal mucosa cells as a source of DNA to study oxidative stress. Cancer Epidemiol Biomarkers Prev. 2008;17:212–9. doi: 10.1158/1055-9965.EPI-07-0706. [DOI] [PubMed] [Google Scholar]
- 17.Metgud R, Khajuria N, Patel S, Lerra S. Nuclear anomalies in exfoliated buccal epithelial cells of petrol station attendants in Tamil Nadu. South India J Can Res Ther. 2015;11:868–73. doi: 10.4103/0973-1482.146058. [DOI] [PubMed] [Google Scholar]
- 18.Harris RE. Vol 1. Massachusetts: Jones and Bartlett Learning; 2015. Global Epidemiology of Cancer; pp. 4–6. [Google Scholar]
- 19.Karia R, Gupta I, Khandait H, Yadav A, Yadav A. COVID-19 and its modes of transmission. SN Compr Clin Med. 2020;2:1798–801. doi: 10.1007/s42399-020-00498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang WK, Chen SY, Liu IJ, Chen YC, Chen HL, Yang CF, et al. Detection of SARS-associated coronavirus in throat wash and saliva in early diagnosis. Emerg Infect Dis. 2004;10:1213–9. doi: 10.3201/eid1007.031113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong M, Lin B, Pathak JL, Gao H, Young AJ, Wang X, et al. ACE2 and furin expressions in oral epithelial cells possibly facilitate COVID-19 infection via respiratory and fecal–oral routes. Front Med. 2020;7:580796. doi: 10.3389/fmed.2020.580796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azubuike NC, Onwukwe OS, Onyemelukwe AO, Maduakor UC, Udoh IP, Ikele IT, et al. Micronucleus evaluation of exfoliated buccal epithelial cells from automobile spray painters –A preliminary study. Anat J Afr. 2019;8:1460–9. [Google Scholar]
- 23.Dey P, Samanta S, Susheilia S. Micronucleus assay in buccal smears of breast carcinoma patients. Diagn Cytopathol. 2010;40:664–6. doi: 10.1002/dc.21589. [DOI] [PubMed] [Google Scholar]
- 24.Kamini K, Padmanidhi A, Shailesh K, Jain K. Micronuclei as a predictor for oral carcinogenesis. J Cytol. 2018;35:233–6. doi: 10.4103/JOC.JOC_141_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah S, Singaraju S, Bertin ET, Singaraju M, Sharma A. Quantification of micronuclei in exfoliated cells of human immunodeficiency virus/AIDS-infected female patients. J Oral Maxillofac Pathol. 2019;23:301. doi: 10.4103/jomfp.JOMFP_251_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leite ST, Silva MB, Pepato MA, Souto FJ, Santos RA, Bassi-Branco CL. Increased frequency of micronuclei in the lymphocytes of patients chronically infected with hepatitis B or hepatitis C virus. Mem Inst Oswaldo Cruz. 2014;109:15–20. doi: 10.1590/0074-0276140183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto TG, Alpire MES, Ribeiro DA. Cytogenetic biomonitoring in buccal mucosa cells of COVID-19 patients:Preliminary findings. In Vivo. 2021;35:3495–9. doi: 10.21873/invivo.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cimini D, Degrassi F. Aneuploidy:A matter of bad connections. Trends Cell Biol. 2005;15:442–51. doi: 10.1016/j.tcb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Fenech M, Kirsch-Volders M, Natarajan AT, Surralles J, Crott JW, Parry J, et al. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis. 2011;26:125–32. doi: 10.1093/mutage/geq052. [DOI] [PubMed] [Google Scholar]
- 30.Norppa H, Falck GC. What do human micronuclei contain? Mutagenesis. 2003;18:221–3. doi: 10.1093/mutage/18.3.221. [DOI] [PubMed] [Google Scholar]
- 31.Jeffers L, Webster-Cyriaque JY. Viruses and salivary gland disease (SGD):Lessons from HIV SGD. Adv Dent Res. 2011;23:79–83. doi: 10.1177/0022034510396882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily:Structure-function relationship(s) Microsc Res Tech. 2000;50:184–95. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 33.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141–6. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 34.Nowsheen S, Yang ES. The intersection between DNA damage response and cell death pathway. Exp Oncol. 2012;34:243–54. [PMC free article] [PubMed] [Google Scholar]
- 35.Botros N, Iyer P, Ojcius DM. Is there an association between oral health and severity of COVID-19 complications? Biomed J. 2020;43:325–7. doi: 10.1016/j.bj.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin B, Savic V, Juntilla MM, Bredemeyer AL, Yang-Iott KS, Helmink BA, et al. Histone H2AX stabilizes broken DNA strands to suppress chromosome breaks and translocations during V(D)J recombination. J Exp Med. 2009;206:2625–39. doi: 10.1084/jem.20091320. [DOI] [PMC free article] [PubMed] [Google Scholar]


