Abstract
Healthy Gambian children, children with clinical Plasmodium falciparum malaria, and children with asymptomatic P. falciparum infections were studied to investigate whether antitoxic activities may contribute to protection against malarial symptoms. Markers of inflammatory reactions, soluble tumor necrosis factor receptor I, and C-reactive protein were found in high concentrations in children with symptomatic P. falciparum malaria compared with levels in children with asymptomatic P. falciparum infections or in healthy children, indicating that inflammatory reactions are induced only in children with clinical symptoms. Concentrations of soluble tumor necrosis factor receptor I and C-reactive protein were associated with levels of parasitemia. We detected antitoxic activities in sera as measured by their capacity to block toxin-induced Limulus amoebocyte lysate (LAL) activation. Symptomatic children had decreased capacity to block induction of LAL activation by P. falciparum exoantigen. The decreased blocking activity was restored in the following dry season, when the children had no clinical malaria. Symptomatic children also had the highest immunoglobulin G (IgG) reactivities to conserved P. falciparum erythrocyte membrane protein 1 and “Pfalhesin” (band #3) peptides, indicating that such IgG antibodies are stimulated by acute disease but are lost rapidly after the disease episode. Half of the children with symptomatic infections had low levels of haptoglobin, suggesting that these children had chronic P. falciparum infections which may have caused symptoms previously. Only a few of the children with asymptomatic P. falciparum infections had high parasite counts, and antitoxic immunity in the absence of antiparasite immunity appears to be rare among children in this community.
Asymptomatic Plasmodium falciparum infections are common among African children (10, 19). The risk of developing clinical symptoms increases with increasing levels of parasitemia, but a number of African children carry a high level of parasitemia without having symptoms. Markers of inflammatory reactions are not found in these asymptomatic children (16). It is possible that these children have acquired some degree of antitoxic immunity through the production of neutralizing molecules, such as antibodies to the malaria toxins, believed to be released at schizogony, which can stimulate cytokine production in host mononuclear cells (3, 18, 22). Parasite virulence is also determined by cytoadherence patterns of the parasite, mediated at least in part by P. falciparum erythrocyte membrane protein 1 (EMP-1) and the “Pfalhesin” epitope of band 3 (a band 3-derived neoantigen with cytoadherent properties) (2, 6, 8).
C-reactive protein (CRP) and tumor necrosis factor (TNF) alpha are markers of inflammatory reactions, but TNF has a short half-life in serum (5) while soluble TNF (sTNF) receptors circulate in serum longer than TNF and may therefore be a more reliable marker of cytokine activation. Haptoglobin binds and clears free hemoglobin released from ruptured infected erythrocytes, and a low level of haptoglobin is a marker of chronic malaria (20). Malaria parasite toxin activity can, like lipopolysaccharide (LPS) toxin activity, be measured in a number of ways, including after a pyrogenic reaction, by induction of TNF, interleukin 1 (IL-1), or IL-6 secretion and by activation of Limulus amoebocyte lysate (LAL). To investigate whether the development of antitoxic activities may contribute to the control of malarial symptoms, we have collected sera from Gambian children with clinical P. falciparum malaria, from children with asymptomatic P. falciparum infections, and from healthy noninfected children. We measured markers of inflammatory reactions and of chronic infections in sera as well as their toxin-neutralizing activities by the LAL assay. In addition, we measured antibody reactivities against Pfalhesin and against a conserved and a semiconserved peptide sequence of P. falciparum EMP-1.
MATERIALS AND METHODS
Donors and blood sampling.
The study was carried out between October 1993 and May 1994 in a rural area near the town of Farafenni, The Gambia. Parents or guardians gave informed consent for the participation of their children in the study, which was approved by the Medical Research Council Ethical Committee of The Gambia.
Three donor groups were defined by their clinical status at the time of blood collection, which took place during the rainy season. Group i consisted of children with symptomatic P. falciparum infections. These children had axillary temperatures of >37.5°C, P. falciparum parasitemia, and no other obvious causes for their fevers. Some of these children had an additional blood sample collected during the dry season in May 1994, none of whom had fever at that time. Group ii consisted of children with asymptomatic P. falciparum infections. These children had P. falciparum parasitemia and axillary temperatures of <37.5°C and were well. Group iii consisted of healthy children without fever and without demonstrable parasites in their peripheral blood.
Children with malaria or with asymptomatic P. falciparum infections were treated with chloroquine at a dose of 25 mg/kg of body weight given over three days. Treatment started approximately 24 h after blood films were collected.
Thick blood smears were stained with Field’s stain, and thin blood smears were stained with Giemsa. Parasite density was calculated per 100 high-power fields as described previously (9). Serum samples were frozen and kept at −20°C for 1 to 2 months in The Gambia. The samples were then transported on dry ice to Denmark and stored at −70°C until they were analyzed.
Determination of sTNF-RI.
Enzyme-linked immunosorbent assay (ELISA) kits were used as specified by the manufacturer to measure human sTNF receptor I (sTNF-RI; Research and Development Systems, Minneapolis, Minn.). Assay sensitivity was 25 pg/ml.
ELISA for haptoglobin and CRP.
Nunc (Roskilde, Denmark) Maxisorp plates were used. Rabbit antibodies (100 μl) against haptoglobin or against CRP (10 μg/ml; DAKO, Glostrup, Denmark) diluted in carbonate buffer, pH 9.6, were added to wells, and plates were incubated overnight at 4°C. Sera (1:10,000 for haptoglobin and 1:2,000 for CRP) were added and incubated at room temperature for 1 h. After being washed, peroxidase-conjugated rabbit anti-human CRP (DAKO) or biotinylated rabbit anti-human haptoglobin (DAKO), diluted 1:4,000, was incubated for 1 h at room temperature. For the haptoglobin ELISA, after a further wash, 100 μl of peroxidase-conjugated streptavidin (DAKO) diluted 1:5,000 in dilution buffer was added and plates were incubated for 1 h at room temperature. After a final wash, 100 μl of the substrate was added and coloration was stopped after 15 min with the addition of H2SO4. Absorbance was read at a wavelength of 492 nm. Dilutions of haptoglobin and CRP standards (Sigma and DAKO, respectively) were tested on the same plates. All tests were performed in duplicate.
ELISA for chloroquine.
Chloroquine levels were measured by an ELISA method with antibodies directed against the 7-chloroquinoline structure of the chloroquine molecule as described previously (7, 23). The antibodies recognize chloroquine metabolites and amodiaquine. Briefly, 50-μl serum samples (diluted 1:5) were each mixed with 200 μl of a chloroquine horseradish peroxidase conjugate, added to plates precoated with chloroquine-specific antibodies, and incubated for 1 h at room temperature. After the plates were washed, 100 μl of o-phenylenediamine in citrate buffer was added to each well and optical densities were determined. Samples from healthy individuals who had not received chloroquine and diluent were included on each plate as negative controls. Standards of chloroquine phosphate dissolved to various concentrations in normal serum were included on each plate. The ELISA detected chloroquine in individuals with levels higher than 5 ng/ml in sera.
Pfalhesin and P. falciparum EMP-1 peptide ELISA.
Peptides were obtained from Schafer (Copenhagen, Denmark). Two peptides derived from the published sequence of the P. falciparum var-1 gene of the Malayan Camp strain (2, 24) and the cytoadhesive band 3 peptide Pfalhesin (6) were synthesized. Amino acid sequences of the peptides given in the one-letter code are LENNLIEIFKKIHENL for the 1391 peptide, YDELLKRKENELF for the 1393 peptide, and DHPLQKTNY for the Pfalhesin peptide. All peptides were synthesized by the fluorenylmethoxycarbonyl method and analyzed by reversed-phase high-pressure liquid chromatography. Antibody reactivities to the peptides were measured by ELISA as described by Jakobsen (15). Maxisorp plates (Nunc) were coated with the synthetic peptides at 5 μg per well. Plates were washed after serum samples, diluted 1:100, were incubated for 1 h and after peroxidase-conjugated rabbit anti-human immunoglobulin G (IgG; DAKO) was incubated for 1 h. Finally, substrate was added, and the plates were read at a wavelength of 492 nm.
Exoantigens of P. falciparum for LAL activation.
The P. falciparum isolate HB-3/Honduras was kept in continuous culture with RPMI 1640 supplemented with 21 mM sodium bicarbonate, 25 mM HEPES buffer, and 5% human serum. The parasites were grown in a 4% (vol/vol) suspension of group O-positive human erythrocytes.
Exoantigens were affinity purified from culture medium as described previously (13) with a pool of IgG from clinically immune African adults as the ligand. Before chromatography, the culture medium was centrifuged at 7,000 × g for 10 min, filtered through a 0.22-μm-pore-size membrane, and dialyzed overnight at 4°C against column buffer.
The LAL assay for toxin activity.
This assay has been described in detail previously (26). The principle of the LAL peptide C ELISA is that toxins activate a cascade of enzymes in LAL and that this activation results in cleavage of the protein coagulogen, with subsequent exposure of an antigenic epitope residing in its peptide C region. The level of generation of peptide C immunoreactivity is directly proportional to the concentration of LAL-activating toxin (25).
All glassware was rendered pyrogen free by heating it to 250°C for at least 3 h. Sterile pyrogen-free tips and microplates were purchased from Eppendorf, Hamburg, Germany, and Nunc, respectively.
Commercial LAL preparations studied included Pyrotell (lot 42-99-541) from Associates of Cape Cod, Woods Hole, Mass., and LAL (lot 2L0860) from Whittaker Bioproducts, Walkersville, Md. The control standard endotoxin was NP-3, which is a purified preparation of LPS from Salmonella abortusequi purchased from Pyroquant Diagnostik, Waldorf, Germany. Its potency was confirmed to be 10 endotoxin units/ng in comparison with that of USP reference standard endotoxin EC-5. The monoclonal antibody against peptide C has been described in detail previously (25).
Twenty-microliter samples of LAL diluted 10-fold in LAL buffer (0.1 M Tris-HCl [pH 8.0] containing 0.15 M NaCl and 0.02 M MgCl2) were added to the wells of a pyrogen-free microplate and mixed with 10 μl of exoantigens or endotoxin and 10 μl of serum diluted 1:100. Some wells contained LAL mixed with exoantigens or endotoxin standards to estimate maximum stimulation. Other wells contained LAL mixed with sera alone to test the serum-mediated stimulation of LAL reactivity. Plates were then placed in a thermal incubator at 37°C for 45 min. The reaction was stopped by adding 200 μl of 50 mM NaOH to each well, and 50-μl aliquots of the mixtures were transferred to a microtiter plate (Maxiscorp; Nunc) to which 50 mM NaOH (50 μl per well) had previously been added. After incubation for 30 min, the plate was washed four times with washing buffer (phosphate-buffered saline containing 0.5 M NaCl and 0.05% Triton X-100 [pH 7.3]) and 100 μl of a biotinylated monoclonal antibody against peptide C mixed with streptavidin peroxidase (DAKO) at 1:2,000 in the dilution buffer (washing buffer containing 1% bovine serum albumin) was added to each well. After incubation for 30 min, the plate was washed four times and 100 μl of the substrate solution (0.4 mg of o-phenylenediamine dihydrochloride per ml and 0.014% H2O2 in 0.1 M sodium phosphate–citric acid buffer [pH 5.0]) was added. The color development was stopped by addition of 150 μl of 1 M H2SO4, and the plate was read at a wavelength of 490 nm with a microplate reader (Molecular Devices Inc., Menlo Park, Calif.).
All assays were performed in duplicate, and test values were averaged.
Statistical methods.
The Mann-Whitney rank sum test was used for intergroup comparisons of concentrations of sTNF-RI, CRP, haptoglobin, and chloroquine and of antibody reactivities because of the skewed distributions of data (Kolmogorow-Smironow test). P values lower than 0.05 were considered significant.
The Student t test was used for intergroup comparisons of inhibitory activities in the LAL assays. P values lower than 0.05 were considered significant.
The Spearman rank order correlation coefficient (r) was used for evaluation of parameter associations. P values lower than 0.01 combined with r values higher than 0.5 were considered significant. All calculations were performed with SigmaStat software (Jandel Scientific, San Rafael, Calif.).
RESULTS
Characteristics of the donor groups.
A total of 126 children were enrolled in the study. Thirty children (16 females and 14 males) had symptomatic malaria, 50 children (19 females and 31 males) had asymptomatic P. falciparum infections, and 46 children were healthy aparasitemic children (19 females and 27 males). Some of their characteristics are summarized in Table 1.
TABLE 1.
Donor characteristics of children with symptomatic P. falciparum infections, children with asymptomatic P. falciparum infections, and healthy aparasitemic children
| Groupa | No. of children | Mean age (years) ± SD | Parasitemiab (no. of parasites/μl)
|
Mean axillary temp (°C) ± SD | Mean PCVc ± SD | ||
|---|---|---|---|---|---|---|---|
| Median | 25% | 75% | |||||
| i | 30 | 3.5 ± 2.1 | 90,000 | 20,000 | 140,000 | 39.4 ± 0.7 | 29.6 ± 5.6 |
| ii | 50 | 2.7 ± 1.3 | 3,000 | 295 | 15,000 | 36.6 ± 0.3 | 28.3 ± 4.2 |
| iii | 46 | 2.5 ± 1.3 | 36.7 ± 0.4 | 31.9 ± 3.6 | |||
Group i, children with symptomatic P. falciparum infections; group ii, children with asymptomatic P. falciparum infections; and group iii, healthy aparasitemic children.
25% and 75%, 25th and 75th percentiles, respectively.
PCV, packed cell volume.
Parasite density was significantly higher in children with clinical malaria than in children with asymptomatic infections (P < 0.001). Twenty-eight of 30 children with symptomatic infections and 21 of 50 children with asymptomatic infections had parasite levels exceeding 5,000/μl. Axillary temperature was correlated with the level of parasitemia (r = 0.505, P < 0.001).
The mean packed cell volume was significantly higher in healthy children than in children with asymptomatic infections (P < 0.001) but not in children with symptomatic infections.
Chloroquine levels were measured in some of the serum samples. Two of 19 samples from children with clinical malaria, 3 of 27 samples from children with asymptomatic infections, and 6 of 37 samples from healthy children had chloroquine levels exceeding 10 ng/ml.
Markers of acute disease (sTNF-RI and CRP concentrations).
Table 2 shows the concentrations of sTNF-RI and CRP in sera. The concentrations of sTNF-RI in sera were significantly higher in children with clinical malaria than in children with asymptomatic infections or in healthy children (P < 0.001). Children with asymptomatic infections had higher levels of sTNF-RI than healthy aparasitemic children (P = 0.008).
TABLE 2.
sTNF-RI, CRP, and haptoglobin concentrations in Gambian children with symptomatic P. falciparum infections or asymptomatic P. falciparum infections and in healthy aparasitemic children
| Groupa | Median amt (25–75%; n)b of:
|
||
|---|---|---|---|
| sTNF-RI (ng/ml) | CRPc (μg/ml) | Haptoglobin (μg/ml) | |
| i | 4.8 (3.9–5.5; 17) | 68 (33–95; 27) | 702 (349–1,069; 19) |
| ii | 1.9 (1.5–2.2; 25) | 11 (3–48; 32) | 346 (21–673; 31) |
| iii | 1.4 (0.9–1.6; 17) | 1 (0–2; 40) | 1,024 (582–1,742; 40) |
Group i, children with symptomatic P. falciparum infections; group ii, children with asymptomatic P. falciparum infections; group iii, healthy aparasitemic children.
Values in the 25th to 75th percentiles (25–75%) and the numbers of samples tested are shown in parentheses.
The percentages of children with CRP concentrations of 8 μg/ml or above were 96.3, 56.3, and 12.5% for groups i, ii, and iii, respectively.
Levels of sTNF-RI were correlated with levels of parasitemia (r = 0.55, P < 0.005) and with axillary temperature (r = 0.705, P < 0.005).
Concentrations of CRP in sera were significantly higher in children with clinical malaria than in children with asymptomatic infections or in healthy aparasitemic children (P < 0.001). Children with asymptomatic infections had higher levels of CRP than healthy aparasitemic children (P < 0.001). The majority of children with clinical malaria had CRP concentrations of 8 μg/ml or above (Table 2).
Levels of CRP were correlated with levels of parasitemia (r = 0.58, P < 0.005) and with levels of sTNF-RI (r = 0.77, P = 0.003).
Marker of chronic infection (haptoglobin concentrations).
Table 2 shows the concentrations of haptoglobin in sera. The haptoglobin concentrations in serum were significantly lower in children with asymptomatic infections than in children with clinical malaria or in healthy aparasitemic children (P = 0.02 or 0.001, respectively). There were no significant differences in haptoglobin concentrations between children with clinical malaria and healthy children (P = 0.26). Fifteen of 29 children with asymptomatic infections, 3 of 21 children with clinical malaria, and 4 of 40 healthy children had haptoglobin concentrations less than 180 μg/ml. Seven of 15 children with haptoglobin concentrations less than 180 μg/ml had parasite numbers exceeding 5,000/μl, while 4 of 14 children with haptoglobin concentrations of more than 180 μg/ml had parasite numbers exceeding 5,000/μl.
Antibodies to Pfalhesin (band 3) and P. falciparum EMP-1 peptides.
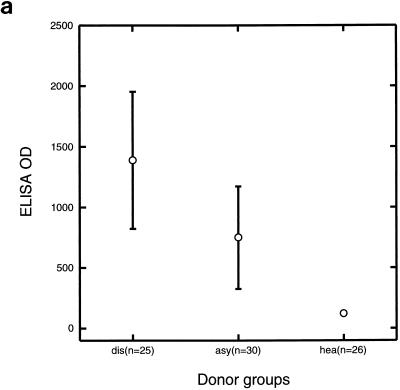
Sera from children with symptomatic infections had higher IgG reactivities to the Pfalhesin peptide than sera from children with asymptomatic infections (P = 0.001) or sera from healthy children (P < 0.001) (Fig. 1a). Sera from children with asymptomatic infections had higher IgG reactivities to the Pfalhesin peptide than sera from healthy children (P < 0.001).
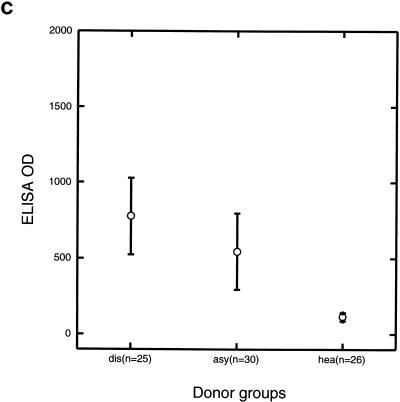
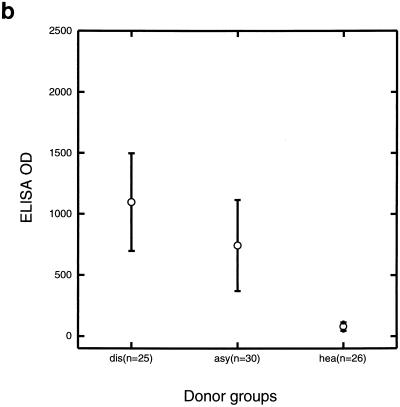
FIG. 1.
IgG reactivities against Pfalhesin peptide (a), P. falciparum EMP1 1391 peptide (b), and P. falciparum EMP-1 1393 peptide (c) measured by ELISA of sera from three groups of Gambian children, those with symptomatic malaria (dis), those with asymptomatic P. falciparum infections (asy), and healthy aparasitemic children (hea). Means and 95% confidence intervals are shown.
Sera from children with symptomatic infections and sera from children with asymptomatic infections had higher IgG reactivities to the P. falciparum EMP-1 1391 peptide than sera from healthy children (P < 0.001) (Fig. 1b). There were no significantly different IgG reactivities between sera from children with symptomatic infections and sera from children with asymptomatic infections (P = 0.06). IgG reactivities to the EMP-1 1391 peptide correlated with sTNF-RI concentrations (r = 0.605, P < 0.03).
Sera from children with symptomatic infections had higher IgG reactivities to the P. falciparum EMP-1 1393 peptide than sera from children with asymptomatic infections (P = 0.02) or sera from healthy children (P < 0.001) (Fig. 1c). Sera from children with asymptomatic infections had higher IgG reactivities to the EMP-1 1393 peptide than sera from healthy children (P < 0.001).
Blocking of LAL reactivity.
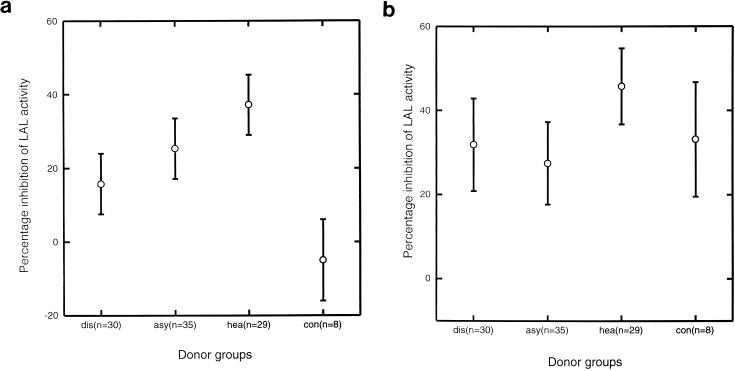
Sera from children with symptomatic infections were less effective in blocking malaria antigen-induced LAL activation than sera from children with asymptomatic infections (P = 0.05) or sera from healthy children (P < 0.001 ) (Fig. 2a). Sera from children with asymptomatic infections were less effective in blocking malaria antigen-induced LAL activation than sera from healthy children (P = 0.04). Sera from healthy children were also more effective in blocking LPS-induced LAL activation than sera from children with symptomatic infections (P = 0.01 ) and sera from children with asymptomatic infections (P = 0.02) (Fig. 2b). The ability of sera to block malaria antigen-induced LAL activation correlated with the ability to block LPS-induced LAL activation (r = 0.507, P < 0.005), but no associations with levels of parasitemia or temperature were found.
FIG. 2.
Serum-mediated inhibition of LAL activity induced by P. falciparum exoantigens (a) or LPS (b) in three groups of Gambian children, those with symptomatic malaria (dis), those with asymptomatic P. falciparum infections (asy), and healthy aparasitemic children (hea), as well as in eight Danish adults serving as controls (con). Means and 95% confidence intervals are shown.
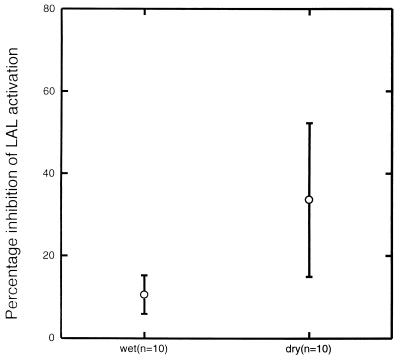
Sera collected in the dry season from seven of the children with symptomatic infections in the wet season were studied. They were more effective in blocking malaria antigen-induced LAL activity than the paired sera collected in the wet season (P = 0.001) (Fig. 3). There was no significant difference between the paired serum samples in their capacities to block LPS-induced LAL activation.
FIG. 3.
Serum-mediated inhibition of LAL activity induced by P. falciparum exoantigens among paired serum samples collected in the wet and in the dry season from Gambian children. Means and 95% confidence intervals are shown.
DISCUSSION
African children with clinical malaria normally carry high parasite loads, which induce inflammatory reactions. This study shows that children with symptomatic P. falciparum infections have much higher concentrations of sTNF-RI and CRP, markers of inflammatory reactions, than do children with asymptomatic infections, as has been noted previously (12). The increased concentrations of sTNF-RI and CRP in children with symptomatic infections correlated with levels of parasitemia. The higher parasite burdens in children with symptomatic infections may trigger the secretion of sTNF-RI and CRP, while the lower parasite burdens in children with asymptomatic infections may not be sufficient to trigger high concentrations of sTNF-RI and CRP. High concentrations of IL-10 and IL-1Ra in these children have also been reported (16). IL-10, IL-1Ra, and sTNF-RI do not have inflammatory activities themselves but are induced by inflammatory mediators, have longer half-lives than inflammatory mediators such as TNF, and are thus markers of inflammatory reactions.
Half of the children with asymptomatic infections had very low haptoglobin levels, indicating that these children carried chronic P. falciparum infections (20). These children may have had an episode of fever days or weeks previously that had resolved without treatment or on treatment with an only partially effective drug. The majority of study children had no detectable chloroquine in their sera, ruling out the possibility that self-medication affected the division of children into different clinical groups. We cannot exclude the possibility that some of the asymptomatic children would have become symptomatic if their levels of parasitemia increased, but the children were well the day after inspection, when treatment was given. Very few of the children with asymptomatic P. falciparum infections had high levels of parasitemia or high levels of inflammatory markers. Thus, chronicity of infection and a low parasite burden appear to be major reasons for the lack of symptoms in these children. The few asymptomatic children with high parasite densities probably had higher fever thresholds than children with symptomatic infections.
Modulation of parasite toxic activities may protect children against disease manifestations such as fever. Parasites have different capacities to induce inflammatory mediators such as TNF (1), and children have different capacities to mount a TNF response (21). In addition, the existence of antitoxic immunity has been suggested but not verified (22). Different factors may mediate antitoxic immunity (14). IgM antibodies from two malaria patients, which were reactive with phosphatidylinositol, have been shown to block TNF induction in vitro (4). It is noteworthy that it has been suggested that TNF may also function as a cryogen (antipyretic), despite its established categorization as an endogenous pyrogen (17), making interpretations of TNF assays more difficult. Malaria antigens activate the LAL assay, as do endotoxins from gram-negative bacteria (13). In this study, we have found evidence for antitoxic responses in children exposed to malaria parasites as determined by the ability of sera to inhibit malaria parasite activation of the LAL. These antitoxic responses may be able to block parasite toxicity without harming the parasite itself. Sera were collected from some of the children with clinical symptoms in the wet season and again in the dry season at a time with no malaria transmission. These dry-season sera were more inhibitory of parasite-induced LAL activation than the paired sera collected in the wet season and had inhibitory activities similar to those of sera from healthy children and sera from children with asymptomatic infections. Children with symptomatic malaria had lower anti-LAL activities than asymptomatic children. This finding might be explained in one of two ways. First, it may indicate that children with low levels of anti-LAL activity are at a greater risk of developing symptoms when they are infected with malaria parasites. Second, it may indicate consumption of anti-LAL activity during acute symptomatic malaria attacks, which are generally associated with higher levels of parasitemia than asymptomatic attacks. When studied during the following dry season, children with symptomatic malaria showed increased anti-LAL activities, which is compatible with the idea of consumption of anti-LAL activity during acute attacks. However, this observation does not exclude the other hypothesis, as this increase in activity may reflect stimulation by the attack of malaria that occurred some months previously. Further, prospective studies will be needed to resolve this issue, but it seems that a high LAL blocking activity in connection with a high parasite density is rare. The anti-LAL activities may be different from the IgM-mediated antitoxic immune reactions detected in two patients (4). These IgM antibodies are stimulated by acute disease, are of short duration, and do not protect patients from fever despite their TNF blocking activities (4). The facts that healthy children and children with asymptomatic infections had lower antibody reactivities to the Pfalhesin and P. falciparum EMP-1 peptides but that they had higher LAL blocking activities indicate that the kinetics of LAL blocking activity are different from the kinetics of the antibodies and may be mediated by factors different from antibodies.
Malaria parasites cause disease by their replication, their cytoadherence to endothelial cells, and their toxic activities. Cytoadherence of infected erythrocytes is mediated, at least in part, by P. falciparum EMP-1 and the Pfalhesin epitope of band 3 (2, 6, 8). Antibodies to the Pfalhesin epitope have been detected previously among Liberian donors (11). Antibody reactivities to P. falciparum EMP-1 among African children are believed to be predominantly strain specific, while the Pfalhesin epitope is conserved. We found that antibodies against Pfalhesin and against conserved parts of EMP-1 were produced during acute episodes of malaria. They are probably lost rapidly, as healthy aparasitemic children had very low antibody reactivities. These healthy children also had lower IgG reactivities to rhoptry-associated protein 1 of P. falciparum (data not shown), indicating that the children had not recently been exposed to P. falciparum parasites. Thus, it is likely that such peptide-specific antibodies do not protect against subsequent clinical episodes of malaria. It is, however, possible that older children may develop a more sustained antibody reactivity to these peptides.
In conclusion, we detected antitoxic activities in sera from Gambian children as evaluated by their capacity to block toxin-induced LAL activation in vitro. Children with symptomatic P. falciparum infections had high parasite loads and high levels of markers of inflammatory reactions, and their sera had low toxin-blocking activities in the LAL assay, possibly because of consumption. The data indicate that consumption of antitoxic responses may be one factor leading to disease progression in infected children. Half of the children with asymptomatic infections probably had chronic infections, and only a few of the remaining children had high levels of parasitemia, suggesting that the proportion of asymptomatic children with antitoxic immunity and no antiparasite immunity was probably low.
ACKNOWLEDGMENTS
Jimmy Weng is thanked for excellent technical assistance.
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases and the Danish Medical Research Council.
REFERENCES
- 1.Allan R J, Rowe A, Kwiatkowski D. Plasmodium falciparum varies in its ability to induce tumor necrosis factor. Infect Immun. 1993;61:4772–4776. doi: 10.1128/iai.61.11.4772-4776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baruch D I, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 3.Bate C A W, Taverne J, Playfair J H L. Malarial parasites induce TNF production by macrophages. Immunology. 1988;64:227–231. [PMC free article] [PubMed] [Google Scholar]
- 4.Bate C A W, Kwiatkowski D. Inhibitory immunoglobulin M antibodies to tumor necrosis factor-inducing toxins in patients with malaria. Infect Immun. 1994;62:3086–3091. doi: 10.1128/iai.62.8.3086-3091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blick M, Sherwin S A, Rosenblum M, Gutterman J. Phase I study of recombinant tumor necrosis factor in cancer patients. Cancer Res. 1987;47:2986–2989. [PubMed] [Google Scholar]
- 6.Crandall I, Collins W E, Gysin J, Sherman I W. Synthetic peptides based on motifs present in human band III protein inhibit cytoadherence/sequestration of the malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 1993;90:4703–4707. doi: 10.1073/pnas.90.10.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eggelte T A. Production of monoclonal antibodies against antimalarial drugs for use in immunoassays. In: Navaratnam V, Payne D, editors. The validation of chemical and immunochemical tests for antimalarials in body fluids. International Monograph Series no. 3. Penang, Malaysia: Universiti Sains Malaysia; 1990. pp. 35–63. [Google Scholar]
- 8.Gardner J P, Pinches R A, Roberts D J, Newbold C I. Variant antigens and endothelial receptor adhesion in Plasmodium falciparum. Proc Natl Acad Sci USA. 1996;93:3503–3508. doi: 10.1073/pnas.93.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwood B M, Armstrong J R M. Comparison of two simple methods for determining malaria parasite density. Trans R Soc Trop Med Hyg. 1991;85:186–188. doi: 10.1016/0035-9203(91)90015-q. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood B M, Marsh K, Snow R. Why do some African children develop severe malaria? Parasitol Today. 1987;7:277–281. doi: 10.1016/0169-4758(91)90096-7. [DOI] [PubMed] [Google Scholar]
- 11.Hogh B, Petersen E, Crandall I, Gottschau A, Sherman I W. Immune responses to band 3 neoantigens on Plasmodium falciparum-infected erythrocytes in subjects living in an area of intense malaria transmission are associated with low parasite density and high hematocrit value. Infect Immun. 1994;62:4362–4366. doi: 10.1128/iai.62.10.4362-4366.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurt N, Smith T, Tanner M, Mwankusye S, Bordmann G, Weiss N A, Teuscher T. Evaluation of C-reactive protein and haptoglobin as malaria episode markers in an area of high transmission in Africa. Trans R Soc Trop Med Hyg. 1994;88:182–186. doi: 10.1016/0035-9203(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 13.Jakobsen P H, Baek L, Jepsen S. Demonstration of soluble Plasmodium falciparum antigens reactive with Limulus amebocyte lysate and polymyxin B. Parasite Immunol. 1988;10:593–606. doi: 10.1111/j.1365-3024.1988.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 14.Jakobsen P H, Bate C A W, Taverne J, Playfair J H L. Malaria: toxins, cytokines and disease. Parasite Immunol. 1995;17:223–231. doi: 10.1111/j.1365-3024.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 15.Jakobsen P H, Hviid L, Theander T G, Afare E A, Ridley R G, Heegaard P M H, Stuber D, Dalsgaard K, Nkrumah F K. Specific T-cell recognition of the merozoite proteins rhoptry-associated protein 1 and erythrocyte-binding antigen 1 of Plasmodium falciparum. Infect Immun. 1993;61:268–273. doi: 10.1128/iai.61.1.268-273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakobsen P H, McKay V, Njie R, Olaleye B O, D’Alessandro U, Bendtzen K, Schousboe I, Greenwood B M. Soluble products of inflammatory reactions are not induced in children with asymptomatic Plasmodium falciparum infections. Clin Exp Immunol. 1996;105:69–73. doi: 10.1046/j.1365-2249.1996.d01-718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozak W, Conn C A, Klir J J, Wong G H W, Kluger M J. TNF soluble receptor and antiserum against TNF enhance lipopolysaccharide fever in mice. Am J Physiol. 1995;269:R23–R29. doi: 10.1152/ajpregu.1995.269.1.R23. [DOI] [PubMed] [Google Scholar]
- 18.Kwiatkowski D, Cannon J G, Manogue K R, Cerami A, Dinarello C A, Greenwood B M. Tumour necrosis factor production in Falciparum malaria and its association with schizont rupture. Clin Exp Immunol. 1989;77:361–366. [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh, K. 1992. Malaria—a neglected disease? Parasitology 104(Suppl.):s53–s69. [DOI] [PubMed]
- 20.McGuire W, D’Alessandro U, Olaleye B O, Thomson M C, Langerock P, Greenwood B M, Kwiatkowski D. C-reactive protein and haptoglobin in the evaluation of a community-based malaria control programme. Trans R Soc Trop Med Hyg. 1996;90:10–14. doi: 10.1016/s0035-9203(96)90461-7. [DOI] [PubMed] [Google Scholar]
- 21.McGuire W, Hill A V S, Allsopp C E M, Greenwood B M, Kwiatkowski D. Variation in the TNF-alpha promotor region associated with susceptibility to cerebral malaria. Nature. 1994;371:508–511. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- 22.Playfair J H L, Taverne J, Bate C A W, de Souza J B. The malaria vaccine: anti-parasite or anti-disease? Immunol Today. 1990;11:25–27. doi: 10.1016/0167-5699(90)90007-v. [DOI] [PubMed] [Google Scholar]
- 23.Shenton F C, Bots M, Menon A, Eggelte T A, de Wit M, Greenwood B M. An ELISA test for detecting chloroquine in urine. Trans R Soc Trop Med Hyg. 1988;82:216–220. doi: 10.1016/0035-9203(88)90415-4. [DOI] [PubMed] [Google Scholar]
- 24.Su X, Heaatwole V M, Wertheimer S P, Guinet F, Herrfeldt J A, Peterson D S, Ravetch J A, Wellems T E. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhang G-H, Baek L, Nielsen P E, Buchardt O, Koch C. Sensitive quantification of endotoxin by enzyme-linked immunosorbent assay with a monoclonal antibody against Limulus peptide C. J Clin Microbiol. 1994;32:416–422. doi: 10.1128/jcm.32.2.416-422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang G-H, Bæk L, Berthelsen T, Koch C. Quantification of the endotoxin-neutralizing capacity of serum and plasma. APMIS. 1995;103:721–730. doi: 10.1111/j.1699-0463.1995.tb01429.x. [DOI] [PubMed] [Google Scholar]