Abstract
a). Purpose of review:
The modifiable and non-modifiable determinants and the currently available methods of assessment of menstrual blood flow will be discussed, with the goal of helping healthcare providers, researchers, and those interested in public health.
b). Recent findings:
Several factors can impact menstruation. The determinants include modifiable factors such as smoking, nutrition, exercise, stress, weight fluctuation, and benign gynecologic diseases, and non-modifiable factors such as age, race, and the individual’s genes. The intertwined dynamic among these determinants needs more critical attention. Currently, the methods for the assessment of menstruation all have advantages and disadvantages, often with a tradeoff between practicality and accuracy.
c). Summary:
Considered by many as the fifth vital, menstruation provides a window to an individual’s general health. The discussion of its determinants and assessment can be more appropriate for individual contexts, especially from a public health perspective as it can improve the reproductive health of the population.
Keywords: Menstruation, Menstrual blood flow, Determinants, Assessment
1.0. Introduction
Menstruation is the monthly shedding of blood and tissues from the uterine lining, known as the endometrium, during the reproductive years and in the absence of pregnancy. The days of bleeding are referred to as a “period” or “menses” while the duration between the first day of two successive periods is referred to as the “menstrual cycle”. The menstrual cycle is considered the “fifth vital sign” to reflect an individual’s overall health status[1].
Regular menstruation usually indicates a healthy reproductive system[2]. The average cycle length is 28 days while the length of normal cycles may range from 21 to 35 days, and the range of normal periods can be from 2 to 8 days [3]. Due to the effects of endometrial lytic enzymes, menstrual effluent contains prostaglandins, lysed blood clots, and tissue debris. If the menstrual flow is heavy, individuals can see blood clots as the flow is too fast to allow the normal lysis of blood clots by the lytic enzymes. According to one source, the average amount of menstrual flow during the entire period in ovulatory individuals is 33.2 mL[4]. More than 80 mL of effluent is considered heavy menstrual bleeding (HMB) and is typically referred to as menorrhagia[4]. During reproductive years, irregularities in the lengths and frequency of the menstrual cycle and the amount of menstrual flow are common and often collectively called abnormal uterine bleeding (AUB)[5–7]. AUB is one of the most common complaints in gynecologic practice and can impair general health by causing iron deficiency anemia, which can be severe, and impair the quality of life. One-third of menstruating individuals suffer from AUB during their lifetime[5,7].
Menstrual disorders are very common globally, reflecting an under-recognized public health problem. Identifying and understanding determinants of abnormal menstruation (Fig. 1a) not only helps reach the correct diagnosis but also provides effective treatment options for individuals suffering from abnormal menstruation. At the same time, convenient and accurate assessment of menstrual blood loss (Fig. 1b) is a high priority both in research and clinical practice. This review aims to discuss both the modifiable and non-modifiable determinants and various assessment methods of menstrual blood flow and cycle irregularities (Box 1.).
Fig. 1a.
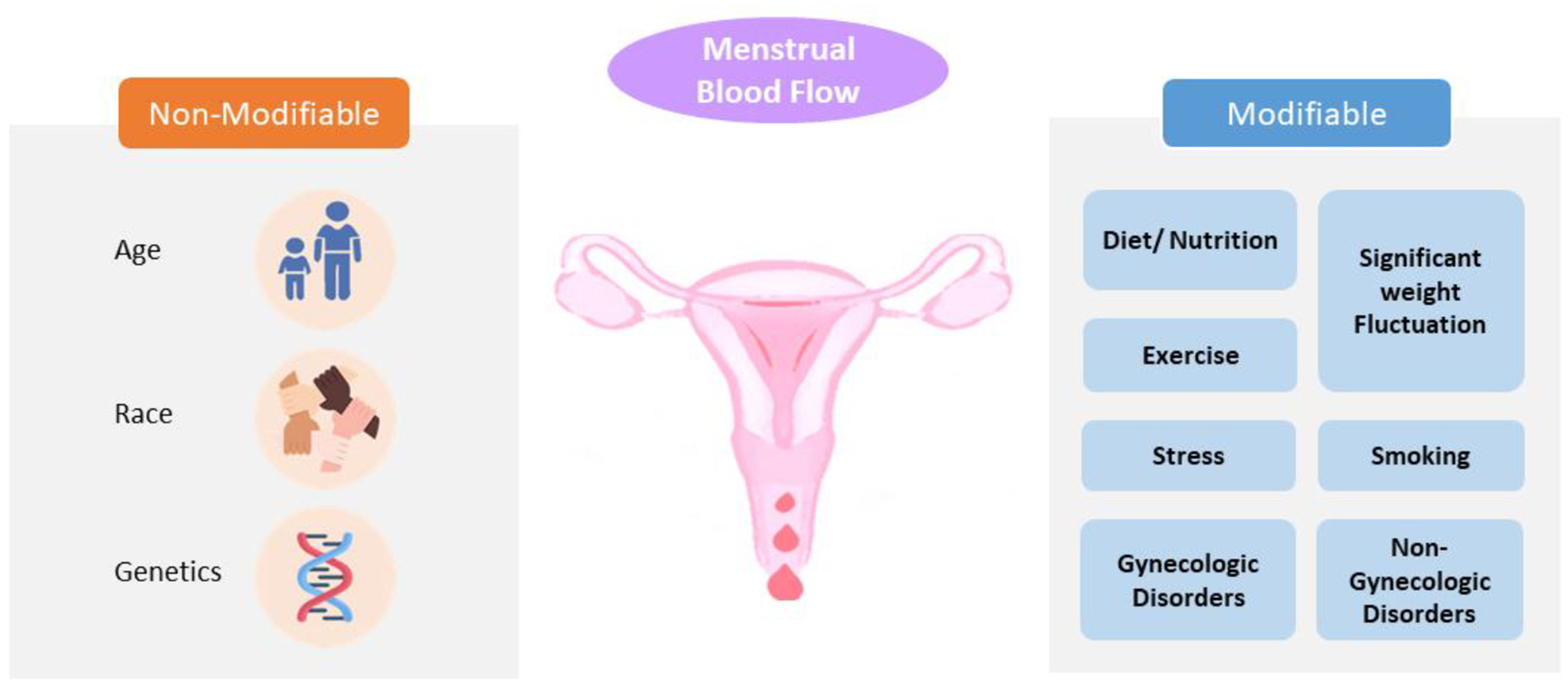
The determinants of menstrual blood flow
Non-modifiable determinants are inherent and cannot be changed, while modifiable determinants can potentially be changed.
Fig. 1b.
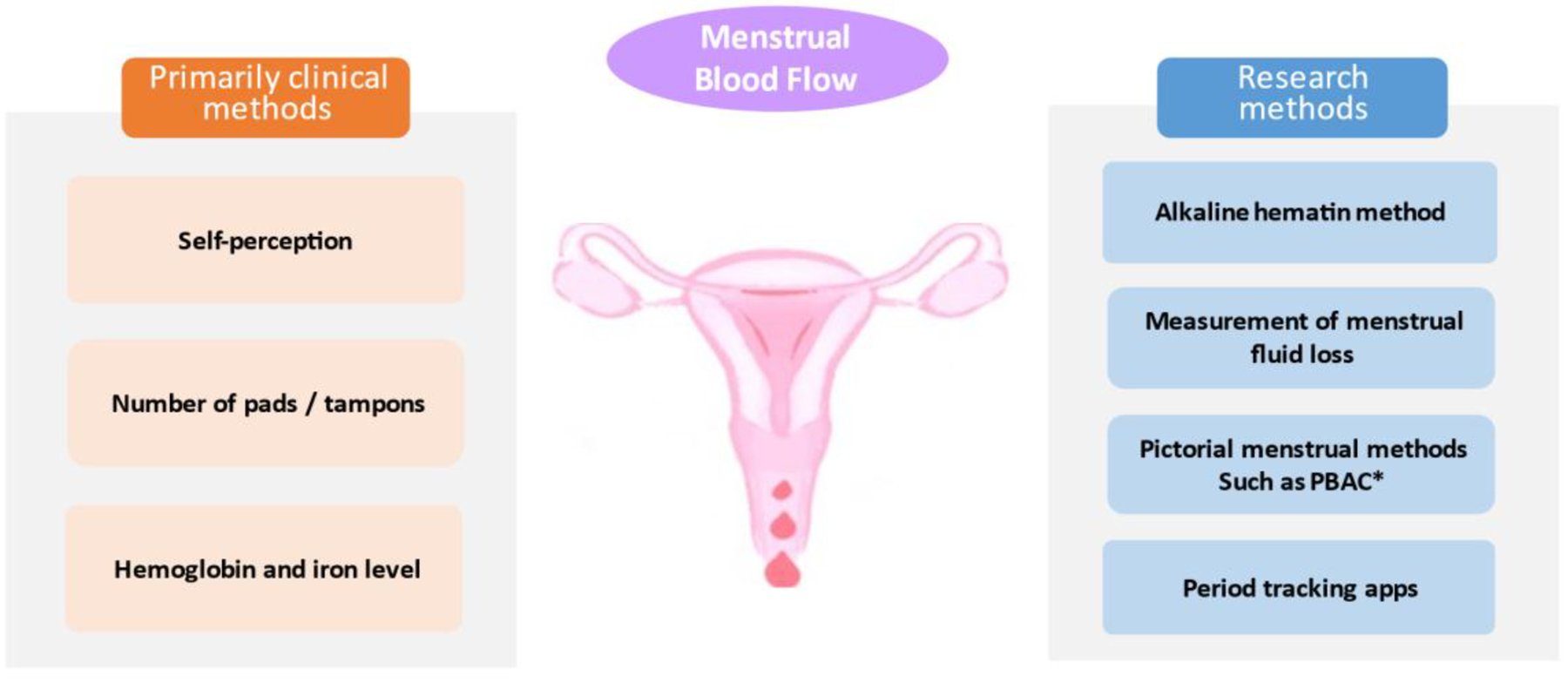
The assessment of menstrual blood flow
The assessment of menstruation includes primarily clinical and research methods. These methods usually doen’t satisfy convenience and accuracy at the same time.
• * PBAC (pictorial blood loss assessment chart) is semi-quantitative with a scoring system accounting for the number and the degree of staining of of feminine hygiene products.
Box 1. Overview of Determinants and Assessment of Menstruation.
| 1.0 Introduction 2.0 A Brief Review of the Regulation of Menstruation 3.0 Determinants of Menstrual Blood Flow (Fig. 1a) 3.1 Non-modifiable Determinants 3.2 Modifiable Determinants 3.2.1 Smoking 3.2.2 Diet and Nutrition 3.2.3 Eating Patterns, BMI, and Significant Weight Fluctuation 3.2.4 Exercise 3.2.5 Stress 3.2.6 Gynecologic Disorders 3.2.6.1 Uterine Fibroids 3.2.6.2 Adenomyosis 3.2.6.3 Endometrial and Cervical Polyps 3.2.6.4 Gynecologic Malignancies and Precancerous Conditions, Hyperplasia 3.2.6.5 Ovulatory Dysfunction 3.2.6.6 Other Functional Menstrual Disorders 3.2.6.7 Iatrogenic 3.2.7 Non-gynecologic Conditions 4.0 Methods of Assessing Menstrual Blood Flow (Fig. 1b) 4.1 Primarily Clinical Methods 4.1.1 Self-Perception 4.1.2 Menstrual Diaries 4.1.3 Measurement of Hemoglobin and Iron Levels 4.2 Primarily Research Methods 4.2.1 Alkaline Hematin Method 4.2.2 Estimated Menstrual Fluid Volume 4.2.3 Validated Pictorial Menstrual Methods 4.2.4 Period Tracking Apps 5.0 Future Directions |
2.0. A Brief Overview of the Regulation of Menstruation
The menstrual cycle can be divided into three phases: the ovary proceeds follicular (preovulatory), ovulatory and luteal (postovulatory) phases, while the endometrium cycles menstrual, proliferative and secretory phases[8]. Menstrual cycle, as a process, is strictly regulated by fluctuating levels of the female sex hormones (estrogens and progesterone) throughout all phases. As the result of declining female sex hormone levels, menstrual bleeding marks the start of a menstrual cycle. The follicular phase varies more than other phases and measures around 14 days if an individual has a 28-day menstrual cycle[9]. This phase is characterized by the development of ovarian follicles and increasing estrogen secretion through an upregulation of Follicle Stimulating Hormone (FSH) receptors. High estrogen levels initiate mucosal proliferation, and increases stroma, glands, and depth of arteries in the endometrium[10]. The thickening of the endometrium is able to support implantation and pregnancy in the case of fertilization[11]. Positive feedback of estrogen, FSH and luteinizing hormone (LH) levels [3] causes the “LH surge,” a peak in LH level that triggers follicle rupture which releases an oocyte, also called “ovulation” [10]. The ruptured follicle forms the corpus luteum, which secretes estrogen and progesterone. Estradiol levels fall at the end of ovulation[4]. The lutealphase occurs after ovulatary phase until the end of the cycle. The length of luteal phase is quite stable and lasts around 14 days[4]. During this phase, the corpus luteum and the endometrium are maintained to support a potential fertilization, implantation, and ongoing pregnancy. If implantation does not occur, the corpus luteum begins to degenerate and the secretion of estrogen and progesterone is halted, leading to menstruation. At menstruation, inflammatory processes are initiated in the endometrium, due to the absence of progesterone[12]. Symptoms of menstruation — pain, heat, swelling — can be attributed to this inflammation, and are regulated by the declining progesterone level[12]. This is due to the activation of the pro-inflammatory Nuclear Factor Kappa B(NF-kB) pathway, which is inhibited by high progesterone levels[12]. Activation of this inflammatory pathway increases levels of cytokines, prostaglandins, and matrix metalloproteinases, which in turn induces lysis of connective tissue and bleeding. Endometrial regeneration and healing will be regulated by estrogen during the proliferative phase, resulting in re-epithelialization of the luminal lining, the growth of stromal tissue, vessel repair, and finally the cessation of endometrial bleeding[12].
3.0. Determinants of Menstrual Blood Flow (Fig. 1a)
3.1. Non-modifiable Determinants
Non-modifiable determinants include inherent characteristics, such as age, race, and genetic/family history. While such risk factors cannot be changed, they are very helpful in understanding which populations are more vulnerable to menstrual problems. Numerous studies report an association between menstrual regularity and genetics, suggesting that characteristics affecting menstrual cycle duration are, at least in part, heritable[3,11]. The follicle-stimulating hormone beta subunit (FSHB) locus, in particular, has been shown to affect menstrual cycle lengths and menopause timing[3,11]. Race has been associated with age at menarche and blood flow[13–15]. For example, these studies report that black menstruators often experience a relatively earlier age of menarche. Menstrual regularity increases with age, and many studies highlight the prevalence of irregularity in adolescence[13–15].
3.2. Modifiable Determinants
3.2.1. Smoking
Smoking has been associated with menstrual irregularity[2,16–19]. Menstrual irregularity in smokers is 1.4 times that of nonsmokers [17]. Smokers are more likely to have infrequent periods, with 3 or more months between cycles [18,19], to have short menstrual cycles of 21–24 days or less[16,19], and to have heavy periods[2].
It is worth noting that multiple factors can affect period regularity among smokers, such as age at initiation and frequency of smoking. One study found that starting to smoke before the age of 19 was associated with more menstrual irregularity than starting later[17]. Therefore, smoking cessation is strongly advised to promote menstrual regularity and overall health[2,17].
3.2.2. Diet and Nutrition
Accumulating evidence suggests that diet and nutrition have an impact on the menstrual cycle[20,21]. While a balanced diet including all food groups provides a wide range of health benefits during the menses, excessive consumption of certain nutrients, or lack thereof, can result in harmful effects[22]. Certain nutritional food groups have been associated with varying levels of menstrual regularity and blood flow. A Spanish study investigated the effect of adherence to Mediterranean diet and found that individuals who consumed olive oil daily were more likely to have a lower percentage of heavy bleeding flow, and alcohol consumption was associated with longer cycle length than normal[23]. Research also indicates that carbohydrate intake can play a role in menstrual dysfunction. For instance, individuals following a low-carb or ketogenic diet tend to have increased menstrual dysfunction, particularly amenorrhea[24,25]. Hence, nutritional intake does have an effect on menstrual cycles[24,25].
3.2.3. Eating Patterns, BMI, and Significant Weight Fluctuation
Evidence points to a link between energy balance and reproductive health, revealing the need for proper caloric intake and consistent mealtime habits for people who menstruate[26]. A higher prevalence of anovulatory cycles has been reported for individuals with a history of eating disorders such as anorexia nervosa, binge eating, and bulimia[27–30]. Binge eating and anorexia in particular were associated with secondary amenorrhea[31–33]. Individuals with a BMI above the range of 18.5 to 24.9 are more prone to AUB[28,30,34–37]. However, the type of dysfunction present is quite variable. Individuals with a BMI from 25 to 25.9 have a heavier menstrual blood flow[35,36] and more irregular periods than those who have a BMI from 18.5 to 24.9[36,37]. For example, a prospective study of college-aged menstruators found overweight students were at an increased risk of having a cycle longer than 43 days[37]. Additional studies report menstrual irregularity, particularly amenorrhea, among individuals with a history of significant weight loss or are underweight (BMI < 18.5)[28,30]. Eating patterns, body weight, and menstruation are intertwined.
3.2.4. Exercise
Functional hypothalamic amenorrhea (FHA) is a menstrual disorder in which low weight, excessive exercise, or stress lead to the absence of menses [38]. Regular menses requires a minimum fat storage requirement of about 22%, and therefore, factors that lower one’s body fat percentage such as excessive exercise will contribute to FHA development[38]. This is commonly seen in athletes who need to maintain low body weight for success in their sport, though research shows a similar trend in the general female population as well[39]. Researchers of a prospective study found about 50% of cycles in exercising menstruating individuals were anovulatory compared to only 4% in sedentary menstruating individuals[40]. Additionally, menstruating individuals participating in moderate to vigorous physical activity (3–6 or greater than 6 metabolic equivalent hours per week) had a significantly higher prevalence of anovulatory cycles[37,40–42]. This included both longer cycle durations (i.e. exceeding at least 35 day) and even amenorrhea[39].
However, the impact of lack of exercise on menstruation cannot be overlooked. In a study centered on Danish menstruating individuals, researchers found a strong association between low physical activity levels and irregular cycles[43]. In particular, obese individuals (BMI >= 30) with sedentary lifestyles experienced the greatest menstrual irregularity compared to other groups[43].
3.2.5. Stress
Stress, which often induces hormonal changes within the body, is known to have an effect on menstrual dysfunction[37,44–49]. Major life changes, high intensity work environments, depressive moods, and stress more generally increases the risk for irregular menstruation[48]. A few studies found a correlation between stress and longer cycles with more than 33 days[37,45–48,50]. Additionally, stress seemed to contribute to other types of menstrual dysfunction. While some studies uncovered an association between psychological stressors and heavier/prolonged blood flow during menses, others found those stressors to have an isolated effect on cycle regularity[48]. While the specific etiology may be inconclusive, stress-induced changes in menstruation affect both cycle duration and blood flow.
3.2.6. Gynecologic Disorders
Most causes of gynecologic disorders which affect menstrual blood flow are due to AUB. Fortunately, most patients with AUB can be treated with safe, efficient, and noninvasive medication such as hormonal contraceptives and levonorgestrel intrauterine system (LNG IUS)[6,44,49,50].
3.2.6.1. Uterine Fibroids
Also known as leiomyomas, uterine fibroids are the most common benign pelvic tumor in reproductive-aged individuals, arising from smooth muscle cells within the myometrium[6]. No less than 70% of individuals are diagnosed with uterine fibroids before age 50[51]. Risk factors include race/ethnicity, early menarche, obesity, diet issues, family history, et al[52]. Though most individuals with fibroids are asymptomatic, some do present with abnormal menstruation, the most common being heavy menstrual bleeding[5]. The possible reasons include distortion and/or increased volume of the endometrial cavity, as well as the impacted endometrial function, especially for the submucosal fibroids[53]. Medical treatment can be effective for heavy menstrual bleeding [52], while surgeries such as myomectomy, endometrial ablation, and hysterectomy may be needed for cases with additional symptoms.
3.2.6.2. Adenomyosis
Adenomyosis is characterized by the growth of endometrial glands and stroma that proliferate focally or globally inside the uterine wall. Its prevalence is about 5% - 70%[54] The etiologies are unclear, but could possibly be related to abnormal gene expression[55]. Around one third of individuals with adenomyosis present no symptoms, though heavy menstrual bleeding and multiple irregular menses (i.e., intermenstrual bleeding, prolonged menstrual bleeding) are often seen[5,56]. Hormone based therapies may be effective but hysterectomy remains an option for those with failed medical methods[6].
3.2.6.3. Endometrial and Cervical Polyps
Focal hyperplasia of the endometrium, including glands and stroma, surrounding a blood-vessels core forms endometrial polyp. Once they are large enough, these polyps can extend towards the internal os[57]. The prevalence of endometrial polyps ranges from 7.8% - 34.9%[58]. While the causes are still unclear, it is speculated that genetic and hormonal factors may play a role in polyps development [6]. Risk factors often include age, obesity, excessive estrogen exposure, and Lynch syndrome [59]. Both endometrial polyps and cervical polyps can result in AUB. For instance, intermenstrual bleeding or other types of AUB are seen in more than 60% of premenopausal patients with endometrial polyps[5,6]. Most endometrial and cervical polyps can be diagnosed and removed easily, though surgeries may not be necessary for asymptomatic individuals.
3.2.6.4. Gynecologic Malignancies and Precancerous Conditions, Hyperplasia
Uterine malignancies and precancerous conditions can cause AUB. Therefore, it is important to discern the etiologies via both pelvic examinations, including speculum and rectal inspection, and pathologic examination such as pap smears and biopsy sampling[6]. Fortunately, non-benign diseases are often identified in the early stages in more than 70% of individuals presenting with AUB [6].
3.2.6.5. Ovulatory Dysfunction
Ovulatory dysfunction refers to infrequent and irregular ovulation. This gynecologic disorder has various causes, including hyperprolactinemia, hypothalamic-pituitary disorders, obesity, extreme exercise/weight loss, and hypothyroidism[5,60]. However, as the most common endocrine disorder in reproductive-age menstruating individuals, polycystic ovarian syndrome (PCOS) is the leading cause of ovulatory dysfunction[61]. PCOS is a complex, multigenic endocrinopathy linked to menstrual irregularities (mostly anovulatory)[5,62], which has impacts across the lifespan including reproductive, metabolic and psychological health, affecting 5%−18% of menstruating individuals[63]. The related symptoms with menstruation range from amenorrhea, irregular menstrual bleeding to extreme HMB[6]. As one of PCOS’s most common symptoms, amenorrhea could also be caused by overexercising, undereating and stress and therefore leads to a misdiagnosis[64]. Detailed medical records of the bleeding pattern can narrow down differential diagnoses. PCOS can be well-managed based on individuals’ varying treatment goals[65]. Also, individuals with PCOS can gain regular menstrual cycles with age[66]. Lifestyle optimization including a balanced diet and regular exercise can ameliorate the metabolic abnormalities associated with PCOS and improve the menstrual irregularity and anovulation [63]. PCOS in individuals with a BMI >= 25 [67] may be treated with calorie-restricted diets[68,69] because even a small weight loss, 5% - 10%, can improve menstrual irregularities[68]. In cases of individuals with a BMI >= 35 kg/m2, bariatric surgery could offer an option for sustained weight loss, allowing for restoration of menstrual regularity[67]. Medical therapy such as combined oral contraceptives is also used as the most efficient and first-line option to manage menstrual disorders for those not attempting pregnancy[70]. In over-weight individuals with suspected ovulatory dysfunction, endometrial intraepithelial neoplasia and/or endometrial cancer may need to be ruled out by endometrial sampling [6].
3.2.6.6. Other Functional Menstrual Disorders
Anovulation, usually being caused by Hypothalamic-Pituitary-Ovarian (HPO) axis, dysfunction, seems to explain why around 95% of otherwise healthy adolescents suffer from AUB[71]. It could take up to 2 years for regular ovulation to begin after menarche[65].
Menopause is identified retrospectively: at least 12 months of amenorrhea in an individual who is at least 40 years old[72]. As individuals approach their final menopause, anovulation is also very common, with short bleeding and spotting episodes being the most frequent symptoms. Meanwhile, HMB usually is present in individuals transitioning into menopause[73]. Furthermore, BMI and nutritional status such as low BMI, high BMI/obesity could increase number of heavy bleeding days[73].
Another disorder to be mentioned is luteal phase defect - a clinical diagnosis with less than 10 days of luteal phase, compared to the normal length of about 14 days[74]. The related symptoms include short menstrual cycles and premenstrual spotting. Proposed treatment is usually centered on hormone therapy[74,75].
3.2.6.7. Iatrogenic
Side effects of medical interventions are called “iatrogenesic”[76]. Abnormal menstruation could be caused by iatrogenic factors such as use of steroid hormones, intrauterine devices (IUD), medication affecting dopamine metabolism, and anticoagulants[5,7]. Among these, steroid hormones (such as stopping oral contraceptives) are the most common reason[60] and can include HMB, an unpredictable pattern of bleeding, or intermenstrual bleeding[5]. Additionally, other non-gynecologic medicines including corticosteroid-related drugs, anti-depressants, or anticoagulants may contribute to HMB and/or prolonged menses[6]. Amenorrhea and hypomenorrhea could also be a result of infection, cervical stenosis, or intrauterine adhesion due to gynecologic surgeries [77,78]. The treatment options commonly involve use of hysteroscopic resection, followed by mechanical and/or hormone therapy[77]. The treatment plan is based on the individual’s specific symptoms and etiologies.
3.2.7. Non-gynecologic Conditions
Thyroid function and prolactin concentration should be assessed to individuals with abnormal menstrual bleeding such as amenorrhea and hypermenorrhea[77]. While hypomenorrhea and polymenorrhea may also be caused by other uncommon etiologies such as female genital tuberculosis[79]. Abnormal bleeding may be caused by coagulation disorders, such as Von Willebrand disease, qualitative platelet disorders, and congenital fibrinogen deficiency[7,80].
4.0. Methods of Assessing Menstrual Blood Flow (Fig. 1b)
4.1. Primarily Clinical Methods
4.1.1. Self-Perception
Gauging the true amount of blood loss during menstruation based on self-perception can be difficult, since in many cases, the subjective assessment of the blood flow does not correspond to the actual blood loss[6,81][82]. However, subjective assessment is very important as: 1) it is usually the factor that prompts the patient to seek medical care; and 2) it largely determines the quality of life[83]. In addition, detailed menstrual history including the pattern of bleeding episodes, the appearance of clots, and total numbers of tampons/pads used, rather than simply inquiring if the period is light or heavy, should be obtained to better predict the volume of menstrual blood loss[82].
4.1.2. Menstrual Diaries
Menstrual cycle length and bleeding, the number of pads and/or tampons used in the last 24 hours, and how soaked the pads/tampons can all be recorded in menstrual diaries, whether on paper or electronically. Even though menstrual blood loss is difficult to accurately quantify, more than 80 ml of effluent per period is considered excessive[82]. To simplify, excessive bleeding can be defined as changing soaked pads or tampons every one to two hours[65], or the occurrence of three soaked pads or six soaked regular tampons per day over three days [71,80].
4.1.3. Measurement of Hemoglobin and Iron Levels
HMB often results in iron deficiency and secondary anemia. However, due to the unawareness of the condition and less willingness to consult a doctor for HMB symptoms, iron deficiency and iron-deficiency anemia remain underdiagnosed and underreported[53]. Assessments involve the use of circulating iron deficiency markers such as serum ferritin and transferrin saturation, as well as measurement of hemoglobin, hematocrit and red cell counts[84].
4.2. Primarily Research Methods
The Alkaline Hematin (AH) method has been considered the gold standard for menstrual effluent measurement in research, since its development in 1964[85]. However, in recent years, the use of validated pictorial methods and estimated menstrual fluid volume have been adopted as alternative methods[53][86]. Furthermore, the advent of menstrual period tracking applications and the rise of “Femtech” may present opportunities for research teams to use period-tracking technology to measure menstrual blood loss in studies[87,88].
4.2.1. Alkaline Hematin Method
Individuals must collect their menstrual sanitary products, from which blood content is chemically measured. Tampons and pads are soaked for 20 hours in sodium hydroxide, which extracts hemoglobin and converts it to hematin. The hematin is then measured with a spectrophotometer. This method has an accuracy of measuring menstrual blood within 5%[52]; however, it may not be acceptable or feasible for many individuals as they must collect, store, and submit all used products[53]. This aspect of the AH method limits its use in clinical settings, though it is well-validated for research purposes [53] [22].
4.2.2. Estimated Menstrual Fluid Volume
Measuring menstrual fluid loss (MFL) weight or volume is a simple alternative to AH method[53]. The specific gravity of menstrual fluid is assumed to be 1; thus, the weight can be measured in grams and converted to milliliters[53]. One study found that, in a clinical context, the estimated blood volume from MFL weight correlated with menstrual blood loss as measured by the AH method[89]. Furthermore, Schumacher et al. developed a statistical model in which the estimated blood volume from MFL weight correlated with menstrual blood loss as measured by AH (r = 0.73, p <0.0001)[90]. Further investigation is warranted to determine the degree of interchangeability between AH and weighing MFL.
4.2.3. Validated Pictorial Menstrual Methods
Various methods exist for assessing menstrual blood loss pictorially. PBAC has been validated as diagnostic of menorrhagia, with a specificity and sensitivity of over 80%[86]. PBAC is inexpensive and semi-quantitative, as it uses a scoring system qualitatively measured variables which accounts for number of feminine hygiene products and the degree of saturation of each item[91]. Use of this method as a substitute for the AH method is increasing in clinical studies, as it is less involved for participants and less expensive for research teams. The limitations of this method are that there is a wide variety in pictorial charts used, and a systematic review found that charts are not all standardized to account for all 4 variables (pads, tampons, clots, and flooding). In order to be validated, each pictorial chart must be verified against AH for each absorbency category (i.e. light/spotting, normal, heavy)[53].
4.2.4. Period Tracking Apps
“Femtech”, first released in 2013, is known as a category of developing technology aimed at supporting and improving individuals’ health[87,88]. Individuals can record when their period begins, the degree of flow throughout the period, and the timing of the end of their period. Tracking this information allows individuals to receive an estimate for the beginning of the next cycle, the day of ovulation, and their fertile window, which are helpful to monitor fertility or increase the probability of pregnancy[87]. Femtech use has expanded to approximately 50 million individuals worldwide[92]. The top 10 of over 90 apps were discussed in a systematic review[87] and a review article addressed limitations of these applications[93]. For example, Pichon et al. described how the design of some applications assumes every individual is menstruating on a 28-day cycle as opposed to accounting for the variation in normal menstrual cycles[93]. Applications may rely on what is considered “normal” to provide predictions to users for their next cycle, day of ovulation, and fertile window[93]; however, a study by Worsfold et al found that tracking app predictions were inaccurate for cycles that were not 28 days long or were irregular [87]. Furthermore, Pichon et al. state that some menstrual tracking applications have been criticized as failing to account for variations in the gender of menstruators — for example, application aesthetics are typically pink, pastel and/or have flowers. While these applications exist for the user, there is an opportunity for expanding research on measuring menstrual blood flow in a systematic way in order to accurately track menstrual blood loss [93].
5.0. Future Directions
Nearly half of an individual’s life may be characterized by menstruation. Despite the negative impact of abnormal menstrual bleeding on one’s quality of life[60], the number of affected individuals visiting their gynecologists is under presented[94]. Currently, there are still many challenges regarding the determinants and assessment of menstruation. For example, the causes of menstrual bleeding disorder are often intertwined and complicated. On the one hand, obesity is associated with prolonged cycles [95] and PCOS is positively associated with obesity[96,97]. On the other hand, race and ethnicity are associated with obesity and PCOS[98]. Therefore, more research related to the etiology of menstrual disorders is needed. Assessment of menstrual blood loss is a key component of an individual’s reproductive health and can facilitate identification of pathologic conditions[60], but it is not easy to estimate and often relies on subjective assessment. Accordingly, not only general practitioners but also obstetrics and gynecology specialists need to be educated with a higher awareness of the menstrual disorders[94]. And more feasible and accurate methods are needed to assess menstrual bleeding in the future.
Funding
This work was supported, in part, by NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grant 3R01HD094380-04S1.
Footnotes
Conflict of Interest
The authors declare that they have no actual or potential competing financial interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- [1].Menstrual Cycles as a Fifth Vital Sign | NICHD - Eunice Kennedy Shriver National Institute of Child Health and Human Development 2021. https://www.nichd.nih.gov/about/org/od/directors_corner/prev_updates/menstrual-cycles (accessed May 16, 2023).
- [2].Bae J, Park S, Kwon J-W. Factors associated with menstrual cycle irregularity and menopause. BMC Womens Health 2018;18:36. 10.1186/s12905-018-0528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Reed BG, Carr BR. The Normal Menstrual Cycle and the Control of Ovulation. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al. , editors. Endotext, South Dartmouth (MA): MDText.com, Inc.; 2000. [PubMed] [Google Scholar]
- [4].Mihm M, Gangooly S, Muttukrishna S. The normal menstrual cycle in women. Animal Reproduction Science 2011;124:229–36. 10.1016/j.anireprosci.2010.08.030. [DOI] [PubMed] [Google Scholar]
- [5].Diagnosis of Abnormal Uterine Bleeding in Reproductive-Aged Women n.d. https://www.acog.org/en/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women (accessed February 19, 2023).
- [6].Marnach ML, Laughlin-Tommaso SK. Evaluation and Management of Abnormal Uterine Bleeding. Mayo Clin Proc 2019;94:326–35. 10.1016/j.mayocp.2018.12.012. [DOI] [PubMed] [Google Scholar]
- [7].Munro MG, Critchley HOD, Fraser IS, FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet 2018;143:393–408. 10.1002/ijgo.12666. [DOI] [PubMed] [Google Scholar]
- [8].Female Reproductive Endocrinology - Gynecology and Obstetrics. Merck Manuals Professional Edition n.d. https://www.merckmanuals.com/professional/gynecology-and-obstetrics/female-reproductive-endocrinology/female-reproductive-endocrinology (accessed July 16, 2023).
- [9].Monis CN, Tetrokalashvili M. Menstrual Cycle Proliferative And Follicular Phase. StatPearls, Treasure Island (FL): StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- [10].Thiyagarajan DK, Basit H, Jeanmonod R. Physiology, Menstrual Cycle. StatPearls, Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- [11].Graafian Follicles - an overview | ScienceDirect Topics n.d. https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/graafian-follicles (accessed November 21, 2022).
- [12].Granot I, Gnainsky Y, Dekel N. Endometrial inflammation and effect on implantation improvement and pregnancy outcome. Reproduction 2012;144:661–8. 10.1530/REP-12-0217. [DOI] [PubMed] [Google Scholar]
- [13].Chumlea WC, Schubert CM, Roche AF, Kulin HE, Lee PA, Himes JH, et al. Age at menarche and racial comparisons in US girls. Pediatrics 2003;111:110–3. 10.1542/peds.111.1.110. [DOI] [PubMed] [Google Scholar]
- [14].Ruth KS, Beaumont RN, Tyrrell J, Jones SE, Tuke MA, Yaghootkar H, et al. Genetic evidence that lower circulating FSH levels lengthen menstrual cycle, increase age at menopause and impact female reproductive health. Hum Reprod 2016;31:473–81. 10.1093/humrep/dev318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Harlow SD, Campbell B. Ethnic Differences in the Duration and Amount of Menstrual Bleeding during the Postmenarcheal Period. American Journal of Epidemiology 1996;144:980–8. 10.1093/oxfordjournals.aje.a008868. [DOI] [PubMed] [Google Scholar]
- [16].Lawson CC, Whelan EA, Lividoti Hibert EN, Spiegelman D, Schernhammer ES, Rich-Edwards JW. Rotating shift work and menstrual cycle characteristics. Epidemiology 2011;22:305–12. 10.1097/EDE.0b013e3182130016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jung AN, Park JH, Kim J, Kim SH, Jee BC, Cha BH, et al. Detrimental Effects of Higher Body Mass Index and Smoking Habits on Menstrual Cycles in Korean Women. J Womens Health (Larchmt) 2017;26:83–90. 10.1089/jwh.2015.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grossman MP, Nakajima ST. Menstrual cycle bleeding patterns in cigarette smokers. Contraception 2006;73:562–5. 10.1016/j.contraception.2006.01.016. [DOI] [PubMed] [Google Scholar]
- [19].Rowland AS, Baird DD, Long S, Wegienka G, Harlow SD, Alavanja M, et al. Influence of Medical Conditions and Lifestyle Factors on the Menstrual Cycle. Epidemiology 2002;13:668. [DOI] [PubMed] [Google Scholar]
- [20].Magnay JL, O’Brien S, Gerlinger C, Seitz C. Pictorial methods to assess heavy menstrual bleeding in research and clinical practice: a systematic literature review. BMC Womens Health 2020;20:24. 10.1186/s12905-020-0887-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shaw ST, Aaronson DE, Moyer DL. Quantitation of menstrual blood loss — Further evaluation of the alkaline hematin method. Contraception 1972;5:497–513. 10.1016/0010-7824(72)90015-7. [DOI] [PubMed] [Google Scholar]
- [22].Magnay JL, O’Brien S, Gerlinger C, Seitz C. A systematic review of methods to measure menstrual blood loss. BMC Womens Health 2018;18:142. 10.1186/s12905-018-0627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Onieva-Zafra MD, Fernández-Martínez E, Abreu-Sánchez A, Iglesias-López MT, García-Padilla FM, Pedregal-González M, et al. Relationship between Diet, Menstrual Pain and other Menstrual Characteristics among Spanish Students. Nutrients 2020;12:1759. 10.3390/nu12061759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Payne NE, Cross JH, Sander JW, Sisodiya SM. The ketogenic and related diets in adolescents and adults--a review. Epilepsia 2011;52:1941–8. 10.1111/j.1528-1167.2011.03287.x. [DOI] [PubMed] [Google Scholar]
- [25].Mady MA, Kossoff EH, McGregor AL, Wheless JW, Pyzik PL, Freeman JM. The ketogenic diet: adolescents can do it, too. Epilepsia 2003;44:847–51. 10.1046/j.1528-1157.2003.57002.x. [DOI] [PubMed] [Google Scholar]
- [26].Pirke KM, Schweiger U, Laessle R, Dickhaut B, Schweiger M, Waechtler M. Dieting influences the menstrual cycle: vegetarian versus nonvegetarian diet**Supported by a grant from the German Federal Government (Ministerium für Forschung und Technologie) grant no. 187490. Fertility and Sterility 1986;46:1083–8. 10.1016/S0015-0282(16)49884-5. [DOI] [PubMed] [Google Scholar]
- [27].Drosdzol-Cop A, Bąk-Sosnowska M, Sajdak D, Białka A, Kobiołka A, Franik G, et al. Assessment of the menstrual cycle, eating disorders and self-esteem of Polish adolescents. Journal of Psychosomatic Obstetrics & Gynecology 2017;38:30–6. 10.1080/0167482X.2016.1216959. [DOI] [PubMed] [Google Scholar]
- [28].Pedersen AB, Bartholomew MJ, Dolence LA, Aljadir LP, Netteburg KL, Lloyd T. Menstrual differences due to vegetarian and nonvegetarian diets. Am J Clin Nutr 1991;53:879–85. 10.1093/ajcn/53.4.879. [DOI] [PubMed] [Google Scholar]
- [29].Bajalan Z, Alimoradi Z, Moafi F. Nutrition as a Potential Factor of Primary Dysmenorrhea: A Systematic Review of Observational Studies. Gynecol Obstet Invest 2019;84:209–24. 10.1159/000495408. [DOI] [PubMed] [Google Scholar]
- [30].Ålgars M, Huang L, Von Holle AF, Peat CM, Thornton LM, Lichtenstein P, et al. Binge eating and menstrual dysfunction. Journal of Psychosomatic Research 2014;76:19–22. 10.1016/j.jpsychores.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Martini MG, Solmi F, Krug I, Karwautz A, Wagner G, Fernandez-Aranda F, et al. Associations between eating disorder diagnoses, behaviors, and menstrual dysfunction in a clinical sample. Arch Womens Ment Health 2016;19:553–7. 10.1007/s00737-015-0576-2. [DOI] [PubMed] [Google Scholar]
- [32].Roberts RE, Farahani L, Webber L, Jayasena C. Current understanding of hypothalamic amenorrhoea. Therapeutic Advances in Endocrinology 2020;11:204201882094585. 10.1177/2042018820945854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wiksten-Almströmer M, Lindén Hirschberg A, Hagenfeldt K. Menstrual disorders and associated factors among adolescent girls visiting a youth clinic. Acta Obstet Gynecol Scand 2007;86:65–72. 10.1080/00016340601034970. [DOI] [PubMed] [Google Scholar]
- [34].Mena GP, Mielke GI, Brown WJ. Prospective associations between physical activity and BMI with irregular periods and heavy menstrual bleeding in a large cohort of Australian women. Human Reproduction 2021;36:1481–91. 10.1093/humrep/deab055. [DOI] [PubMed] [Google Scholar]
- [35].Vyver E, Steinegger C, Katzman DK. Eating Disorders and Menstrual Dysfunction in Adolescents. Annals of the New York Academy of Sciences 2008;1135:253–64. 10.1196/annals.1429.013. [DOI] [PubMed] [Google Scholar]
- [36].Poyastro Pinheiro A, Thornton LM, Plotonicov KH, Tozzi F, Klump KL, Berrettini WH, et al. Patterns of menstrual disturbance in eating disorders. Int J Eat Disord 2007;40:424–34. 10.1002/eat.20388. [DOI] [PubMed] [Google Scholar]
- [37].Seif MW, Diamond K, Nickkho-Amiry M. Obesity and menstrual disorders. Best Practice & Research Clinical Obstetrics & Gynaecology 2015;29:516–27. 10.1016/j.bpobgyn.2014.10.010. [DOI] [PubMed] [Google Scholar]
- [38].Stoegerer–Hecher E, Kirchengast S, Huber JC, Hartmann B. Amenorrhea and BMI as independent determinants of patient satisfaction in LNG-IUD users: Cross-sectional study in a Central European district. Gynecological Endocrinology 2012;28:119–24. 10.3109/09513590.2011.588751. [DOI] [PubMed] [Google Scholar]
- [39].Assessing Your Weight and Health Risk n.d. https://www.nhlbi.nih.gov/health/educational/lose_wt/risk.htm (accessed February 19, 2023).
- [40].Bryant M, Truesdale KP, Dye L. Modest changes in dietary intake across the menstrual cycle: implications for food intake research. Br J Nutr 2006;96:888–94. 10.1017/BJN20061931. [DOI] [PubMed] [Google Scholar]
- [41].Huhmann K Menses Requires Energy: A Review of How Disordered Eating, Excessive Exercise, and High Stress Lead to Menstrual Irregularities. Clinical Therapeutics 2020;42:401–7. 10.1016/j.clinthera.2020.01.016. [DOI] [PubMed] [Google Scholar]
- [42].Huhmann K Menses Requires Energy: A Review of How Disordered Eating, Excessive Exercise, and High Stress Lead to Menstrual Irregularities. Clin Ther 2020;42:401–7. 10.1016/j.clinthera.2020.01.016. [DOI] [PubMed] [Google Scholar]
- [43].Song S, Choi H, Pang Y, Kim O, Park H-Y. Factors associated with regularity and length of menstrual cycle: Korea Nurses’ Health Study. BMC Women’s Health 2022;22:361. 10.1186/s12905-022-01947-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nagma S To Evaluate the Effect of Perceived Stress on Menstrual Function. JCDR 2015. 10.7860/JCDR/2015/6906.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].De Souza MJ, Toombs RJ, Scheid JL, O’Donnell E, West SL, Williams NI. High prevalence of subtle and severe menstrual disturbances in exercising women: confirmation using daily hormone measures. Human Reproduction 2010;25:491–503. 10.1093/humrep/dep411. [DOI] [PubMed] [Google Scholar]
- [46].Bernstein L, Ross RK, Lobo RA, Hanisch R, Krailo MD, Henderson BE. The effects of moderate physical activity on menstrual cycle patterns in adolescence: implications for breast cancer prevention. Br J Cancer 1987;55:681–5. 10.1038/bjc.1987.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hahn K, Wise L, Riis, Mikkelsen, Rothman, Banholzer, et al. Correlates of menstrual cycle characteristics among nulliparous Danish women. CLEP 2013:311. 10.2147/CLEP.S46712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Motzer SA, Hertig V. Stress, stress response, and health. Nursing Clinics of North America 2004;39:1–17. 10.1016/j.cnur.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [49].Jung E-K, Kim S-W, Ock S-M, Jung K-I, Song C-H. Prevalence and related factors of irregular menstrual cycles in Korean women: the 5th Korean National Health and Nutrition Examination Survey (KNHANES-V, 2010–2012). J Psychosom Obstet Gynaecol 2018;39:196–202. 10.1080/0167482X.2017.1321631. [DOI] [PubMed] [Google Scholar]
- [50].Yu M, Han K, Nam GE. The association between mental health problems and menstrual cycle irregularity among adolescent Korean girls. J Affect Disord 2017;210:43–8. 10.1016/j.jad.2016.11.036. [DOI] [PubMed] [Google Scholar]
- [51].Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–7. 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- [52].Vercellini P, Frattaruolo MP. Uterine fibroids: from observational epidemiology to clinical management. BJOG 2017;124:1513. 10.1111/1471-0528.14730. [DOI] [PubMed] [Google Scholar]
- [53].Vannuccini S, Jain V, Critchley H, Petraglia F. From menarche to menopause, heavy menstrual bleeding is the underrated compass in reproductive health. Fertil Steril 2022;118:625–36. 10.1016/j.fertnstert.2022.07.021. [DOI] [PubMed] [Google Scholar]
- [54].Dueholm M Transvaginal ultrasound for diagnosis of adenomyosis: a review. Best Pract Res Clin Obstet Gynaecol 2006;20:569–82. 10.1016/j.bpobgyn.2006.01.005. [DOI] [PubMed] [Google Scholar]
- [55].Benagiano G, Brosens I, Habiba M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum Reprod Update 2014;20:386–402. 10.1093/humupd/dmt052. [DOI] [PubMed] [Google Scholar]
- [56].Smolarz B, Szyłło K, Romanowicz H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int J Mol Sci 2021;22:10554. 10.3390/ijms221910554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Savelli L, De Iaco P, Santini D, Rosati F, Ghi T, Pignotti E, et al. Histopathologic features and risk factors for benignity, hyperplasia, and cancer in endometrial polyps. Am J Obstet Gynecol 2003;188:927–31. 10.1067/mob.2003.247. [DOI] [PubMed] [Google Scholar]
- [58].Salim S, Won H, Nesbitt-Hawes E, Campbell N, Abbott J. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol 2011;18:569–81. 10.1016/j.jmig.2011.05.018. [DOI] [PubMed] [Google Scholar]
- [59].Nappi L, Indraccolo U, Di Spiezio Sardo A, Gentile G, Palombino K, Castaldi MA, et al. Are diabetes, hypertension, and obesity independent risk factors for endometrial polyps? J Minim Invasive Gynecol 2009;16:157–62. 10.1016/j.jmig.2008.11.004. [DOI] [PubMed] [Google Scholar]
- [60].Matteson KA, Zaluski KM. Menstrual Health as a Part of Preventive Health Care. Obstet Gynecol Clin North Am 2019;46:441–53. 10.1016/j.ogc.2019.04.004. [DOI] [PubMed] [Google Scholar]
- [61].Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 2016;106:6–15. 10.1016/j.fertnstert.2016.05.003. [DOI] [PubMed] [Google Scholar]
- [62].Lentscher JA, Decherney AH. Clinical Presentation and Diagnosis of Polycystic Ovarian Syndrome. Clinical Obstetrics & Gynecology 2021;64:3–11. 10.1097/GRF.0000000000000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol 2022;10:668–80. 10.1016/S2213-8587(22)00163-2. [DOI] [PubMed] [Google Scholar]
- [64].Copp T, Doust J, McCaffery K, Hersch J, Jansen J. Polycystic ovary syndrome: why widening the diagnostic criteria may be harming women. BMJ 2021;373:n700. 10.1136/bmj.n700. [DOI] [PubMed] [Google Scholar]
- [65].Rundell K, Panchal B. Being Reproductive. Primary Care: Clinics in Office Practice 2018;45:587–98. 10.1016/j.pop.2018.07.003. [DOI] [PubMed] [Google Scholar]
- [66].Elting MW, Korsen TJ, Rekers-Mombarg LT, Schoemaker J. Women with polycystic ovary syndrome gain regular menstrual cycles when ageing. Hum Reprod 2000;15:24–8. 10.1093/humrep/15.1.24. [DOI] [PubMed] [Google Scholar]
- [67].Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertility and Sterility 2009;92:1966–82. 10.1016/j.fertnstert.2008.09.018. [DOI] [PubMed] [Google Scholar]
- [68].Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2013;98:4565–92. 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Walker K, Decherney AH, Saunders R. Menstrual Dysfunction in PCOS. Clinical Obstetrics and Gynecology 2021;64:119. 10.1097/GRF.0000000000000596. [DOI] [PubMed] [Google Scholar]
- [70].Polycystic Ovary Syndrome n.d. https://www.acog.org/en/clinical/clinical-guidance/practice-bulletin/articles/2018/06/polycystic-ovary-syndrome (accessed February 19, 2023).
- [71].Deligeoroglou E, Karountzos V. Abnormal Uterine Bleeding including coagulopathies and other menstrual disorders. Best Pract Res Clin Obstet Gynaecol 2018;48:51–61. 10.1016/j.bpobgyn.2017.08.016. [DOI] [PubMed] [Google Scholar]
- [72].Wallace RB, Sherman BM, Bean JA, Treloar AE, Schlabaugh L. Probability of menopause with increasing duration of amenorrhea in middle-aged women. American Journal of Obstetrics & Gynecology 1979;135:1021–4. 10.1016/0002-9378(79)90729-4. [DOI] [PubMed] [Google Scholar]
- [73].Harlow SD. Menstrual Cycle Changes as Women Approach the Final Menses: What Matters? Obstetrics and Gynecology Clinics of North America 2018;45:599–611. 10.1016/j.ogc.2018.07.003. [DOI] [PubMed] [Google Scholar]
- [74].Diagnosis and treatment of luteal phase deficiency: a committee opinion. Fertility and Sterility 2021;115:1416–23. 10.1016/j.fertnstert.2021.02.010. [DOI] [PubMed] [Google Scholar]
- [75].Deligeoroglou E, Karountzos V, Creatsas G. Abnormal uterine bleeding and dysfunctional uterine bleeding in pediatric and adolescent gynecology. Gynecological Endocrinology 2013;29:74–8. 10.3109/09513590.2012.705384. [DOI] [PubMed] [Google Scholar]
- [76].Peer RF, Shabir N. Iatrogenesis: A review on nature, extent, and distribution of healthcare hazards. J Family Med Prim Care 2018;7:309–14. 10.4103/jfmpc.jfmpc_329_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Healy MW, Schexnayder B, Connell MT, Terry N, DeCherney AH, Csokmay JM, et al. Intrauterine adhesion prevention after hysteroscopy: a systematic review and meta-analysis. Am J Obstet Gynecol 2016;215:267–275.e7. 10.1016/j.ajog.2016.05.001. [DOI] [PubMed] [Google Scholar]
- [78].Matsuoka M, Taga A, Hata S, Yamamoto R, Ando Y, Kozono Y, et al. Abnormal menstruation after radical trachelectomy and its effects on fertility. Journal of Obstetrics and Gynaecology Research 2019;45:1906–12. 10.1111/jog.14032. [DOI] [PubMed] [Google Scholar]
- [79].Djuwantono T, Permadi W, Septiani L, Faried A, Halim D, Parwati I. Female genital tuberculosis and infertility: serial cases report in Bandung, Indonesia and literature review. BMC Research Notes 2017;10:683. 10.1186/s13104-017-3057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Brown DL. Congenital bleeding disorders. Current Problems in Pediatric and Adolescent Health Care 2005;35:38–62. 10.1016/j.cppeds.2004.12.001. [DOI] [PubMed] [Google Scholar]
- [81].Hallberg L, Hôgdahl A-M, Nilsson L, Rybo G. Menstrual Blood Loss–A Population Study. Acta Obstetricia et Gynecologica Scandinavica 1966;45:320–51. 10.3109/00016346609158455. [DOI] [PubMed] [Google Scholar]
- [82].Warner PE, Critchley HOD, Lumsden MA, Campbell-Brown M, Douglas A, Murray GD. Menorrhagia I: measured blood loss, clinical features, and outcome in women with heavy periods: a survey with follow-up data. American Journal of Obstetrics & Gynecology 2004;190:1216–23. 10.1016/j.ajog.2003.11.015. [DOI] [PubMed] [Google Scholar]
- [83].Heavy menstrual bleeding: assessment and management 2021. [PubMed]
- [84].Camaschella C Iron deficiency. Blood 2019;133:30–9. 10.1182/blood-2018-05-815944. [DOI] [PubMed] [Google Scholar]
- [85].van Eijkeren MA, Scholten PC, Christiaens GCML, Alsbach GPJ, Haspels AA. The alkaline hematin method for measuring menstrual blood loss — a modification and its clinical use in menorrhagia. European Journal of Obstetrics & Gynecology and Reproductive Biology 1986;22:345–51. 10.1016/0028-2243(86)90124-3. [DOI] [PubMed] [Google Scholar]
- [86].Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol 1990;97:734–9. 10.1111/j.1471-0528.1990.tb16249.x. [DOI] [PubMed] [Google Scholar]
- [87].Worsfold L, Marriott L, Johnson S, Harper JC. Period tracker applications: What menstrual cycle information are they giving women? Womens Health (Lond) 2021;17:17455065211049904. 10.1177/17455065211049905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Krishnamurti T, Birru Talabi M, Callegari LS, Kazmerski TM, Borrero S. A Framework for Femtech: Guiding Principles for Developing Digital Reproductive Health Tools in the United States. J Med Internet Res 2022;24:e36338. 10.2196/36338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Fraser IS, Warner P, Marantos PA. Estimating menstrual blood loss in women with normal and excessive menstrual fluid volume. Obstet Gynecol 2001;98:806–14. 10.1016/s0029-7844(01)01581-2. [DOI] [PubMed] [Google Scholar]
- [90].Schumacher U, Schumacher J, Mellinger U, Gerlinger C, Wienke A, Endrikat J. Estimation of menstrual blood loss volume based on menstrual diary and laboratory data. BMC Womens Health 2012;12:24. 10.1186/1472-6874-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wyatt KM, Dimmock PW, Walker TJ, O’Brien PMS. Determination of total menstrual blood loss. Fertility and Sterility 2001;76:125–31. 10.1016/S0015-0282(01)01847-7. [DOI] [PubMed] [Google Scholar]
- [92].What Your Period Tracker App Knows About You Consumer Reports n.d. https://www.consumerreports.org/health-privacy/what-your-period-tracker-app-knows-about-you-a8701683935/ (accessed November 26, 2022).
- [93].Pichon A, Jackman KB, Winkler IT, Bobel C, Elhadad N. The messiness of the menstruator: assessing personas and functionalities of menstrual tracking apps. J Am Med Inform Assoc 2021;29:385–99. 10.1093/jamia/ocab212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Dkeidek A, Mattinson R, Grover SR. Bleeding disorders: Are gynaecologists testing women at risk? Haemophilia 2021;27:e534–6. 10.1111/hae.14283. [DOI] [PubMed] [Google Scholar]
- [95].Rochester D, Jain A, Polotsky AJ, Polotsky H, Gibbs K, Isaac B, et al. Partial recovery of luteal function after bariatric surgery in obese women. Fertil Steril 2009;92:1410–5. 10.1016/j.fertnstert.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Simons PIHG, Cornelissen MEB, Valkenburg O, Onland-Moret NC, van der Schouw YT, Stehouwer CDA, et al. Causal relationship between polycystic ovary syndrome and coronary artery disease: A Mendelian randomisation study. Clin Endocrinol (Oxf) 2022;96:599–604. 10.1111/cen.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Álvarez-Blasco F, Botella-Carretero JI, San Millán JL, Escobar-Morreale HF. Prevalence and Characteristics of the Polycystic Ovary Syndrome in Overweight and Obese Women. Archives of Internal Medicine 2006;166:2081–6. 10.1001/archinte.166.19.2081. [DOI] [PubMed] [Google Scholar]
- [98].Li R, Zhang Q, Yang D, Li S, Lu S, Wu X, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod 2013;28:2562–9. 10.1093/humrep/det262. [DOI] [PubMed] [Google Scholar]


