Abstract
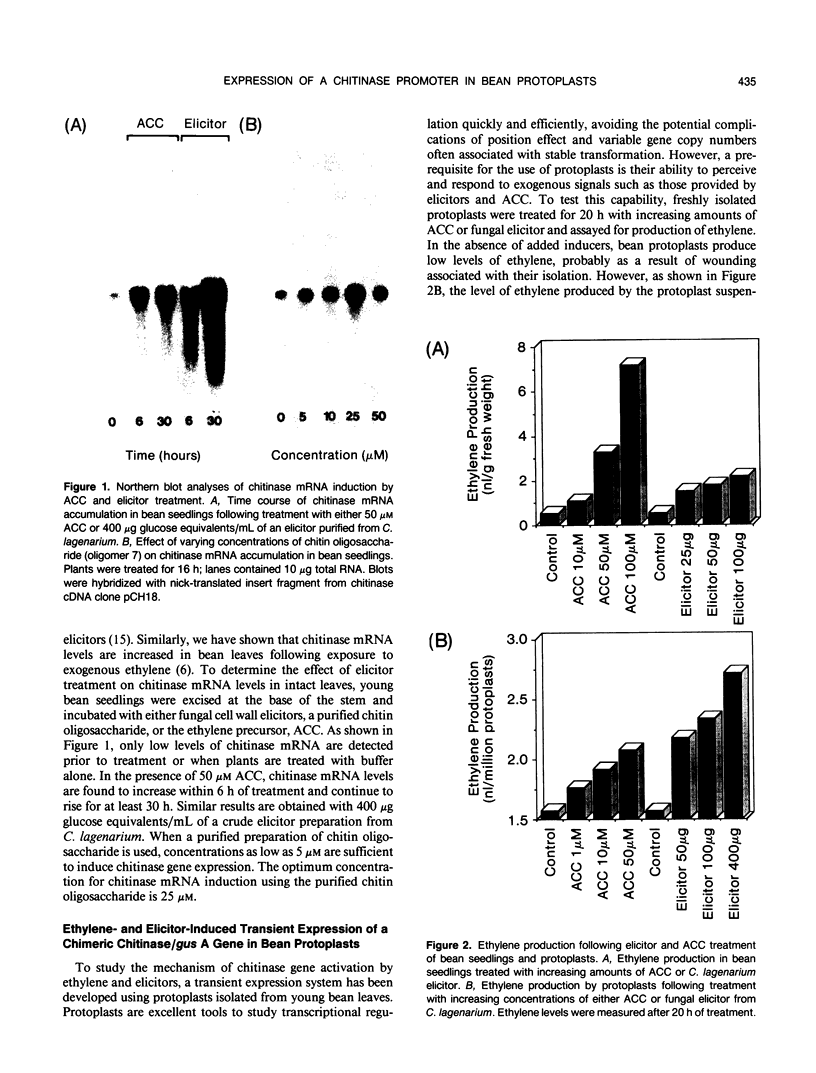
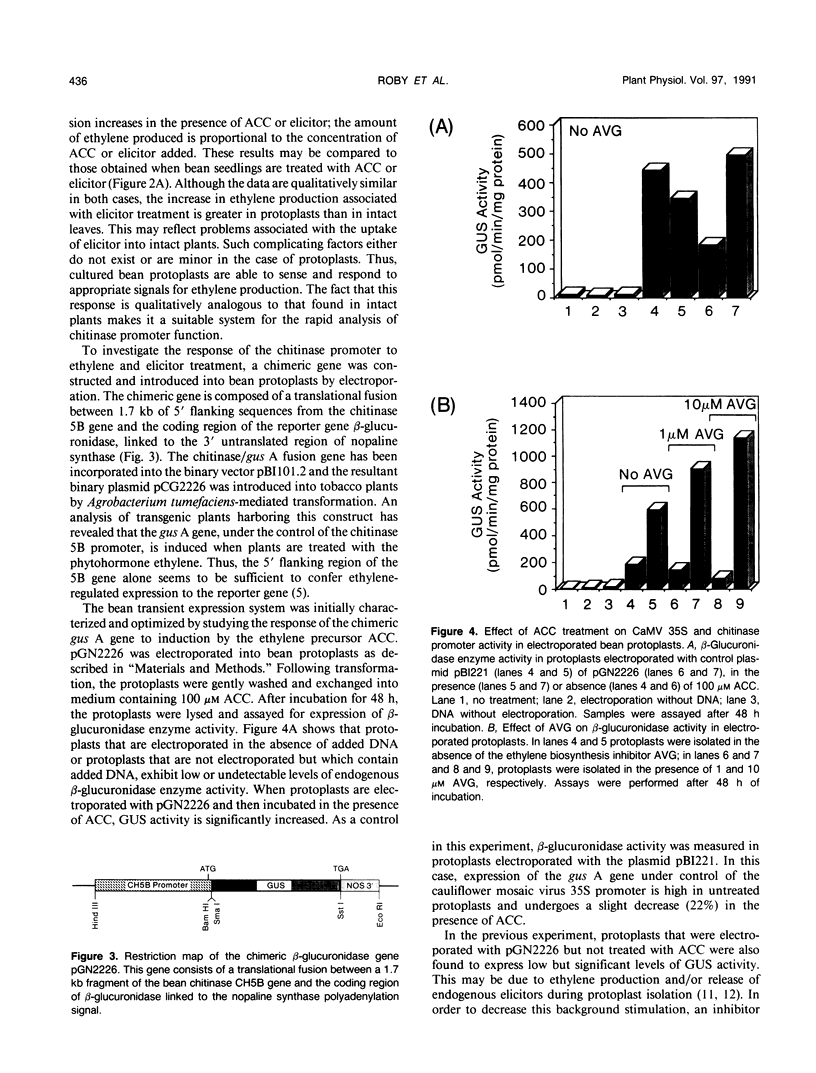
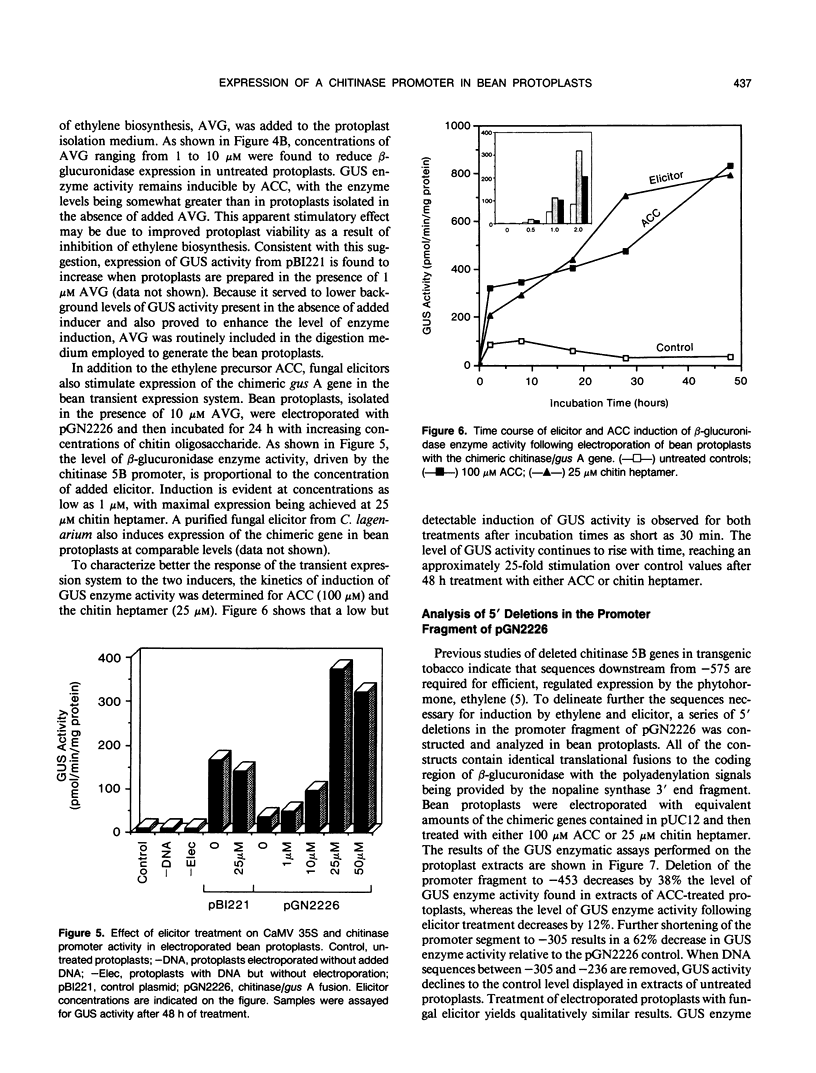
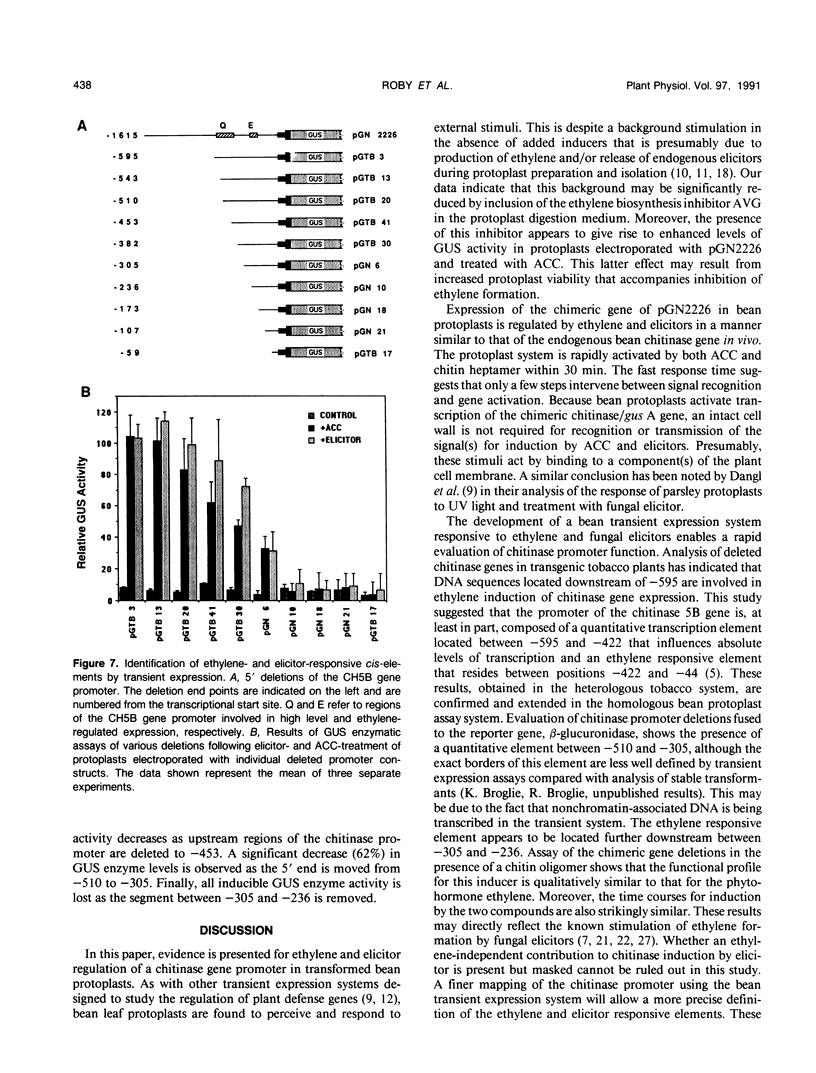
Chitinase gene expression has been shown to be transcriptionally regulated by a number of inducers, including ethylene, elicitors, and pathogen attack. To investigate the mechanism(s) responsible for induction of chitinase gene expression in response to various stimuli, we have developed a transient gene expression system in bean (Phaseolus vulgaris) protoplasts that is responsive to ethylene and elicitor treatment. This system was used to study the expression of a chimeric gene composed of the 5′ flanking sequences of a bean endochitinase gene fused to the reporter gene β-glucuronidase linked to a 3′ fragment from nopaline synthase. Addition of 1-aminocyclopropane-1-carboxylic acid, the direct precursor of ethylene, or elicitors such as chitin oligosaccharides or cell wall fragments derived from Colletotrichum lagenarium, to transformed protoplasts resulted in a rapid and marked increase in the expression of the chimeric gene. The kinetics and dose response for these treatments were similar to those observed for the native gene in vivo. Analyses of 5′ deletion mutants in the protoplast system indicated that DNA sequences located between −305 and −236 are important for both ethylene and elicitor induction of the reporter gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Bosshart R. P., Forrence L. E., Habig W. H. Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol. 1971 Jan;47(1):129–134. doi: 10.1104/pp.47.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglie K. E., Biddle P., Cressman R., Broglie R. Functional analysis of DNA sequences responsible for ethylene regulation of a bean chitinase gene in transgenic tobacco. Plant Cell. 1989 Jun;1(6):599–607. doi: 10.1105/tpc.1.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglie K. E., Gaynor J. J., Broglie R. M. Ethylene-regulated gene expression: molecular cloning of the genes encoding an endochitinase from Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6820–6824. doi: 10.1073/pnas.83.18.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl J. L., Hauffe K. D., Lipphardt S., Hahlbrock K., Scheel D. Parsley protoplasts retain differential responsiveness to u.v. light and fungal elicitor. EMBO J. 1987 Sep;6(9):2551–2556. doi: 10.1002/j.1460-2075.1987.tb02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. R., Hahlbrock K. Induction of defense responses in cultured parsley cells by plant cell wall fragments. Plant Physiol. 1987 Aug;84(4):1286–1290. doi: 10.1104/pp.84.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dron M., Clouse S. D., Dixon R. A., Lawton M. A., Lamb C. J. Glutathione and fungal elicitor regulation of a plant defense gene promoter in electroporated protoplasts. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6738–6742. doi: 10.1073/pnas.85.18.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert P. R., Ha S. B., An G. Identification of an essential upstream element in the nopaline synthase promoter by stable and transient assays. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5745–5749. doi: 10.1073/pnas.84.16.5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger L. A., Beckman J. M. Chitosan as a Component of Pea-Fusarium solani Interactions. Plant Physiol. 1980 Aug;66(2):205–211. doi: 10.1104/pp.66.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. A., Bell J. N., Boller T., Lamb C. J. Chitinase cDNA cloning and mRNA induction by fungal elicitor, wounding, and infection. Plant Physiol. 1988 Jan;86(1):182–186. doi: 10.1104/pp.86.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols E. J., Beckman J. M., Hadwiger L. A. Glycosidic Enzyme Activity in Pea Tissue and Pea-Fusarium solani Interactions. Plant Physiol. 1980 Aug;66(2):199–204. doi: 10.1104/pp.66.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby D., Gadelle A., Toppan A. Chitin oligosaccharides as elicitors of chitinase activity in melon plants. Biochem Biophys Res Commun. 1987 Mar 30;143(3):885–892. doi: 10.1016/0006-291x(87)90332-9. [DOI] [PubMed] [Google Scholar]
- Roby D., Toppan A., Esquerré-Tugayé M. T. Cell Surfaces in Plant-Microorganism Interactions : VI. Elicitors of Ethylene from Colletotrichum lagenarium Trigger Chitinase Activity in Melon Plants. Plant Physiol. 1986 May;81(1):228–233. doi: 10.1104/pp.81.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby D., Toppan A., Esquerré-Tugayé M. T. Cell surfaces in plant-microorganism interactions : v. Elicitors of fungal and of plant origin trigger the synthesis of ethylene and of cell wall hydroxyproline-rich glycoprotein in plants. Plant Physiol. 1985 Mar;77(3):700–704. doi: 10.1104/pp.77.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C. B., Labavitch J. M., Yang S. F. The induction of ethylene production from pear cell culture by cell wall fragments. Plant Physiol. 1986 Jul;81(3):929–930. doi: 10.1104/pp.81.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppan A., Esquerré-Tugayé M. T. Cell Surfaces in Plant-Microorganism Interactions : IV. Fungal Glycopeptides Which Elicit the Synthesis of Ethylene in Plants. Plant Physiol. 1984 Aug;75(4):1133–1138. doi: 10.1104/pp.75.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. C., Howard E. A., Dennis E. S., Peacock W. J. DNA sequences required for anaerobic expression of the maize alcohol dehydrogenase 1 gene. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6624–6628. doi: 10.1073/pnas.84.19.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]