Abstract
Periodontitis, which is widespread in the adult population, is a persistent bacterial infection associated with Porphyromonas gingivalis. Gingival epithelial cells are among the first cells encountered by both P. gingivalis and commensal oral bacteria. The chemokine interleukin 8 (IL-8), a potent chemoattractant and activator of polymorphonuclear leukocytes, was secreted by gingival epithelial cells in response to components of the normal oral flora. In contrast, P. gingivalis was found to strongly inhibit IL-8 accumulation from gingival epithelial cells. Inhibition was associated with a decrease in mRNA for IL-8. Antagonism of IL-8 accumulation did not occur in KB cells, an epithelial cell line that does not support high levels of intracellular invasion by P. gingivalis. Furthermore, a noninvasive mutant of P. gingivalis was unable to antagonize IL-8 accumulation. Invasion-dependent destruction of the gingival IL-8 chemokine gradient at sites of P. gingivalis colonization (local chemokine paralysis) will severely impair mucosal defense and represents a novel mechanism for bacterial colonization of host tissue.
Persistent bacterial infection of host tissue is gaining recognition as a major and previously unappreciated factor in chronic diseases, including gastric cancer (7), coronary heart disease (17, 27, 32), and preterm delivery of low-birth-weight infants (18). Periodontal diseases that result from persistent bacterial infection in the gingival sulcus have also been linked to an increased incidence of preterm delivery of low-birth-weight infants (30) and cardiovascular disease (5). Local persistent infections may exert systemic effects by the release of antigens or modulation of systemic cytokine levels. However, the mechanisms that bacteria employ to overcome innate host defense and persist in host tissue are still largely unknown.
Adult periodontitis is a highly destructive chronic inflammatory disease that is highly prevalent in human populations and a major cause of tooth loss (37). The microbiology of the disease is complex, and the periodontal pocket can contain more than 300 species that may reach concentrations exceeding 108 bacteria per site. Only a small subset of these organisms, however, is considered pathogenic due to their ability to elaborate various enzymes and toxic products that directly impinge upon periodontal tissues and provide a stimulus for host inflammatory reactions. Under certain circumstances, the inflammatory response mediates destruction of the tissue and alveolar bone surrounding the tooth root. Porphyromonas gingivalis is considered one of the foremost periodontal pathogens due to a strong clinical correlation (37) and its ability to induce disease in primates (20). This bacterium possesses pathogenic properties, including the ability to invade host epithelial cells, that are consistent with its clinically defined role (8, 23). However, an understanding of the pathogenic mechanisms by which this organism causes disease at the molecular and cellular levels is still incomplete.
Molecular mediators of the inflammatory arm of innate host defense, such as E-selectin, intracellular adhesion molecule 1 (ICAM-1), and interleukin 8 (IL-8), are expressed in clinically healthy periodontal tissue (26, 29, 39). The expression of these molecules is consistent with the characteristic low-level inflammatory cellular infiltrate of neutrophils and monocytes found in healthy periodontal tissue in response to bacterial colonization (31). IL-8 forms a gradient of expression in clinically healthy periodontal tissue that is highest at the bacterial cell-epithelial cell interface and decreases deeper in the periodontium (39). This gradient directs neutrophils to the site of bacterial colonization, thereby protecting normal periodontal tissue from neutrophil mediated damage. Low-level expression of these inflammatory mediators is believed to be key for the maintenance of clinically healthy tissue, although the factors that regulate their expression are not known.
Recent studies have pointed out the important contribution that epithelial cells make to innate host defense (1, 2, 12, 21, 25, 34). Gastrointestinal and uroepithelial cells express a limited spectrum of proinflammatory cytokines in response to both invasive and noninvasive bacteria. This limited proinflammatory response has been proposed to “limit the consequences of microbial exposure at the mucosal surface and help maintain the integrity of other tissue compartments” (1). This is especially relevant in the periodontium, where epithelial cells are constantly exposed to microbial antigens. However, little is known about the response of gingival epithelial cells (GEC) to periodontal bacteria. In this report, the ability of primary GEC to secrete IL-8 in response to periodontal bacteria was examined. It was found that a variety of periodontal bacteria not normally associated with disease were potent stimulators of IL-8 from GEC. In contrast, not only did P. gingivalis fail to elicit the accumulation of this chemokine but it also inhibited IL-8 accumulation in response to other bacteria. Based upon these observations, a novel mechanism of subversion of innate host defenses by P. gingivalis is described.
MATERIALS AND METHODS
Reagents and buffers.
Reagent-grade chemicals were obtained from Sigma Chemical Co., St. Louis, Mo. Pooled human serum was obtained from Gemini Bioproducts, Calabasas, Calif. Lipopolysaccharide (LPS) was purified as described previously (10).
Bacteria and culture conditions.
P. gingivalis 33277 was maintained as frozen stock cultures. P. gingivalis 381 and DPG3, kindly provided by R. J. Genco (State University of New York), are a parent laboratory strain and a fimbria-deficient mutant, respectively. DPG3 was created by insertional inactivation of the fimA gene (24). Bacteria were grown anaerobically at 37°C in Trypticase soy broth supplemented with hemin and menadione (22). P. gingivalis MP4-504 is a low-passage clinical isolate obtained from periodontal tissues (23). Fusobacterium nucleatum 25586, Neisseria flavescens 13120, Haemophilus parainfluenzae BMS C128, Eikenella corrodens 23834, and Leptotrichia buccalis 14201 are maintained as frozen stock cultures and grown as described previously (9).
Epithelial cell culture.
Primary cultures of GEC were obtained from gingival explants and maintained in tissue culture in keratinocyte growth medium (KGM) (Clonetics) as described previously (23). KB cells, an oral epithelial line, were maintained as frozen stocks and cultured in Dulbecco modified Eagle medium (Gibco).
Bacterial coincubation with GEC and detection of IL-8.
Bacteria, washed and suspended in phosphate-buffered saline (pH 7.2), were added (108 cells/well, unless otherwise noted) to primary (passage 3 or 4) GEC in KGM or KB cells containing 1% (final concentration) pooled human serum as a source of human lipopolysaccharide binding protein and soluble CD14. After 18 h of coincubation with bacteria, the culture supernatant was removed and IL-8 was detected with Cytoscreen IL-8 Immunoassay Kit (Biosource International, Camarillo, Calif.) as described by the manufacturer. The number of bacteria added to GEC was calculated by a predetermined conversion factor that related the optical density to the number of bacteria and confirmed retroactively by viable counting. Bacterial viability experiments demonstrated that the bacteria did not grow in the epithelial cell culture medium during the experiments, so the number added represents the highest number of bacteria exposed to the GEC. In experiments which contained the addition of P. gingivalis and other bacteria (for example, F. nucleatum plus P. gingivalis), the other bacteria (108), and P. gingivalis bacteria (106) were mixed before addition to the GEC. Where indicated, 40 ng of anti-CD14 antibody (MY4; Coulter Immunology, Hialeah, Fla.) per ml was added to the reaction mixture along with the bacteria.
Epithelial cell viability was determined on duplicate plates by several methods: the intracellular esterase hydrolysis of calcein-acetomethyl ester method as described by the manufacturer (Molecular Probes, Inc., Eugene, Oreg.), trypan blue exclusion, and calcium responses to ionomycin.
Reverse transcription-PCR (RT-PCR) analysis of IL-8.
Total RNA from approximately 2 × 106 GEC was purified by using the RNA-Stat 30 kit (Tel-Test “B”, Inc., Friendswood, Tex.) according to the manufacturer’s protocol. cDNA was synthesized from 3 μg of total RNA using the SUPERSCRIPT Preamplification System (GIBCO-BRL) according to the manufacturer’s instructions. Five microliters of a 1:30 dilution of cDNA in a total volume of 50 μl was used for PCR analysis. Oligonucleotide primers were used at a final concentration of 1 μM. The PCR was performed for 35 cycles, with 1 cycle consisting of denaturation at 94°C for 1 min, annealing at 54°C for 2 min, and polymerization at 72°C for 3 min. The oligonucleotide primers used in the PCR for IL-8 are as follows: 5′ oligonucleotide, TTTCTGATGGAAGAGAGCTCTGTCTGGAACC, and 3′ oligonucleotide, AGTGGAACAAGGACTTGTGG TGGCTA. Oligonucleotides specific for human B-actin used as controls for mRNA and cDNA synthesis were as follows: 5′ oligonucleotide, GTCGGTTGGAGCGAGCATC, and the 3′ oligonucleotide, AGCCCTGGCTGCCTCCAC. The amplified PCR products were then analyzed by electrophoresis on 1% agarose gels. The identities of the bands were confirmed by sequence analysis.
RESULTS
GEC secrete IL-8 in response to periodontal plaque bacteria.
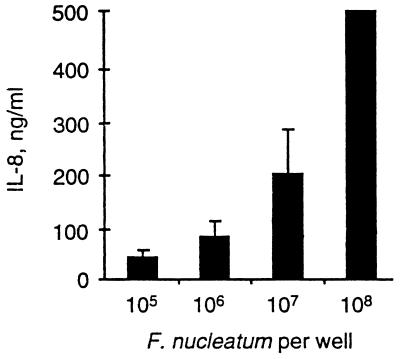
The ability of GEC to secrete IL-8 in response to several different periodontal plaque bacteria was examined. During the assay period, bacteria did not multiply significantly, and the epithelial cell monolayer remained adherent, with cells displaying their characteristic morphology. Several common dental plaque bacteria were able to elicit accumulation of IL-8 into the GEC culture supernatant (Table 1). Coincubation of bacteria and GEC was sufficient to elicit IL-8 accumulation, and centrifugation to promote adherence was not required. It was not determined if whole bacteria or released cell wall material was responsible for the activation. IL-8 accumulation increased with increasing concentrations of F. nucleatum (Fig. 1). Activation of GEC by F. nucleatum required CD14, a key mediator of innate host defense that recognizes and funnels bacterial antigens to activation pathways in host cells including epithelial cells (33, 41, 42). The component of F. nucleatum responsible for CD14-dependent IL-8 accumulation by GEC is currently under investigation.
TABLE 1.
IL-8 accumulation by GEC in response to periodontal bacteriaa
| Bacterium | IL-8 level (ng/ml)b |
|---|---|
| Porphyromonas gingivalis | 0 ± 0 |
| Fusobacterium nucleatum | 383 ± 25 |
| Fusobacterium nucleatum + MY4c | 0 ± 0 |
| Fusobacterium nucleatum + P. gingivalis | 0 ± 0 |
| Neisseria flavescens | 353 ± 28 |
| Neisseria flavescens + P. gingivalis | 0 ± 0 |
| Haemophilus parainfluenzae | 383 ± 11 |
| Haemophilus parainfluenzae + P. gingivalis | 0 ± 0 |
| Eikenella corrodens | 328 ± 37 |
| Eikenella corrodens + P. gingivalis | 0 ± 0 |
| Leptrotrichia buccalis | 400 ± 14 |
| Leptrotrichia buccalis + P. gingivalis | 0 ± 0 |
IL-8 in the culture supernatant was assayed after 18 h of incubation with bacteria (108 cells/well). In experiments which contained P. gingivalis and other bacteria, the other bacteria (108) and P. gingivalis bacteria (106) were mixed prior to addition to the GEC.
The means and standard deviations from three separate experiments are reported.
MY4 is an anti-CD14 monoclonal antibody and was added at 40 ng/ml.
FIG. 1.
F. nucleatum induces IL-8 accumulation from GEC. Various amounts of F. nucleatum were coincubated with GEC as described in the text. After 18 h, the culture supernatant was removed and the level of IL-8 in the culture supernatant was determined. The amount of IL-8 in culture supernatants without the addition of bacteria was 40 (±9) ng/ml. Three separate experiments were performed, and the data are presented as the averages and interassay standard deviations (with 108 F. nucleatum cells, more than 500 ng of IL-8 per ml was detected in each experiment).
In contrast, exposure of GEC to P. gingivalis did not result in the production of IL-8 (Table 1). Addition of 102 to 109 cells of P. gingivalis failed to result in IL-8 accumulation in the supernatant (not shown). Lack of IL-8 accumulation was not due to GEC toxicity. Consistent with a previous study (23), GEC exposed to P. gingivalis maintained their characteristic morphology, remained adherent, excluded trypan blue, were able to respond to ionomycin (10−5 M) with an increase in intracellular calcium levels indistinguishable from that in control cells, and remained viable as determined by the calcein hydrolysis method.
Periodontal bacterium coincubation with P. gingivalis resulted in the lack of IL-8 accumulation.
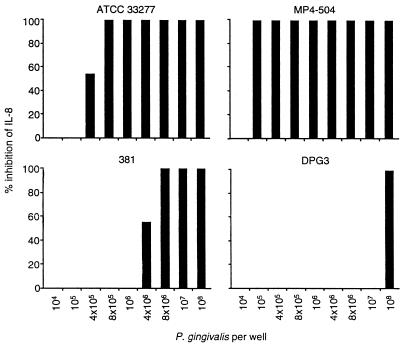
In addition to the lack of IL-8 accumulation, P. gingivalis also prevented IL-8 accumulation when GEC were exposed to mixtures containing this bacterium and other subgingival plaque bacteria (Table 1). P. gingivalis inhibition of the IL-8 response to F. nucleatum was investigated further. Various amounts of four different strains of P. gingivalis were examined for their ability to inhibit F. nucleatum-induced IL-8 (Fig. 2). Although all strains were able to inhibit IL-8 accumulation, there was a wide variability in the number of bacteria necessary to produce inhibition. For example, the most potent strain, P. gingivalis MP4-504, inhibited IL-8 accumulation when more than 105 bacteria were used, whereas 3 log units more of P. gingivalis DPG3, a fimbria-negative mutant, were required for inhibition (Fig. 2). A sharp titration curve of inhibition was observed for all strains, with complete inhibition occurring within a range of bacterial concentration of 1 log unit.
FIG. 2.
P. gingivalis inhibition of IL-8 accumulation. F. nucleatum (108 bacteria) and various amounts of P. gingivalis 33277, MP4-504, 381, or DPG3 were mixed before addition to GEC. After coincubation with GEC for 18 h, the level of IL-8 was determined as described in the text. The amount of IL-8 inhibition was determined by comparing the amount of IL-8 detected after incubation with the combination of P. gingivalis and F. nucleatum to that obtained with F. nucleatum alone. Three separate experiments were performed for each strain. In each experiment, at most datum points, there was either complete or no inhibition of IL-8 accumulation, depending upon the concentration of bacteria examined. When partial inhibition occurred, an average of the three experiments is presented.
One possible explanation for the lack of IL-8 accumulation when P. gingivalis and other periodontal bacteria are coincubated with GEC is the potent repertoire of proteases this organism contains (4, 6). This possibility was examined by adding various concentrations of the same four strains of P. gingivalis examined above to culture supernatants containing IL-8 and determining the amount of IL-8 remaining in the supernatant after incubation for 18 h at 37°C. The source of IL-8 for these experiments was spent F. nucleatum-exposed GEC supernatants, which contained approximately 300 ng of IL-8 per ml. In three separate experiments, IL-8 was not detected after the addition of 108 bacteria of any of the strains examined. In addition, no IL-8 was detected after the addition of 107 P. gingivalis MP4-504. In contrast, no reduction in the amount of IL-8 was observed at lower bacterial concentrations of any of the strains examined (concentrations of 104 to 108 were examined). The lack of detectable IL-8 after incubation of high numbers of P. gingivalis is consistent with degradation due to production of extracellular- or cell-associated proteases (4).
P. gingivalis halted ongoing IL-8 accumulation without loss of previously secreted IL-8.
Three of the four P. gingivalis strains examined inhibited IL-8 accumulation when added to GEC at bacterial concentrations significantly lower than necessary for degradation of existing IL-8 in culture supernatants (Fig. 2 and preceding paragraph). For example, strain 33277 completely inhibited IL-8 accumulation when added to GEC at concentrations of 8 × 105 bacteria per well and above, whereas 108 bacteria per well were required to obtain degradation of existing IL-8 in culture supernatants. A similar difference in the inhibition of IL-8 accumulation was observed for strains MP4-504 and 381. In contrast, strain DPG3 did not inhibit IL-8 accumulation when lower concentrations of bacteria were added to GEC.
The ability of P. gingivalis to inhibit IL-8 accumulation without loss of previously secreted IL-8 was investigated next.
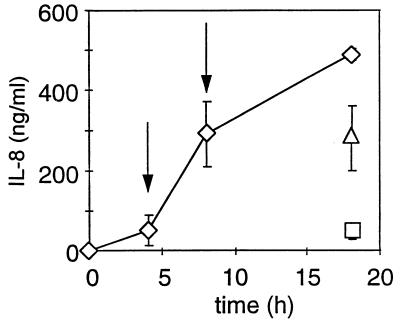
F. nucleatum was added to GEC, and the amount of IL-8 that accumulated in the culture supernatant was determined over an 18-h period (Fig. 3). Separately, P. gingivalis 33277 was added to GEC 4 or 8 h after the addition of F. nucleatum when a significant amount of IL-8 had already accumulated in the culture supernatant. The effect of the addition of P. gingivalis 33277 on the accumulation level of IL-8 was evaluated after 18 h. After the addition of 106 P. gingivalis 33277 bacteria at either the 4- or 8-h time point, no additional IL-8 accumulation occurred and there was no significant loss in the levels of previously secreted IL-8. At higher bacterial concentrations, consistent with the earlier results, no IL-8 was detected in the culture supernatant, indicating degradation of the previously secreted IL-8 (data not shown). In another experiment, P. gingivalis MP4-504 (105 bacteria) was added 4 and 8 h after the addition of F. nucleatum, and similar to results obtained with P. gingivalis 33277, no further IL-8 accumulation occurred after 18 h. These experiments demonstrated that P. gingivalis was able to terminate IL-8 accumulation by a mechanism which did not involve degradation or sequestration of existing IL-8.
FIG. 3.
P. gingivalis 33277 inhibition of additional IL-8 accumulation without degradation of preexisting IL-8. F. nucleatum (108 bacteria) was added to GEC, and the amount of IL-8 in the culture supernatant was determined at the indicated times (◊). At 4 (□) and 8 h (▵) after F. nucleatum addition to GEC, P. gingivalis (106 bacteria) was added to the wells (indicated by arrows). The cells were allowed to incubate for 18 h, and the level of IL-8 in the culture supernatant was determined. The data are presented as the means and standard deviations from at least three separate experiments.
Two noninvasive mechanisms for P. gingivalis inhibition of IL-8 accumulation were examined.
P. gingivalis LPS is a transcriptional inhibitor of IL-8 and E-selectin expression from human vascular umbilical cord endothelial cells (HUVEC) (9). Therefore, the possibility that LPS inhibited IL-8 accumulation in GEC was examined. The addition of P. gingivalis LPS (three separate experiments with 1 μg of LPS per well, one experiment with 10 μg of LPS per well) to F. nucleatum-exposed GEC (methodology was as described for bacterial cell coincubations; see the legend to Fig. 2) did not inhibit IL-8 accumulation (data not shown). LPS at 1 μg/well is at least 100-fold in excess of the amount present in 106 whole bacterial cells (the amount of LPS present in 106 cells was estimated by assuming an individual cell weight of 2.8 × 10−13 g and LPS representing 3.4% of the dry weight of cells [28]). This number of bacterial cells is sufficient to completely inhibit IL-8 accumulation (Fig. 2) and is more than sufficient to completely inhibit E-selectin expression and IL-8 accumulation with the complete loss of E-selectin and IL-8 mRNA in HUVEC (9, 35). Therefore, exogenously added purified LPS at the concentrations used in these experiments is apparently not an inhibitor of GEC-secreted IL-8.
The possibility that P. gingivalis and GEC cell contact was responsible for the inhibition of IL-8 accumulation was also examined. Although experiments described above demonstrated that 106 P. gingivalis bacteria added to GEC after the addition of F. nucleatum did not degrade existing IL-8, a more thorough examination of the possible induction of P. gingivalis or GEC proteases was performed. IL-8 and 106 P. gingivalis 33277 bacteria were premixed and added to GEC, and the amount of IL-8 remaining in the supernatant after 18 h at 37°C was determined. In two separate experiments, no significant loss of IL-8 was observed (data not shown), demonstrating that bacterial and epithelial cell contact alone was not sufficient for IL-8 degradation in our assay system.
P. gingivalis inhibited IL-8 mRNA accumulation in F. nucleatum-stimulated GEC.
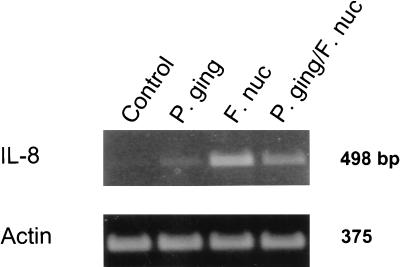
Further experiments revealed that a combination of P. gingivalis (106 cells) and F. nucleatum (108 cells) reduced IL-8 mRNA levels from those obtained when F. nucleatum (108 cells) was added alone (Fig. 4). Relative levels of IL-8 mRNAs were determined from GEC treated with P. gingivalis (106 cells), F. nucleatum (108 cells), or a combination of P. gingivalis (106 cells) and F. nucleatum (108 cells). After 18 h of exposure to the bacteria, mRNA was extracted from GEC and the relative amounts of IL-8 and actin mRNAs were determined by RT-PCR with appropriate probes. Scanning densitometry and quantitation of the gel using NIH Image 1.6 revealed a 75% decrease in the amount of IL-8 mRNA compared to that of actin mRNA in P. gingivalis-infected GEC. Thus, inhibition of IL-8 accumulation by P. gingivalis results in a partial reduction in the steady-state level of IL-8 mRNA, suggesting an internal effect on the GEC.
FIG. 4.
Relative levels of IL-8 mRNAs obtained from GEC treated with P. gingivalis or F. nucleatum. P. gingivalis 33277 (P. ging) (106 cells), F. nucleatum (F. nuc) (108 cells), or a combination of P. gingivalis (106 cells) and F. nucleatum (108 cells) (P. ging/F. nuc) was used. After 18 h of exposure to the bacteria, mRNA was extracted from GEC and the relative amounts of IL-8 and actin mRNAs were determined by RT-PCR with appropriate probes. Scanning densitometry and quantitation of the gel using NIH Image 1.6 revealed a 75% decrease in the amount of IL-8 mRNA compared to that of actin mRNA in P. gingivalis-infected GEC.
P. gingivalis invasion of GEC represents a possible mechanism by which this organism inhibited IL-8 accumulation.
During this investigation, an association between the ability of P. gingivalis to invade GEC and the number of bacteria required to observe IL-8 inhibition when added to GEC was found. For example, P. gingivalis DPG3 is severely impaired in its ability to invade GEC (40), most likely due to an insertion mutation in the fimA gene. As shown above (Fig. 2 and text), in contrast to the other strains examined, strain DPG3 and its isogenic parent, 381, did not inhibit IL-8 accumulation at concentrations of bacteria below 108 per well. Furthermore, this concentration of bacteria was sufficient to degrade previously secreted IL-8, making degradation rather than inhibition of secreted IL-8 the most likely mechanism of IL-8 inhibition for this strain. In addition, strain MP4-504, which is the most invasive strain examined in this study (23), required the fewest bacteria to produce inhibition of IL-8 accumulation (Fig. 2). Consistent with a requirement for invasion (23), chloramphenicol-treated or heat-killed P. gingivalis did not inhibit F. nucleatum-stimulated IL-8 accumulation.
Further evidence for a requirement of invasion to facilitate IL-8 antagonism was provided by the use of KB oral epithelial cells (Fig. 5). P. gingivalis adheres to this epithelial cell line but invades at a very low frequency (11). Similar to primary GEC, F. nucleatum, but not P. gingivalis, stimulated IL-8 accumulation (Fig. 5). However, in contrast to the results obtained with cells permissive for invasion, little or no inhibition of IL-8 accumulation was observed when P. gingivalis 33277 was added to F. nucleatum-stimulated KB cells. The lack of IL-8 antagonism in oral epithelial KB cells is consistent with a requirement for bacterial invasion.
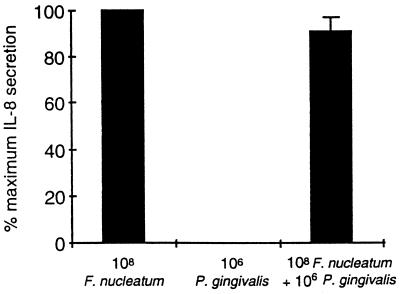
FIG. 5.
Lack of P. gingivalis inhibition of IL-8 accumulation in oral epithelial KB cells. Oral epithelial KB cells were plated as described for GEC in the text, and IL-8 accumulation was examined as described in the text with 108 F. nucleatum and 106 P. gingivalis 33277 bacteria per well. The amount of IL-8 found in the supernatant after 18 h of incubation was determined as described in the text. Three separate experiments were performed. IL-8 accumulation induced by F. nucleatum varied in each experiment (experiment 1 [expt 1], 125 ng/ml; expt 2, 40 ng/ml; and expt 3, 25 ng/ml). No IL-8 was observed when P. gingivalis was added. The amount of IL-8 found when the combination of F. nucleatum and P. gingivalis was used was not significantly less (expt 1, 90 ng/ml; expt 2, 40 ng/ml; and expt 3, 30 ng/ml). The data for the combination are presented as the mean amount of IL-8 accumulation by F. nucleatum and P. gingivalis (90.66% ± 13%) compared to F. nucleatum alone (100%).
DISCUSSION
Clinically healthy periodontal tissue is known to contain a low level of cellular inflammatory infiltrate (31). Accordingly, the expression of several molecular mediators of inflammation has been demonstrated in healthy tissue (26, 29, 39). The low-level expression of mediators such as E-selectin (26, 29) and IL-8 (39) in healthy tissue most likely contributes to the remarkable ability of the host to limit periodontal bacterial growth to the tooth and epithelial cell surface. However, little is known about what regulates expression of these mediators in a clinically healthy environment. Both E-selectin expression and IL-8 accumulation can be elicited by bacteria or their products interacting directly with endothelial (15, 33) or epithelial cells (2, 21, 33, 34). In addition, the production of these mediators can be induced indirectly by cytokines produced by bacterial interactions with monocytes or neutrophils (19, 43). At other mucosal cell-bacterial cell interfaces, epithelial cells have been proposed to be an important component of innate host defense that first detects and responds to the presence of bacteria by accumulation of a limited number of mediators, including IL-8 (21). A recent in situ analysis of clinically healthy periodontal tissue revealed an IL-8 concentration gradient greatest at the epithelial cell-bacterial interface that decreased deeper in the periodontal tissue (39). It is possible that periodontal bacterial interactions with the inflammation arm of innate host defense provide the stimulus and direction for clinically healthy low-level cellular surveillance of periodontal tissue (38). As demonstrated in this study, the ability of a select group of periodontal bacteria not normally associated with disease to directly activate IL-8 accumulation from GEC is consistent with this idea. However, the specific contribution of these or other periodontal bacteria to innate host surveillance is not known. In addition, the relative importance of direct (bacterial) activation or indirect (bacterial to myeloid) activation of nonmyeloid cells in the periodontium remains to be determined. Nevertheless, it appears likely that clinically healthy periodontal tissue is “armed” with low-level inflammatory mediator expression which is brought about by host cell contact with periodontal bacteria.
In contrast to the response elicited by other periodontal bacteria, GEC did not secrete IL-8 when coincubated with several different strains of the periopathogen P. gingivalis. This is consistent with the failure of P. gingivalis to elicit E-selectin expression (9) or IL-8 accumulation (35) from human endothelial cells. The lack of host cell detection of P. gingivalis has been proposed to contribute to bacterial colonization of the host (9, 36). Perhaps more significant, however, was the ability of P. gingivalis to inhibit IL-8 accumulation induced by other bacteria. At high bacterial concentrations (generally 108 bacteria) preexisting IL-8 was destroyed (as evidenced by the failure to detect it by in an enzyme-linked immunosorbent assay), most likely by P. gingivalis protease activity. Support for this contention is provided by a recent report demonstrating that P. gingivalis proteases can degrade IL-1b and IL-6 (14). Evidently, P. gingivalis is able to degrade a variety of different cytokines. In addition, however, P. gingivalis inhibited IL-8 accumulation (Fig. 2 and 3) at bacterial concentrations below those required for loss of existing IL-8 in our assay system. Further experiments with purified LPS suggested that the mechanism of inhibition was not due to exogenously added P. gingivalis LPS.
Two lines of evidence provided in this study support the hypothesis that inhibition of IL-8 accumulation required bacterial invasion. First, P. gingivalis did not inhibit F. nucleatum-induced IL-8 accumulation from oral epithelial KB cells. P. gingivalis adheres to this cell line but invades at a very low frequency (11). We propose that the low frequency of invasion was insufficient to inhibit IL-8 accumulation at the bacterial concentrations employed in this study. In addition, P. gingivalis DPG3, which exhibits impaired invasion capability (40), did not inhibit IL-8 accumulation in GEC. Therefore, inhibition of IL-8 accumulation requires invasive P. gingivalis and epithelial cells permissive to invasion.
The mechanism of IL-8 inhibition appears to involve regulation of IL-8 expression at both the transcriptional and posttranscriptional levels. However, the ability of P. gingivalis to inhibit IL-8 from epithelial cells is apparently different from that observed with inhibition of chemokines and cellular adhesion molecules from endothelial cells. In these instances, the addition of P. gingivalis LPS to HUVEC results in complete inhibition of mRNA for E-selectin and IL-8 (9, 35). The mRNA for IL-8 was not completely inhibited in our study, yet the extracellular IL-8 accumulation was completely halted. It is possible that P. gingivalis inhibits IL-8 secretion but not intracellular accumulation and that this serves as a feedback mechanism and reduces but does not eliminate IL-8 transcription. It is also possible that invasion facilitates the intracellular or membrane delivery of LPS, which then facilitates suppression of IL-8 mRNA synthesis. This is consistent with the recent observation that LPS can be released after bacterial invasion (16). Alternatively, invasion may signal P. gingivalis to secrete an IL-8 inhibitor which is independent of potential protease or LPS effects.
Inhibition of IL-8 accumulation by P. gingivalis at sites of bacterial epithelial cell invasion could have a devastating effect on innate host defense in the periodontium, where bacterial exposure is constant. The host may no longer be able to detect the presence of bacteria and direct leukocytes for their removal. The impairment of the innate host defense inflammatory surveillance system may facilitate bacterial overgrowth. In some respects, this potentially pathogenic mechanism of P. gingivalis is similar to leukocyte adhesion deficiencies I and II (LAD I and II). These congenital deficiencies in leukocyte diapedesis from the vasculature render the host susceptible to potentially any component of the subgingival microbiota and result in severe periodontal disease (3, 13). However, the effect proposed here is bacterially induced and would remain localized to areas where P. gingivalis is present. We have termed this process local chemokine paralysis, since the ability to detect and locate bacterial colonization by IL-8 would be effectively paralyzed and unable to function at sites of P. gingivalis invasion. P. gingivalis-induced local chemokine paralysis represents a novel mechanism for mixed microbial infection of host tissue and provides an additional role for P. gingivalis in the periodontal disease process.
ACKNOWLEDGMENTS
We thank Pam Braham for expert technical assistance; Debby Baxter for preparation of the manuscript; and Aaron Weinberg, Steve Lory and Sam Miller for helpful suggestions.
This work was supported in part by NIDR grant DE11111.
ADDENDUM
While the manuscript was under review, another study demonstrating that P. gingivalis inhibited IL-8 accumulation by gingival epithelial KB cells and inhibited neutrophil transmigration in a Transwell system (23a) was published. Our study, using primary GEC, is consistent with those results and provides additional data demonstrating both a protease-dependent and a protease-independent mechanism for inhibition of IL-8 accumulation.
REFERENCES
- 1.Agace W, Hedges S, Andersson U, Andersson J, Ceska M, Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993;61:602–609. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agace W W, Hedges S R, Svanborg C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Invest. 1993;92:780–785. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson D C, Schmalsteig F C, Finegold M J, Hughes B J, Rothlein R, Miller L J, Kohl S, Tosi M F, Jacobs R L, Waldrop T C, Goldman A S, Shearer W T, Springer T A. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985;152:668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- 4.Barkocy-Gallagher G A, Han N, Patti J M, Whitlock J, Progulske-Fox A, Lantz M S. Analysis of the prtP gene encoding porphypain, a cysteine proteinase of Porphyromonas gingivalis. J Bacteriol. 1996;178:2734–2741. doi: 10.1128/jb.178.10.2734-2741.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck J, Garcia R, Heiss G, Vokonas P S, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodont. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Potempa J, Polanowski A, Wilkstrom M, Travis J. Purification and characterization of a 50-kDa cysteine proteinase (gingipain) from Porphyromonas gingivalis. J Biol Chem. 1992;267:18896–18901. [PubMed] [Google Scholar]
- 7.Cover T L, Blaser M J. Helicobacter pylori: a bacterial cause of gastritis, peptic ulcer disease, and gastric cancer. ASM News. 1995;61:21–26. [Google Scholar]
- 8.Cutler C W, Kalmar J R, Genco C A. Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis. Trends Microbiol. 1995;3:45–51. doi: 10.1016/s0966-842x(00)88874-5. [DOI] [PubMed] [Google Scholar]
- 9.Darveau R P, Cunningham M D, Bailey T, Seachord C, Ratcliffe K, Bainbridge B, Dietsch M, Page R C, Aruffo A. Ability of bacteria associated with chronic inflammatory disease to stimulate E-selectin expression and promote neutrophil adhesion. Infect Immun. 1995;63:1311–1317. doi: 10.1128/iai.63.4.1311-1317.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darveau R P, Hancock R E W. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983;155:831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan M J, Nakao S, Skobe Z, Xie H. Interactions of Porphyromonas gingivalis with epithelial cells. Infect Immun. 1993;61:2260–2265. doi: 10.1128/iai.61.5.2260-2265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etzioni A, Frydman M, Pollack S, Avidor I, Phillips M L, Paulson J C, Gershoni-Baruch R. Brief report: recurrent severe infections caused by a novel leukocyte adhesion deficiency. N Engl J Med. 1992;327:1789–1792. doi: 10.1056/NEJM199212173272505. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher J, Reddi K, Poole S, Nair S, Henderson B, Tabona P, Wilson M. Interactions between periodontopathogenic bacteria and cytokines. J Periodontal Res. 1997;32:200–205. doi: 10.1111/j.1600-0765.1997.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 15.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-del Portillo F, Stein M A, Finlay B B. Release of lipopolysaccharide from intracellular compartments containing Salmonella typhimurium to vesicles of the host epithelial cell. Infect Immun. 1997;65:24–34. doi: 10.1128/iai.65.1.24-34.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grayston J T. Chlamydia pneumoniae and atherosclerosis. Rev Med Interne. 1996;17:45S–47S. doi: 10.1016/0248-8663(96)86505-2. [DOI] [PubMed] [Google Scholar]
- 18.Hillier S L, Nugent R P, Eschenbach D A, Krohn M A, Gibbs R S, Martin D H, Cotch M F, Edelman R, Pastorek J G I, Rao A V, McNellis D, Regan J A, Carey C, Klebanoff M A. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 19.Hofstad T, Skaug N, Sveen K. Stimulation of B lymphocytes by lipopolysaccharide from anaerobic bacteria. Clin Infect Dis. 1993;16:200–202. doi: 10.1093/clinids/16.supplement_4.s200. [DOI] [PubMed] [Google Scholar]
- 20.Holt S C, Ebersole J, Felton J, Brunsvold M, Kornman K S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988;239:55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- 21.Jung H C, Eckmann L, Yang S-K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamont R J, Bevan C A, Gil S, Persson R E, Rosan B. Involvement of Porphyromonas gingivalis fimbriae in adherence to Streptococcus gordonii. Oral Microbiol Immunol. 1993;8:272–276. doi: 10.1111/j.1399-302x.1993.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 23.Lamont R J, Chan A, Belton C M, Izutsu K T, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Madianos P N, Papapanou P N, Sandros J. Porphyromonas gingivalis infection of oral epithelium inhibits neutrophil transepithelial migration. Infect Immun. 1997;65:3983–3990. doi: 10.1128/iai.65.10.3983-3990.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malek R, Fisher J G, Caleca A, Stinson M, van Oss C J, Lee J-Y, Cho M-I, Genco R J, Evans R T, Dyer D W. Inactivation of the Porphyromonas gingivalis fimA gene blocks periodontal damage in gnotobiotic rats. J Bacteriol. 1994;176:1052–1059. doi: 10.1128/jb.176.4.1052-1059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick B A, Hofman P M, Kim J, Carnes D K, Miller S I, Madara J L. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moughal N A, Adonogianaki E, Thornhill M K, Kinane D F. Endothelial cell leukocyte adhesion molecule-1 (ELAM-1) and intercellular adhesion molecule-1 (ICAM-1) expression in gingival tissue during health and experimentally-induced gingivitis. J Periodontal Res. 1992;27:623–630. doi: 10.1111/j.1600-0765.1992.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 27.Muhlestein J B, Hammond E H, Carlquist J F, Radicke E, Thomson M J, Karagounis L A, Woods M L, Anderson J L. Increased incidence of chlamydia species within the coronary arteries of patients with symptomatic atherosclerotic versus other forms of cardiovascular disease. J Am Coll Cardiol. 1996;27:1555–1561. doi: 10.1016/0735-1097(96)00055-1. [DOI] [PubMed] [Google Scholar]
- 28.Neidhardt F C, Umbarger H E. Chemical composition of Escherichia coli. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 13–16. [Google Scholar]
- 29.Nylander K, Danielsen B, Fejerskov O, Dabelsteen E. Expression of the endothelial leukocyte adhesion molecule-1 (ELAM-1) on endothelial cells in experimental gingivitis in humans. J Periodontol. 1993;64:355–357. doi: 10.1902/jop.1993.64.5.355. [DOI] [PubMed] [Google Scholar]
- 30.Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, McKaig R, Beck J. Periodontal infection as a risk factor for preterm low birth weight. J Periodontol. 1996;67:1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 31.Page R C, Schroeder H E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;33:235–249. [PubMed] [Google Scholar]
- 32.Patel P, Mendall M A, Carrington D, Strachan D P, Leatham E, Molineaux N, Levy J, Blakeston C, Seymour C A, Camm A J, Northfield T C. Association of Helicobacter pylori and Chlamydia pneumoniae infections with coronary heart disease and cardiovascular risk factors. Br Med J. 1995;311:711–714. doi: 10.1136/bmj.311.7007.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pugin J, Schurer-Maly C-C, Leturcq D, Moriarty A, Ulevitch R J, Tobias P S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen S J, Eckmann L, Shen L, Zhang Y-X, Fierer J, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reife, R. 1996. Unpublished data.
- 36.Reife R A, Shapiro R A, Bamber B A, Berry K K, Mick G E, Darveau R P. Porphyromonas gingivalis lipopolysaccharide is poorly recognized by molecular components of innate host defense in a mouse model of early inflammation. Infect Immun. 1995;63:4686–4694. doi: 10.1128/iai.63.12.4686-4694.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Socransky S S, Haffajee A D. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 38.Tonetti M S. Molecular factors associated with compartmentalization of gingival immune responses and transepithelial neutrophil migration. J Periodontal Res. 1997;32:104–109. doi: 10.1111/j.1600-0765.1997.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 39.Tonetti M S, Imboden M A, Gerber L, Lang N P, Laisue J, Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun. 1994;62:4005–4014. doi: 10.1128/iai.62.9.4005-4014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberg A, Belton C M, Park Y, Lamont R J. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1997;65:313–316. doi: 10.1128/iai.65.1.313-316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright S D. CD14 and innate recognition of bacteria. J Immunol. 1995;155:6–8. [PubMed] [Google Scholar]
- 42.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 43.Yamazaki K, Ikarashi F. Direct and indirect effects of Porphyromonas gingivalis lipopolysaccharide on interleukin-6 production by human gingival fibroblasts. Oral Microbiol Immunol. 1992;7:218–224. doi: 10.1111/j.1399-302x.1992.tb00028.x. [DOI] [PubMed] [Google Scholar]