Abstract
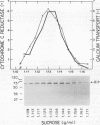
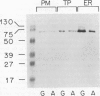
The isolation of a 70-kilodalton protein from barley (Hordeum vulgare L.) aleurone layers that cross-reacts with an antibody against yeast binding protein (BiP) is reported. Endoplasmic reticulum isolated from aleurone layers treated with gibberellic acid contain much higher levels of the BiP cognate than do membranes isolated from layers treated with abscisic acid.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Belanger F. C., Brodl M. R., Ho T. H. Heat shock causes destabilization of specific mRNAs and destruction of endoplasmic reticulum in barley aleurone cells. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1354–1358. doi: 10.1073/pnas.83.5.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. S., Sticher L., van Huystee R., Wagner D., Jones R. L. The calcium requirement for stability and enzymatic activity of two isoforms of barley aleurone alpha-amylase. J Biol Chem. 1989 Nov 15;264(32):19392–19398. [PubMed] [Google Scholar]
- Fargnoli J., Kunisada T., Fornace A. J., Jr, Schneider E. L., Holbrook N. J. Decreased expression of heat shock protein 70 mRNA and protein after heat treatment in cells of aged rats. Proc Natl Acad Sci U S A. 1990 Jan;87(2):846–850. doi: 10.1073/pnas.87.2.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes E. B., Shank B. B., Wrobel R. L., Moose S. P., OBrian G. R., Wurtzel E. T., Boston R. S. Characterization of an immunoglobulin binding protein homolog in the maize floury-2 endosperm mutant. Plant Cell. 1991 May;3(5):483–496. doi: 10.1105/tpc.3.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Herman E. M., Chrispeels M. J. An abundant, highly conserved tonoplast protein in seeds. Plant Physiol. 1989 Nov;91(3):1006–1013. doi: 10.1104/pp.91.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozutsumi Y., Normington K., Press E., Slaughter C., Sambrook J., Gething M. J. Identification of immunoglobulin heavy chain binding protein as glucose-regulated protein 78 on the basis of amino acid sequence, immunological cross-reactivity, and functional activity. J Cell Sci Suppl. 1989;11:115–137. doi: 10.1242/jcs.1989.supplement_11.10. [DOI] [PubMed] [Google Scholar]
- Macer D. R., Koch G. L. Identification of a set of calcium-binding proteins in reticuloplasm, the luminal content of the endoplasmic reticulum. J Cell Sci. 1988 Sep;91(Pt 1):61–70. doi: 10.1242/jcs.91.1.61. [DOI] [PubMed] [Google Scholar]
- Normington K., Kohno K., Kozutsumi Y., Gething M. J., Sambrook J. S. cerevisiae encodes an essential protein homologous in sequence and function to mammalian BiP. Cell. 1989 Jun 30;57(7):1223–1236. doi: 10.1016/0092-8674(89)90059-7. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Misra L. M., Vogel J. P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989 Jun 30;57(7):1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Sambrook J. F. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990 Apr 20;61(2):197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- Vaux D., Tooze J., Fuller S. Identification by anti-idiotype antibodies of an intracellular membrane protein that recognizes a mammalian endoplasmic reticulum retention signal. Nature. 1990 Jun 7;345(6275):495–502. doi: 10.1038/345495a0. [DOI] [PubMed] [Google Scholar]
- Yoshimori T., Semba T., Takemoto H., Akagi S., Yamamoto A., Tashiro Y. Protein disulfide-isomerase in rat exocrine pancreatic cells is exported from the endoplasmic reticulum despite possessing the retention signal. J Biol Chem. 1990 Sep 15;265(26):15984–15990. [PubMed] [Google Scholar]