Abstract
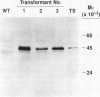
The complete coding sequence for the trichodiene synthase gene from Fusarium sporotrichioides was introduced into tobacco (Nicotiana tabacum) under the regulation of the cauliflower mosiac virus 35S promoter. Expression of trichodiene synthase was demonstrated in the leaves of transformed plants. Leaf homogenates incubated with [3H]farnesyl pyrophosphate produced trichodiene as a major product. Trichodiene was detected in the leaves of a transformed plant at a level of 5 to 10 nanograms per gram fresh weight. The introduction of a fungal sesquiterpene cyclase gene into tobacco has resulted in the expression of an active enzyme and the accumulation of low levels of its sesquiterpenoid product.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cane D. E., Pargellis C. Partial purification and characterization of pentalenene synthase. Arch Biochem Biophys. 1987 May 1;254(2):421–429. doi: 10.1016/0003-9861(87)90120-2. [DOI] [PubMed] [Google Scholar]
- Croteau R., Purkett P. T. Geranyl pyrophosphate synthase: characterization of the enzyme and evidence that this chain-length specific prenyltransferase is associated with monoterpene biosynthesis in sage (Salvia officinalis). Arch Biochem Biophys. 1989 Jun;271(2):524–535. doi: 10.1016/0003-9861(89)90304-4. [DOI] [PubMed] [Google Scholar]
- Dehal S. S., Croteau R. Partial purification and characterization of two sesquiterpene cyclases from sage (Salvia officinalis) which catalyze the respective conversion of farnesyl pyrophosphate to humulene and caryophyllene. Arch Biochem Biophys. 1988 Mar;261(2):346–356. doi: 10.1016/0003-9861(88)90350-5. [DOI] [PubMed] [Google Scholar]
- Hohn T. M., Beremand M. N. Regulation of Trichodiene Synthase in Fusarium sporotrichioides and Gibberella pulicaris (Fusarium sambucinum). Appl Environ Microbiol. 1989 Jun;55(6):1500–1503. doi: 10.1128/aem.55.6.1500-1503.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn T. M., Beremand P. D. Isolation and nucleotide sequence of a sesquiterpene cyclase gene from the trichothecene-producing fungus Fusarium sporotrichioides. Gene. 1989 Jun 30;79(1):131–138. doi: 10.1016/0378-1119(89)90098-x. [DOI] [PubMed] [Google Scholar]
- Hohn T. M., Plattner R. D. Expression of the trichodiene synthase gene of Fusarium sporotrichioides in Escherichia coli results in sesquiterpene production. Arch Biochem Biophys. 1989 Nov 15;275(1):92–97. doi: 10.1016/0003-9861(89)90353-6. [DOI] [PubMed] [Google Scholar]
- Hohn T. M., Plattner R. D. Purification and characterization of the sesquiterpene cyclase aristolochene synthase from Penicillium roqueforti. Arch Biochem Biophys. 1989 Jul;272(1):137–143. doi: 10.1016/0003-9861(89)90204-x. [DOI] [PubMed] [Google Scholar]
- Hohn T. M., Vanmiddlesworth F. Purification and characterization of the sesquiterpene cyclase trichodiene synthetase from Fusarium sporotrichioides. Arch Biochem Biophys. 1986 Dec;251(2):756–761. doi: 10.1016/0003-9861(86)90386-3. [DOI] [PubMed] [Google Scholar]
- Jarvis B. B., Midiwo J. O., Bean G. A., Aboul-Nasr M. B., Barros C. S. The mystery of trichothecene antibiotics in Baccharis species. J Nat Prod. 1988 Jul-Aug;51(4):736–744. doi: 10.1021/np50058a012. [DOI] [PubMed] [Google Scholar]
- Munck S. L., Croteau R. Purification and characterization of the sesquiterpene cyclase patchoulol synthase from Pogostemon cablin. Arch Biochem Biophys. 1990 Oct;282(1):58–64. doi: 10.1016/0003-9861(90)90086-e. [DOI] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Vögeli U., Chappell J. Induction of sesquiterpene cyclase and suppression of squalene synthetase activities in plant cell cultures treated with fungal elicitor. Plant Physiol. 1988 Dec;88(4):1291–1296. doi: 10.1104/pp.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vögeli U., Chappell J. Regulation of a sesquiterpene cyclase in cellulase-treated tobacco cell suspension cultures. Plant Physiol. 1990 Dec;94(4):1860–1866. doi: 10.1104/pp.94.4.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vögeli U., Freeman J. W., Chappell J. Purification and characterization of an inducible sesquiterpene cyclase from elicitor-treated tobacco cell suspension cultures. Plant Physiol. 1990 May;93(1):182–187. doi: 10.1104/pp.93.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]