Abstract
Background
The minimum number of examined lymph nodes (ELN) required for adequate staging and best prediction of survival has not been established in pancreatic ductal adenocarcinoma (PDAC). The aim of the study was to investigate the influence of ELN on staging and survival in PDAC.
Methods
Patients undergoing partial or total pancreatectomy for PDAC at two European university hospitals between 2007 and 2018 were retrospectively reviewed. Multivariate Cox regression model and survival analyses were performed to verify adequate staging.
Results
Overall 341 (73 per cent) patients showed lymph node metastasis (N1/N2), whereas 125 (27 per cent) patients had no lymph node involvement (N0). With increasing number of ELN, the proportion of positive lymph nodes increased. The minimum number of ELN needed to detect lymph node involvement was 21. In multivariate analysis, examination of <21 lymph nodes was a significant negative predictor for survival. Examination of ≥21 ELN reversed this effect and ruled out possible misclassification.
Conclusion
The number of ELN affects survival in PDAC. Possible misclassification was identified when <21 lymph nodes were examined. Therefore, at least 21 lymph nodes must be examined to avoid false lymph node classification in all types of resection.
Four hundred and sixty-six patients undergoing pancreatectomy for pancreatic ductal adenocarcinoma (PDAC) at two European university hospitals were analysed to determine the minimum number of examined lymph nodes required for adequate staging. To avoid nodal misclassification and improve survival in the long term at least 21 lymph nodes must be examined in PDAC.
Introduction
Pancreatic ductal adenocarcinoma is an early metastatic tumour and remains one of the most lethal malignancies1,2. Surgical resection with lymphadenectomy provides the only chance for cure. Lymph node (LN) metastases are common and appear in around 60–90 per cent of all resected patients3,4. In node-negative patients, a median survival of 25–30 months can be achieved, whereas in node-positive patients the median survival decreases to around 15 months4–6. Several studies have demonstrated that the number of positive lymph nodes influences survival3,7,5.
Various randomized controlled trials showed that extended lymphadenectomy does not generate any survival benefit compared to standard regional lymphadenectomy8–10. The number of examined lymph nodes (ELN) might particularly be influenced by variations in lymph node dissection, individual surgeon skills, the gross pathologist expertise, the anatomic constitution of each patient, and neoadjuvant treatment. With the standardization of operation technique, the variation in the number of ELN should theoretically not be pronounced. Due to the fact that not all resected lymph nodes are always examined, it could lead to a misclassification of N status because positive LN can be overlooked. It was also shown that the number of ELN and not only the involved LN is a prognostic factor for survival in patients after distal pancreatectomy11.
There is no clear consensus on the minimum number of ELN needed for adequate staging in pancreatic resections for pancreatic ductal adenocarcinoma (PDAC). Several studies reported numbers varying between 11 and 28 required lymph nodes3,5,11–16.
The aim of the current study was to investigate the influence of ELN on staging and survival in all different types of pancreatic resection and to identify the minimum number of ELN needed for the best prediction of survival in pancreatic cancer.
Methods
Study design and patient selection
Patients with histologically confirmed PDAC who underwent elective curative tumour resection with regional lymphadenectomy at the Department of Surgery, Klinikum rechts der Isar, Munich, Germany or the Department of Digestive Surgery, E. Herriot Hospital, Hospices civils de Lyon, France between 2007 and 2018 were included in the study. All patients were identified from a prospectively maintained pancreatic cancer database containing standard demographic, clinical, operative and pathological data. The institutional review board (IRB) approved the prospective data collection with retrospective data review for the study (Ethics Committee Approval No.: 2022-438-S-NP).
In all patients, a primary curative resection was performed depending on the localization of the tumour, that is pancreaticoduodenectomy (PD), distal pancreatectomy (DP), or total pancreatectomy (TP) with standard lymphadenectomy. Patients with organ metastasis diagnosed during resection (M1), macroscopically incomplete resection (R2 status), neoadjuvant treatment and complication-related postoperative death (≤30 days) were excluded from the study for avoiding bias in the survival analyses (Fig. 1). R0 status was defined as ≥1 mm tumour-free resection margin.
Fig. 1.
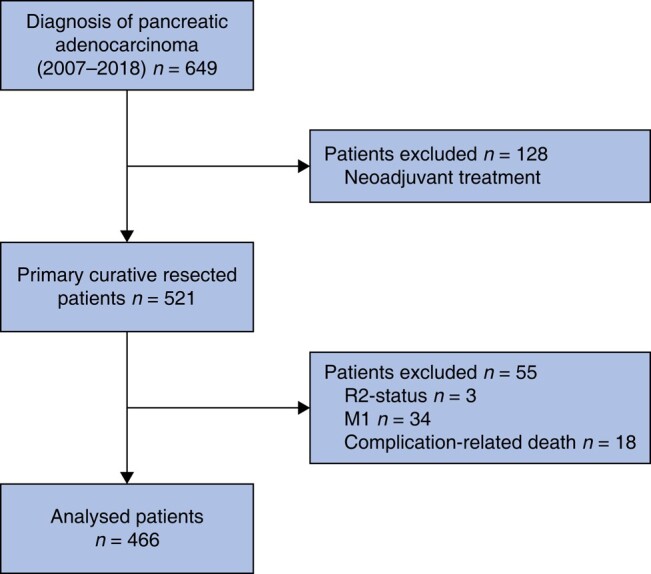
Flowchart detailing inclusion and exclusion criteria of the final study cohort
Overall survival was defined as the time from resection of the tumour to death. Lymph node ratio is calculated with the number of affected lymph nodes divided by the total number of lymph nodes removed. In the pathological report, the latest (8th) edition of the TNM classification of the AJCC was used.
Statistical analysis
IBM SPSS Statistics 28.0 (IBM, New York, USA) and R (release 4.3.0) were used for statistical analyses. Survival analyses were performed with the log-rank test and depicted as Kaplan–Meier curves with the number at risk. An adjusted Cox proportional hazard model with overall survival as a dependent variable was employed for multivariate analysis of prognostic factors with 95 per cent c.i. For correlation analysis, Spearman’s correlation coefficient was used. The model of the binomial probability law was used for the calculation to detect at least one lymph node metastasis with a probability of 95 per cent. It was calculated according to the following mathematical formula: P = 1 − (1 − lymph node ratio)examined lymph nodes, as described previously15. Continuous variables were expressed as median and minimum–maximum. Categorical variables were expressed as frequency and percentage and compared with Chi2-test/Fisher’s exact test. All tests were two-sided, and P < 0.05 was considered to indicate statistical significance.
Results
Demographic and histopathological characteristics
Overall, 466 patients who underwent upfront pancreatic resection due to pancreatic ductal adenocarcinoma at the two hospitals were included in the study. Overall, 309 (66.3 per cent) patients received pancreaticoduodenectomy, 84 (18.0 per cent) patients distal pancreatectomy and 73 (15.7 per cent) patients total pancreatectomy for macroscopically complete (R0/R1) tumour resection. The median number of ELN and the median number of positive LN were 23 (i.q.r. 15) and 2 (i.q.r. 5) in pancreaticoduodenectomy, 17 (i.q.r. 12) and 1 (i.q.r. 3) in distal pancreatectomy and 25 (i.q.r. 15) and 2 (i.q.r. 4) in total pancreatectomy. Overall, 125 (27 per cent) patients had no histological evidence of lymph node metastasis (N0 status), whereas in 186 (40 per cent) patients 1–3 positive LN were detected (N1 status), and in 155 (33 per cent) patients more than 3 LN had tumour metastasis (N2 status). The median follow-up of the study cohort was 62.9 months (i.q.r. 46.3) for surviving patients and 16.8 months (i.q.r. 19.3) for deceased patients. The median overall survival of all patients was 22.7 months (i.q.r. 39.1). In patients without lymph node metastasis (N0), the median survival was 52.3 months (i.q.r. 110.4), whereas in patients with lymph node metastasis the survival was significantly worse with 19.8 months (N1) (i.q.r. 26.8) and 16.5 months (N2) (i.q.r. 21.2) (P < 0.001). The demographic, clinical and pathological characteristics of all patients are outlined in Table 1 (stratified by type of resection) and in Table 2 (stratified by lymph node status). The differences in the study cohorts between centres are shown in Table 3. The survival curves according to the lymph node yield did not differ among centres (Fig. S1).
Table 1.
Lymph node status for different types of resection
| Parameter | Total | Pancreaticoduodenectomy | Distal pancreatectomy | Total pancreatectomy | P |
|---|---|---|---|---|---|
| 466 (100%) | 309 (66.3%) | 84 (18.0%) | 73 (15.7%) | ||
| No. of examined lymph nodes, median (min-max) | 22 (2–85) | 23 (8–69) | 17 (2–45) | 25 (11–85) | <0.01 |
| No. of positive lymph nodes, median (min-max) | 2 (1–25) | 2 (1–23) | 1 (1–9) | 2 (1–25) | 0.03 |
| Lymph node ratio, median | 0.09 | 0.10 | 0.06 | 0.06 | 0.11 |
| Lymph node staging | |||||
| N0 | 125 (26.8) | 79 (25.6) | 29 (34.5) | 17 (23.3) | 0.07 |
| N1 | 186 (39.9) | 117 (37.8) | 38 (45.3) | 31 (42.5) | |
| N2 | 155 (33.3) | 113 (36.6) | 17 (20.2) | 25 (34.2) |
Values are n (%) unless otherwise stated. N1, 1–3 positive lymph nodes; N2, ≥4 positive lymph nodes; lymph node ratio = positive lymph nodes/examined lymph nodes.
Table 2.
Patient characteristics stratified by lymph node status
| Parameter | Total | N0 | N1 | N2 | P |
|---|---|---|---|---|---|
| 466 (100%) | 125 (27%) | 186 (40%) | 155 (33%) | ||
| Sex | |||||
| Male | 255 (54.7) | 65 (52.0) | 100 (53.8) | 90 (58.1) | 0.56 |
| Female | 211 (45.3) | 60 (48.0) | 86 (46.2) | 65 (41.9) | |
| Age (years), median (min-max) | 69.3 (32–93) | 70.4 (34–93) | 69.1 (32–90) | 68.5 (35–87) | 0.51 |
| Type of resection | |||||
| Pancreaticoduodenectomy | 309 (66.3) | 79 (63.2) | 117 (62.9) | 113 (72.9) | 0.057 |
| Distal pancreatectomy | 84 (18.0) | 29 (23.2) | 38 (20.4) | 17 (11.0) | |
| Total pancreatectomy | 73 (15.7) | 17 (13.6) | 31 (16.7) | 25 (16.1) | |
| T stage (AJCC, 8th edition) | |||||
| T1 | 61 (13.1) | 32 (25.6) | 20 (10.7) | 9 (5.8) | < 0.001 |
| T2 | 276 (59.2) | 73 (58.4) | 121 (65.1) | 82 (52.9) | |
| T3 | 105 (22.5) | 18 (14.4) | 38 (20.4) | 49 (31.6) | |
| T4 | 24 (5.2) | 2 (1.6) | 7 (3.8) | 15 (9.7) | |
| Tumour size, median (min-max) | 32 (1–190) | 30 (1–190) | 30 (10–110) | 35 (10–150) | < 0.001 |
| Tumour grading | |||||
| 1 | 48 (10.3) | 15 (12.0) | 19 (10.2) | 14 (9.0) | 0.61 |
| 2 | 238 (51.1) | 66 (52.8) | 95 (51.1) | 77 (49.7) | |
| 3 | 170 (36.5) | 42 (33.6) | 70 (37.6) | 58 (37.5) | |
| 4 | 3 (0.6) | 0 (0.0) | 2 (1.1) | 1 (0.6) | |
| Unknown | 2 (0.4) | 2 (1.6) | 0 (0.0) | 5 (3.2) | |
| Resection margin | |||||
| 0 | 153 (32.8) | 67 (53.6) | 55 (29.6) | 31 (20.0) | < 0.001 |
| 1 | 295 (63.3) | 54 (43.2) | 121 (65.0) | 120 (77.4) | |
| Unknown | 18 (3.9) | 4 (3.2) | 10 (5.4) | 4 (2.6) | |
| Adjuvant treatment | |||||
| Yes | 356 (76.4) | 97 (77.6) | 140 (75.3) | 119 (76.8) | 0.94 |
| No | 59 (12.7) | 14 (11.2) | 26 (14.0) | 19 (12.3) | |
| Unknown | 51 (10.9) | 14 (11.2) | 20 (10.8) | 17 (11.0) | |
| Overall survival (months), median | 22.7 | 52.3 | 19.8 | 16.5 | < 0.001 |
Values are n (%) unless otherwise stated. T1, size ≤ 2 cm; T2, size ≤4 cm; T3, size >4 cm; T4, infiltration of coeliac trunk, A. hepatica or A. mesenterica sup. R1, distance of the tumour < 1 mm to the resection margin.
Table 3.
Centre-specific characteristics of the study cohort
| Parameter | Total | Munich | Lyon | P |
|---|---|---|---|---|
| 466 (100%) | 353 (75.8%) | 113 (24.2%) | ||
| No. of examined lymph nodes, median (min-max) | 22 (2–85) | 22 (5–85) | 22(2–47) | 0.44 |
| No. of positive lymph nodes, median (min-max) | 2 (1–25) | 2 (0–25) | 3 (0–22) | 0.023 |
| Lymph node ratio, median | 0.09 | 0.077 | 0.135 | 0.005 |
| Lymph node status | ||||
| N0 | 125 (26.8) | 103 (29.2) | 22 (19.5) | 0.12 |
| N1 | 186 (39.9) | 136 (38.5) | 50 (44.2) | |
| N2 | 155 (33.3) | 114 (32.3) | 41 (36.3) | |
| Type of resection | ||||
| Pancreaticoduodenectomy | 309 (66.3) | 241 (68.3) | 68 (60.2) | 0.25 |
| Distal pancreatectomy | 84 (18.0) | 61 (17.3) | 23 (20.4) | |
| Total pancreatectomy | 73 (15.7) | 51 (14.4) | 22 (19.4) | |
| Overall survival (months), median | 22.7 | 20.3 (1.9–157.6) | 23.1 (3.3–128) | 0.47 |
Values are n (%) unless otherwise stated.
Influence of the number of examined lymph nodes on nodal staging
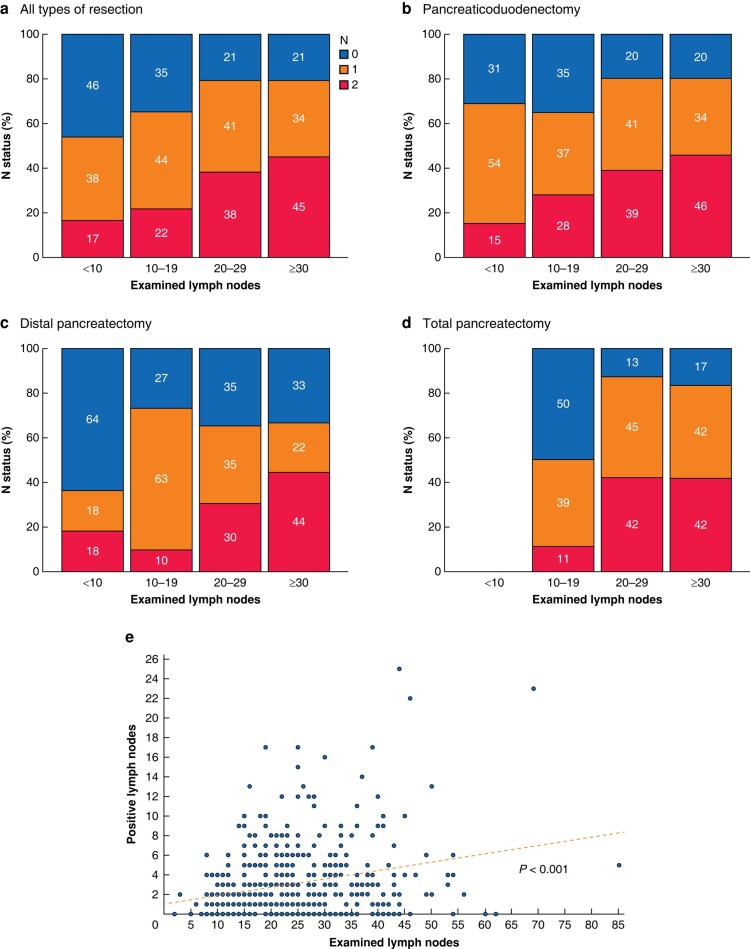
The study cohort was classified into four different groups according to the amount of ELN (group I: < 10 ELN; group II: 10–19 ELN; group III: 20–29 ELN; group IV: ≥ 30 ELN). Concerning all patients, the proportion of lymph node positive status (N1 and N2) increased with increasing amounts of ELN. In patients with ELN <10, 56 per cent (n = 13) showed lymph node metastasis (38 per cent (n = 9) N1 and 17 per cent (n = 4) N2), in patients with 10–19 ELN 66 per cent (n = 108) (44 per cent (n = 72) N1 and 22 per cent (n = 36) N2) compared to 79 per cent (n = 116) (41 per cent (n = 60) N1 and 38 per cent (n = 56) N2) when 20–29 LN were examined, and 79 per cent (n = 104) (34 per cent (n = 45) N1 and 45 per cent (n = 59) N2) when at least 30 LN were histologically examined (Fig. 2a).
Fig. 2.
Distribution of the number of examined lymph nodes (ELN) on nodal status
Patients were categorized into four groups according to the number of ELN (I: <10 ELN; II: 10-19 ELN; III: 20-29 ELN; IV: ≥30 ELN) and analysed for each type of resection (a–d). Correlation analysis of examined lymph nodes and positive lymph nodes with Spearman's correlation (e).
After splitting the cohort based on the type of resection the trend towards a higher proportion of lymph node metastasis with increasing number of ELN persisted (Fig. 2b–d).
Amount of ELN correlated significantly with positive LN detected (P < 0.001) (Fig. 2e), but not with tumour size (P = 0.187).
Calculation of minimum number of examined lymph nodes for adequate nodal staging
The binominal probability law was used to calculate the minimum number of ELN needed to detect at least one positive lymph node. The lymph node ratio was 0.09 in all patients, 0.10 in patients after pancreaticoduodenectomy, 0.06 after distal pancreatectomy and 0.06 after total pancreatectomy. To detect at least one positive lymph node with a probability of 95 per cent, a minimum ELN of 21 was needed in all patients, 20 in pancreaticoduodenectomy, 25 in distal pancreatectomy and 22 for total pancreatectomy.
Impact of nodal staging on survival
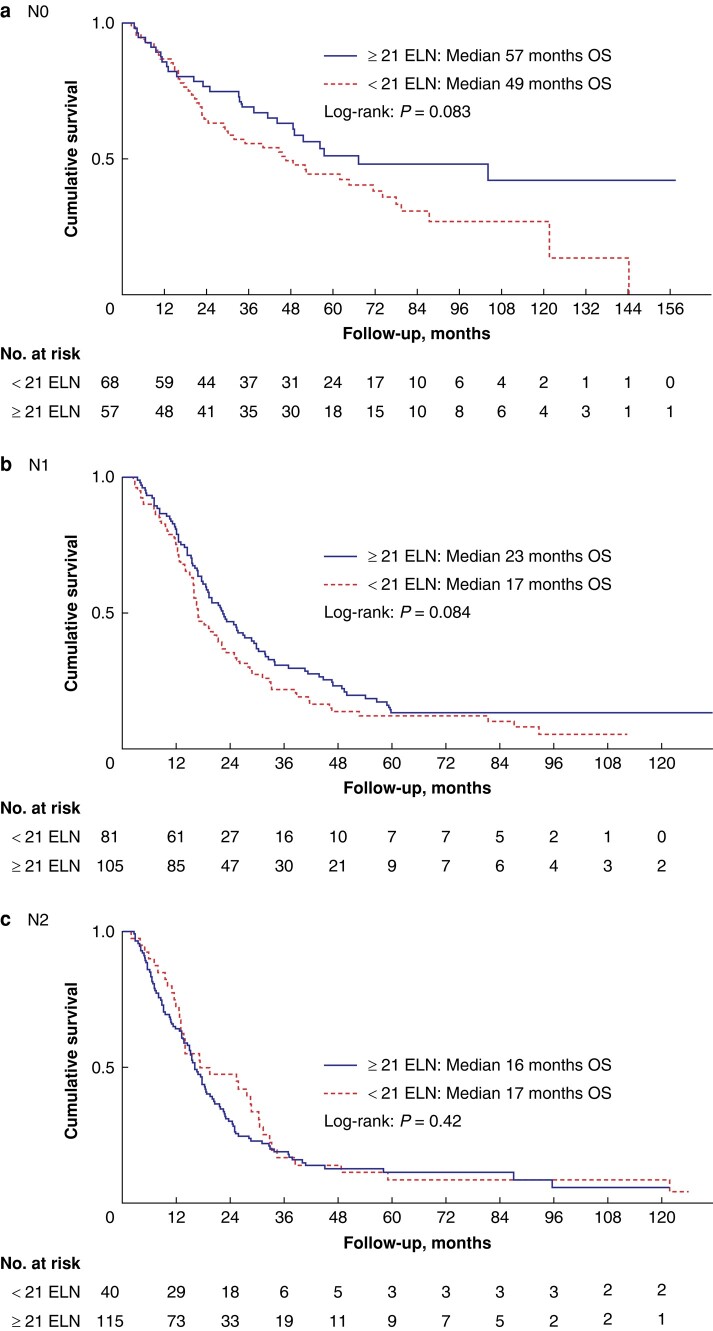
To find a cut-off value that prevents misclassification, the calculated minimum number of ELN needed to detect one positive LN was analysed for its impact on survival. In all patients, the median overall survival did not show significant differences when <21 LN were examined compared to ELN of ≥21 (Fig. 3a–c) (P = 0.10).
Fig. 3.
Overall survival (OS) of all patients stratified by nodal status according to the value of 21 examined lymph nodes (ELN) (a–c)
Univariate and multivariate analysis with Cox regression model was performed including the most affecting factors17–19 for overall survival in pancreatic cancer to analyse the impact of 21 ELN on survival (Tables 4, 5). After adjusting with the other variables, Cox regression analysis showed that the examination of <21 lymph nodes was a significant negative predictor for overall survival (HR 1.27, 95 per cent c.i. 1.02–1.58, P = 0.034). The best cut-off value of ELN was determined when ELN had no significant impact on survival. Interestingly, the examination of <22 LN was not significant in the multivariate analysis (HR 1.23, 95 per cent c.i. 0.99–1.60, P = 0.061). The most influencing factor for worsening overall survival in this cohort was lymph node involvement with N1 status (HR 2.14, 95 per cent c.i. (1.59–2.86), P < 0.001) and N2 status (HR 2.57, 95 per cent c.i. (1.88–3.51), P < 0.001). The multivariate analysis was also performed separately for each lymph node status (N0, N1 and N2) without any differences (Table S1).
Table 4.
Univariate analysis of prognostic factors for overall survival
| Variable | HR | 95% c.i. | P |
|---|---|---|---|
| Sex, female | 0.854 | (0694–1.050) | 0.13 |
| Age (years) | 1.008 | (1.008–1.018) | 0.12 |
| T stage (AJCC, 8th edition) | |||
| T1 | 1.0 (reference) | – | |
| T2 | 2.29 | (1.59–3.31) | < 0.001 |
| T3 | 3.27 | (2.19–4.89) | < 0.001 |
| T4 | 5.03 | (2.94–8.60) | < 0.001 |
| Lymph node status | |||
| N0 | 1.0 (reference) | – | |
| N1 | 2.35 | (0.177–3.12) | < 0.001 |
| N2 | 2.96 | (2.21–3.95) | < 0.001 |
| Tumour grade | |||
| G1 | 1.0 (reference) | – | |
| G2 | 1.39 | (0.96–2.02) | 0.07 |
| G3 | 1.93 | (1.32–2.83) | < 0.001 |
| Resection margin | |||
| R0 | 1.0 (reference) | – | |
| R1 | 1.55 | (1.23–1.95) | < 0.001 |
Table 5.
Multivariate analysis of prognostic factors for overall survival
| Analysis: <21 examined lymph nodes | Analysis: <22 examined lymph nodes | ||||||
|---|---|---|---|---|---|---|---|
| Variable | HR | 95% c.i. | P | Variable | HR | 95% c.i. | P |
| T-stage: T3/T4 | 1.52 | 1.19–1.93 | <0.001 | T-stage: T3/T4 | 1.50 | 1.18–1.91 | <0.001 |
| Grading: G3/G4 | 1.44 | 1.16–1.79 | <0.001 | Grading: G3/G4 | 1.44 | 1.15–1.79 | <0.001 |
| R1-status* | 1.26 | 0.99–1.60 | 0.051 | R1-status* | 1.27 | 0.99–1.53 | 0.051 |
| Lymph node status | Lymph node status | ||||||
| N1 | 2.16 | 1.61–2.89 | <0.001 | N1 | 2.14 | 1.59–2.86 | <0.001 |
| N2 | 2.57 | 1.88–3.51 | <0.001 | N2 | 2.57 | 1.88–3.51 | <0.001 |
| <21 ELN | 1.27 | 1.02–1.58 | 0.034 | <22 ELN | 1.23 | 0.99–1.60 | 0.061 |
*Defined as R0 ≥ 1 mm tumour-free resection margin. Adjusted Cox proportional hazard model, HR with 95 per cent c.i., examined lymph nodes (ELN).
Discussion
The present study highlighted the importance of adequate lymph node dissection and examination in pancreatic cancer surgery. One of the most important findings was that with increasing number of ELN, the proportion of LN metastasis increased. From these results, it was concluded that patients with a low number of ELN are often falsely classified in the N-category, which leads to ‘understaging’ of these patients. In other words, patients classified as N0 are in fact N1, and N1 patients are actually N2 if more LN were removed and examined. A valid prognosis for these patients could have been achieved by more accurate and extensive lymph node sampling. Because lymphadenectomy is supposed to be a standardized procedure, the pathological lymph node examination was probably insufficient. Misclassification can either be caused by inadequate lymphadenectomy, or by insufficient histological examination. Further categorization and analysis of misclassification would require histologic re-evaluation of the resected specimen.
Multivariate analysis of the main factors influencing survival (including tumour size18, surgical resection margin17, pathological grading19 and lymph nodes status5) was used to evaluate a cut-off value of ELN to prevent misclassification. In the adjusted regression model, the examination of <21 LN was a negative predictor for overall survival. Therefore examining at least 21 LN in pancreatic resection prevents possible misclassification and can be seen as the best cut-off point.
Lymph node status is also dependent on the experience of the surgeon. Higher median LN count has been reported in high-volume centres20. Pathological assessment and identification of lymph nodes in the resected tissue is necessary and it depends on the pathologist’s expertise. Larger LN are less likely to be missed, as a lymph node about 4 mm in diameter can be palpable and easily identified. Complete embedding of the fatty tissue in addition can help to increase the number of ELN and was shown to influence nodal staging in oesophageal adenocarcinoma21. The amount of resected LN may also be influenced by the patient’s individual constitution. Autopsy studies revealed different numbers of regional LN with individual differences in LN amount in gastric cancer22,23. It is also known that overweight and high BMI hampers lymphadenectomy in gastric cancer24.
Several studies addressed the role of lymph node examination on survival in pancreatic cancer. In this study the highest median examined lymph node count was collected and all different types of pancreatic resection were considered. Hellan and coworkers12 postulated in a study with 1915 node-negative patients that more than 10 LN should be examined for improved survival, but the median number of ELN in the study was 7, which is relatively low compared to 22 LN in the present cohort. A study by Slidell et al.13 with 1507 node-negative patients proposed that more than 12 LN should be examined to avoid misclassification with a median ELN of 7 LN. Both studies included patients from 1988 to 2003 where quality of lymphadenectomy and histopathological assessment were different than today. Tomlinson et al.14 concluded from a study with 3505 patients that the examination of at least 15 LN is optimal for accurate staging in pancreatic cancer but reported only a median ELN of 7.
The statistical calculation of the minimum number of ELN needed to detect at least one possible LN using the binominal law has been carried out before. For pancreaticoduodenectomy, Vuarnesson et al.15 calculated 16 required ELN and Malleo et al.11 20 LN for distal pancreatectomy. Taking into account that previous studies have a lower median ELN, the present paper will serve as a reference for the minimum needed number of 21 LN for adequate staging. The European Society for Medical Oncology (ESMO) suggests the examination of ≥15 LN in pancreaticoduodenectomy as recommended by the International Study Group on Pancreatic Surgery (ISGPS)25. In the recently updated German S3-Guideline on the treatment of pancreatic cancer (AWMF), the minimum number of ELN needed to classify as N0 was increased to 12 LN26.
The study had several limitations. Due to its retrospective nature, the study was limited by lack of some clinical data and by the impossibility of reassessing all the histological specimens with specific focus on lymph node examination. No data were available on disease-free survival, CA19-9 levels and peri- and vascular invasion. Patients with neoadjuvant treatment were excluded from the final analysis. Neoadjuvant therapy was not the standard treatment during the study period and contained several different treatment protocols.
After splitting the study cohort according to different resection types, there were no significant survival differences detected, probably due to the small number of subgroups.
Standardized lymph node resection and systematic, careful pathological assessment are needed for accurate staging and achieving the best outcome for PDAC patients. From these results, at least a minimum of 21 lymph nodes are needed for adequate examination in all PDAC patients to avoid misclassification of N0 and N1 status by surgical or pathological reasons. This number has to be evaluated in different study cohorts to confirm the results. A uniform recommendation for a minimum need of ELN can improve the comparability of patients and thereby improve survival in the long term.
Supplementary Material
Contributor Information
Ruediger Goess, Department of Surgery, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany; German Cancer Consortium (DKTK), Partner Site Munich, Munich, Germany; CRC 1321 Modelling and Targeting Pancreatic Cancer, Munich, Germany.
Carsten Jäger, Department of Surgery, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany; German Cancer Consortium (DKTK), Partner Site Munich, Munich, Germany; CRC 1321 Modelling and Targeting Pancreatic Cancer, Munich, Germany.
Julie Perinel, Department of Digestive Surgery, E. Herriot Hospital, Hospices civils de Lyon, Lyon, France.
Ilaria Pergolini, Department of Surgery, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany; German Cancer Consortium (DKTK), Partner Site Munich, Munich, Germany; CRC 1321 Modelling and Targeting Pancreatic Cancer, Munich, Germany.
Elke Demir, Department of Surgery, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany; German Cancer Consortium (DKTK), Partner Site Munich, Munich, Germany; CRC 1321 Modelling and Targeting Pancreatic Cancer, Munich, Germany.
Okan Safak, Department of Surgery, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany; German Cancer Consortium (DKTK), Partner Site Munich, Munich, Germany; CRC 1321 Modelling and Targeting Pancreatic Cancer, Munich, Germany.
Florian Scheufele, Department of Surgery, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany; German Cancer Consortium (DKTK), Partner Site Munich, Munich, Germany; CRC 1321 Modelling and Targeting Pancreatic Cancer, Munich, Germany.
Stephan Schorn, Department of Surgery, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany; German Cancer Consortium (DKTK), Partner Site Munich, Munich, Germany; CRC 1321 Modelling and Targeting Pancreatic Cancer, Munich, Germany.
Alexander Muckenhuber, Institute of Pathology, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany.
Mustapha Adham, Department of Digestive Surgery, E. Herriot Hospital, Hospices civils de Lyon, Lyon, France.
Alexander Novotny, Department of Surgery, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany.
Güralp O Ceyhan, Department of General Surgery, HPB-Unit, School of Medicine, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey.
Helmut Friess, Department of Surgery, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany; German Cancer Consortium (DKTK), Partner Site Munich, Munich, Germany; CRC 1321 Modelling and Targeting Pancreatic Cancer, Munich, Germany.
Ihsan Ekin Demir, Department of Surgery, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany; German Cancer Consortium (DKTK), Partner Site Munich, Munich, Germany; CRC 1321 Modelling and Targeting Pancreatic Cancer, Munich, Germany; Department of General Surgery, HPB-Unit, School of Medicine, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey; Else Kröner Clinician Scientist Professorship for Translational Pancreatic Surgery, Munich, Germany.
Funding
I.E.D. has received funding from the Else Kröner Clinician Scientist Professorship Programme. R.G. has received the intramural KKF grant of the Faculty of Medicine of the Tecnical University of Munich.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The data presented in this study are available on request from the corresponding author.
Author contributions
Ruediger Goess (Formal analysis, Investigation, Writing—original draft), Carsten Jäger (Formal analysis, Investigation, Writing—review & editing), Julie Perinel (Data curation, Project administration, Writing—review & editing), Ilaria Pergolini (Data curation, Writing—review & editing), Elke Demir (Data curation, Validation), Okan Safak (Data curation, Validation), Florian Scheufele (Data curation, Validation), Stephan Schorn (Data curation, Validation), Alexander Muckenhuber (Supervision, Writing—review & editing), Mustapha Adham (Conceptualization, Supervision), Alexander Novotny (Data curation, Validation), Güralp O. Ceyhan (Conceptualization, Supervision), Helmut Friess (Conceptualization, Supervision, Writing—review & editing) and Ihsan Ekin Demir (Conceptualization, Supervision, Validation, Writing—review & editing).
References
- 1. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet 2020;395:2008–2020 [DOI] [PubMed] [Google Scholar]
- 2. Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012;148:349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malleo G, Maggino L, Capelli P, Gulino F, Segattini S, Scarpa A et al. Reappraisal of nodal staging and study of lymph node station involvement in pancreaticoduodenectomy with the standard International Study Group of Pancreatic Surgery definition of lymphadenectomy for cancer. J Am Coll Surg 2015;221:367–379e4 [DOI] [PubMed] [Google Scholar]
- 4. Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007;141:610–618 [DOI] [PubMed] [Google Scholar]
- 5. House MG, Gonen M, Jarnagin WR, D'Angelica M, DeMatteo RP, Fong Y et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg 2007;11:1549–1555 [DOI] [PubMed] [Google Scholar]
- 6. Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006;244:10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strobel O, Hinz U, Gluth A, Hank T, Hackert T, Bergmann F et al. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg 2015;261:961–969 [DOI] [PubMed] [Google Scholar]
- 8. Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery 2005;138:618–628, discussion 628–630 [DOI] [PubMed] [Google Scholar]
- 9. Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Sauter PK, Coleman J et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma—part 3: update on 5-year survival. J Gastrointest Surg 2005;9:1191–1204, discussion 1204–1206 [DOI] [PubMed] [Google Scholar]
- 10. Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014;156:591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malleo G, Maggino L, Ferrone CR, Marchegiani G, Mino-Kenudson M, Capelli P et al. Number of examined lymph nodes and nodal status assessment in distal pancreatectomy for body/tail ductal adenocarcinoma. Ann Surg 2019;270:1138–1146 [DOI] [PubMed] [Google Scholar]
- 12. Hellan M, Sun CL, Artinyan A, Mojica-Manosa P, Bhatia S, Ellenhorn JD et al. The impact of lymph node number on survival in patients with lymph node-negative pancreatic cancer. Pancreas 2008;37:19–24 [DOI] [PubMed] [Google Scholar]
- 13. Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008;15:165–174 [DOI] [PubMed] [Google Scholar]
- 14. Tomlinson JS, Jain S, Bentrem DJ, Sekeris EG, Maggard MA, Hines OJ et al. Accuracy of staging node-negative pancreas cancer: a potential quality measure. Arch Surg 2007;142:767–774, discussion 773–774 [DOI] [PubMed] [Google Scholar]
- 15. Vuarnesson H, Lupinacci RM, Semoun O, Svrcek M, Julie C, Balladur P et al. Number of examined lymph nodes and nodal status assessment in pancreaticoduodenectomy for pancreatic adenocarcinoma. Eur J Surg Oncol 2013;39:1116–1121 [DOI] [PubMed] [Google Scholar]
- 16. Warschkow R, Widmann B, Beutner U, Marti L, Steffen T, Schiesser M et al. The more the better—lower rate of stage migration and better survival in patients with retrieval of 20 or more regional lymph nodes in pancreatic cancer: a population-based propensity score matched and trend SEER analysis. Pancreas 2017;46:648–657 [DOI] [PubMed] [Google Scholar]
- 17. Demir IE, Jager C, Schlitter AM, Konukiewitz B, Stecher L, Schorn S et al. R0 versus R1 resection matters after pancreaticoduodenectomy, and less after distal or total pancreatectomy for pancreatic cancer. Ann Surg 2018;268:1058–1068 [DOI] [PubMed] [Google Scholar]
- 18. Moon HJ, An JY, Heo JS, Choi SH, Joh JW, Kim YI. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas 2006;32:37–43 [DOI] [PubMed] [Google Scholar]
- 19. Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA et al. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567–579 [DOI] [PubMed] [Google Scholar]
- 20. Bilimoria KY, Talamonti MS, Wayne JD, Tomlinson JS, Stewart AK, Winchester DP et al. Effect of hospital type and volume on lymph node evaluation for gastric and pancreatic cancer. Arch Surg 2008;143:671–678, discussion 678 [DOI] [PubMed] [Google Scholar]
- 21. Quaas A, Schloesser H, Fuchs H, Zander T, Arolt C, Scheel AH et al. Improved tissue processing in esophageal adenocarcinoma after Ivor Lewis esophagectomy allows histological analysis of all surgically removed lymph nodes with significant effects on nodal UICC stages. Ann Surg Oncol 2021;28:3975–3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herbella FAM, Lourenco LG, Bonini AL, Schlottmann F, Patti MG. Anatomical analysis of gastric lymph nodes in cancer-free individuals. Clin Anat 2019;32:9–12 [DOI] [PubMed] [Google Scholar]
- 23. Wagner PK, Ramaswamy A, Ruschoff J, Schmitz-Moormann P, Rothmund M. Lymph node counts in the upper abdomen: anatomical basis for lymphadenectomy in gastric cancer. Br J Surg 1991;78:825–827 [DOI] [PubMed] [Google Scholar]
- 24. Dhar DK, Kubota H, Tachibana M, Kotoh T, Tabara H, Masunaga R et al. Body mass index determines the success of lymph node dissection and predicts the outcome of gastric carcinoma patients. Oncology 2000;59:18–23 [DOI] [PubMed] [Google Scholar]
- 25. Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goere D et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v56–v68 [DOI] [PubMed] [Google Scholar]
- 26. Seufferlein T, Mayerle J, Bock S, Brunner T, Ettrich TJ, Grenacher L et al. S3-Leitlinie zum exokrinen Pankreaskarzinom—Langversion 2.0. Z Gastroenterol 2022;60:e812–e909 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.