Abstract
It remains unknown whether the expression of cell-mediated protective immunity and the capacity to mount a delayed-type hypersensitivity (DTH) reaction in tuberculosis infection represent two manifestations of a basic response or are dissociable events. In this study, we present data in favor of the latter hypothesis, by showing that tuberculosis infection in the lungs of mice possessing only a truncated form of intracellular adhesion molecule 1 due to gene disruption was still adequately controlled by the expression of protective immunity in the absence of any sustained influx of macrophages and the lack of formation of appreciable granulomas. These animals also had no detectable DTH response to mycobacterial proteins in the footpad assay, indicating that the accumulation of blood-borne macrophages at sites of mycobacterial infection or antigen deposition is not essential to control of the infection. These data support the hypothesis that the DTH component of the cellular response is not protective but contributes by walling off the sites of infection to prevent dissemination and reactivation disease.
The expression of acquired specific resistance to Mycobacterium tuberculosis infection is associated with cessation of the progressive growth of the infection, the formation of a granulomatous structure at the sites of infection, and the concomitant emergence of the capacity of the animal to mount a delayed-type hypersensitivity (DTH) response to mycobacterial antigens inoculated at a separate site. It has long been debated whether these latter two events are separate manifestations of a primary protective immune device or are in fact dissociable mechanisms (10).
When bacilli are engulfed by alveolar macrophages, local chemokine signals attract other macrophages from local tissues, from lymphatics in the bronchial tree, and from the blood. These cells differentiate into epithelioid macrophages that make up the bulk of the developing granuloma. T cells also migrate into these tissues and release gamma interferon (IFN-γ), resulting in macrophage activation and restraint and control of bacterial growth (3, 5, 6, 13). The breakdown of the granuloma with age or due to secondary infections leading to reactivation tuberculosis tends to suggest that the apparent chronic disease state may actually reflect continued active restraint of the infection. This inference, in turn, supports the concept that the granuloma is a containment device, designed to prevent further dissemination of the infection.
In this study, we demonstrate, using gene-disrupted mice in which intracellular adhesion molecule 1 (ICAM-1) had been truncated by targeting of exon 5 (18), that the two mechanisms of control (protective immunity) and containment (massive influx of macrophages from the blood to the site of infection) can in fact be dissociated. In these ICAM-truncated mice, control of the pulmonary infection, resulting in cessation of bacterial growth, proceeded in the same fashion as in controls despite the lack of any discernible epithelioid macrophage influx. In addition, a DTH response to intradermal injection of mycobacterial antigens was not observed in the ICAM-truncated animals. These data therefore indicate that expression of cell-mediated immunity in the lungs to M. tuberculosis represents two overlapping mechanisms. In the first, incoming T cells secrete IFN-γ to activate infected macrophages and restrict bacterial growth, while in the second, large numbers of noninfected macrophages accumulate from the bloodstream and differentiate into a field of epithelioid cells, resulting in the walling off of the infection within the granulomatous lesion.
MATERIALS AND METHODS
Mice.
Eight-week-old female ICAM-1 gene-disrupted (C57BL/6-ICAM1) mice were purchased from The Jackson Laboratory, Bar Harbor, Maine. The ICAM-1 gene-disrupted mice were made by targeted disruption of exon 5 (18). Homozygous mutants were backcrossed to C57BL/6 for six generations. Age- and sex-matched C57BL/6 mice (also purchased from The Jackson Laboratory) were used as wild-type controls. Mice were kept under barrier conditions in an ABL3 facility for the duration of the experiments and maintained with sterile water, bedding, and mouse chow.
Bacteria.
Virulent M. tuberculosis Erdman was grown to mid-log phase in Proskauer-Beck medium containing 0.01% Tween 80 and stored in ampoules frozen at −70°C until use.
Experimental infections.
Mice were infected aerogenically with M. tuberculosis Erdman by using a Glas-Col aerosol generation device (Glas-Col Inc., Terre Haute, Ind.) which gave approximately 100 viable bacilli within the lungs. The course of infection in target organs was monitored against time by harvesting lungs, livers, and spleens and enumerating viable bacilli as described elsewhere (11). Briefly, mice were euthanized, serial dilutions of individual whole organ homogenates were plated on nutrient 7H11 agar, and bacterial colony formation was counted 3 weeks later after incubation at 37°C in humidified air. The data are expressed as the log10 value of the mean number of bacteria recovered from each organ (n = 4).
Detection of cytokine gene expression.
mRNA was isolated from lung samples of each mouse and then reverse transcribed into cDNA as described previously (4, 20). For these analyses, lung tissues from individual mice were homogenized in Ultraspec reagent (BioTecx, Houston, Tex.), and total RNA was isolated by chloroform-phenol extraction and precipitated with cold isopropanol (17). One microgram of total RNA from each preparation was used as template to make cDNA, using Moloney murine leukemia virus reverse transcriptase (GIBCO BRL Life Technologies). cDNA was diluted 1:9 in DNase- and RNase-free water and stored at 4°C. To determine the quantity of readable RNA in the original samples, an aliquot of each cDNA sample was amplified by PCR using primers specific for the housekeeping gene, HPRT (encoding hypoxanthine phosphoribosyltransferase). Using a limited number of PCR cycles (each consisting of denaturation at 95°C for 1 min, annealing at 54°C for 1 min, and an extension period at 72°C for 2 min), the relative amounts of RNA in each sample can be determined. If the signals for HPRT are between one- and twofold of each other, further aliquots of cDNA from each sample are subjected to PCR for specific cytokines. Primer pairs for murine IL-12p40 (p40 subunit of interleukin-12), IFN-γ, and HPRT were published previously (7, 21).
PCR products were subjected to electrophoresis in 1% agarose, Southern blotting, and hybridization with fluorescein-conjugated specific probes which were visualized with a commercial chemiluminescence detection kit (ECL detection system; Amersham Life Sciences, Arlington Heights, Ill.) as described elsewhere (5, 21). Oligonucleotide probes for IL-12p40 (21), IFN-γ, and HPRT (20) were published previously. The density of the signal for each sample on chemiluminescence detection film was measured in pixels with a flatbed scanner (Microtek Scanmaker IIXE) and quantified with NIH Image software (National Institutes of Health, Bethesda, Md.). Each experimental sample is compared to each of the control samples from uninfected mice, and the data points represent the mean of the fold increase of the experimental values over the control values (n = 4 per time point).
DTH response.
The capacity of infected mice to mount a DTH reaction was tested by injection of mycobacterial culture filtrate-derived proteins (CFP) (5 μg in sterile saline) in a hind footpad. Bovine serum albumin (5 μg in sterile saline), injected in the contralateral footpad, was used as the negative control. The two footpads were measured 48 h later, using dial gauge calipers capable of measuring 0.05-mm increments. Data are expressed as mean footpad thickness (millimeters) of CFP-injected footpad minus mean footpad thickness of the negative control (n = 4 per time point).
Histology.
Lung tissues from each mouse were inflated and fixed with 10% neutral buffered formalin. The lower left lobes of four mice from each experimental group were embedded together in paraffin blocks; four sets of slides (each 100 μm apart) were made from each block and stained with hematoxylin and eosin for histological analysis. Slides were coded and scored, for the observed involvement of the lung tissue, by a veterinary pathologist. The scoring was reproducible both in a second blind analysis of the first experiment and in a blind analysis of a second experiment.
RESULTS
Intact ICAM-1 is not required for the control of M. tuberculosis infection in the lung.
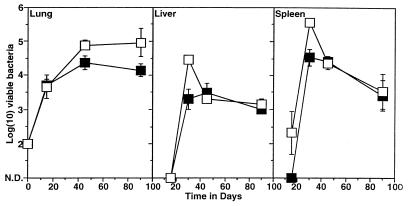
To determine whether ICAM-1 exon 5 gene-disrupted mice could still control a tuberculosis infection, these mice and wild-type control mice were exposed to a low-dose aerosol infection with M. tuberculosis Erdman, and the course of infection was monitored in target organs against time. As shown in Fig. 1, levels of bacillary growth in the lungs were similar in the two groups of mice, with in each case a chronic state of disease being established by day 90 of the infection. Dissemination of the infection from the lungs, which is reflected by bacterial growth in secondary sites of infection such as the liver and spleen, was slightly increased in the mutant mice over the early stages of two separate experiments (Fig. 1), suggesting that bacterial dissemination was initially more prominent in these mice.
FIG. 1.
Growth of M. tuberculosis in lungs, livers, and spleens of wild-type (closed squares) and ICAM-1 gene-disrupted (open squares) mice following exposure to a low-dose aerosol infection. Data shown are representative of two separate experiments and are expressed as log10 mean bacterial count ± standard error of the mean (n = 4). N.D., numbers of mycobacteria not determined (below level of sensitivity of assay).
Defective ICAM-1 expression does not affect the expression of protective cytokines in infected lungs.
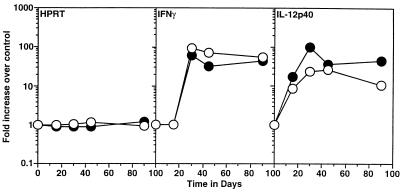
To determine the immunological state of the infected lungs, cytokine gene expression was performed. Expression of mRNA for IFN-γ correlates closely with the control of bacterial proliferation in the lung, and its expression is dependent on the expression of IL-12. These cytokines therefore were monitored by reverse transcription-PCR during the course of the M. tuberculosis infection. In both wild-type and gene-disrupted animals, expression of IFN-γ and IL-12p40 (Fig. 2) correlated closely with the observed control of bacterial growth shown in Fig. 1.
FIG. 2.
Expression of cytokine mRNA in the lungs of wild-type (closed circles) and ICAM-1 gene-disrupted (open circles) mice following exposure to low-dose aerosol of M. tuberculosis. Samples of cDNA from infected lung lobes (n = 4) were amplified individually by using primers specific for HPRT, IFN-γ, and IL-12p40. Product was detected by chemiluminescence and quantitated as pixel values by using a scanner device. Fold increase over control values for uninfected lungs was calculated for each mouse. Data are expressed as mean values for duplicate samples at each time point and are representative of results from two separate experiments.
Intact ICAM-1 expression is required for the expression of DTH in infected mice.
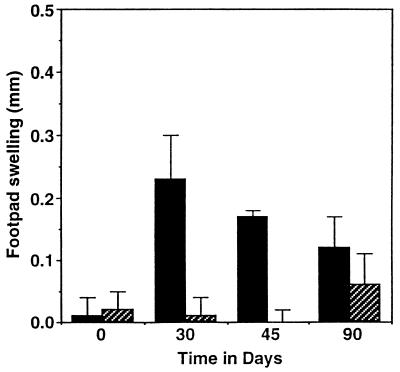
The expression of antigen-specific tissue swelling in response to injection of mycobacterial proteins can be measured in M. tuberculosis-infected mice, although it should be emphasized that such reactions are small (0.2 to 0.5 mm) and do not resemble the much larger reactions seen in other models such as the guinea pig. In this model of DTH, the antigen specificity is mediated by T cells and the swelling is the product of a large influx of mononuclear phagocytes from the blood. The capacity of the ICAM-1 exon 5-disrupted mice to mediate a DTH reaction in response to mycobacterial antigens was compared to that of control animals. As expected, control mice mounted a positive (>0.2-mm) DTH response which peaked at day 30 and then started to wane (Fig. 3); in contrast, the ICAM-1 gene-disrupted mice did not exhibit a DTH response at any time during the course of infection.
FIG. 3.
DTH reactions to CFP antigens in wild-type (solid bars) and ICAM-1 gene-disrupted (hatched bars) mice infected with M. tuberculosis. Mycobacterial CFP was injected into the footpads, and footpad swelling was measured 48 h later. Data are expressed as mean footpad swelling ± standard deviation (n = 4).
ICAM-1 is required for the development of granulomas in the lung.
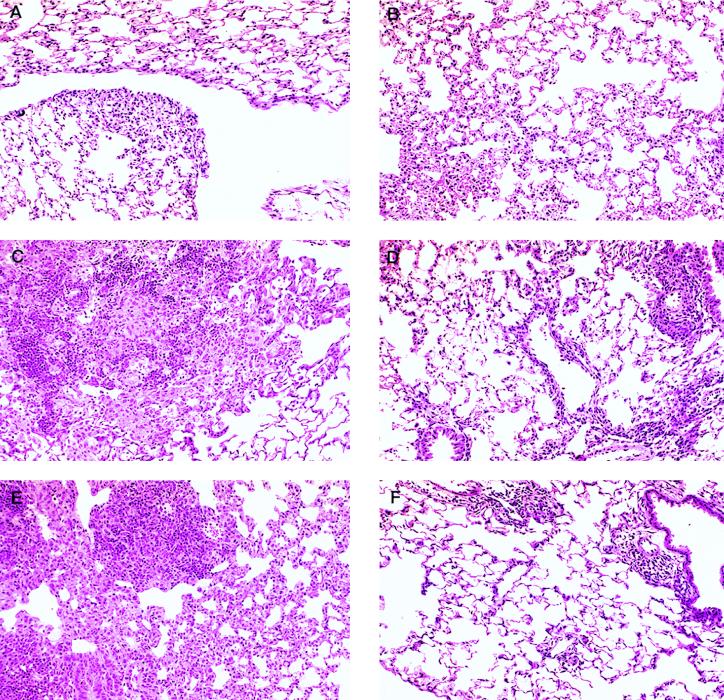
Infected lung tissues were sectioned and stained for histological analysis. Table 1 contains the results of the blinded analysis of four mice for each experimental group over two experiments. In both experiments, there was a marked increase in both the severity and rapidity with which the granulomatous response occurred in the wild-type mice compared to the knockout mice. The lung histology indicated that wild-type mice expressed a mild inflammation early in the infection, day 15 (Fig. 4A), in the form of an interstitial pneumonia characterized by swelling of the septa and the influx of mononuclear cells, with the development of the granuloma becoming obvious by the later time points (Fig. 4C and E). ICAM-1 exon 5 gene-disrupted mice exhibited a similar interstitial pneumonia at day 15 (Fig. 4B); however, inflammation at later time points was very much less severe than in controls (Fig. 4D and F). Considerable lymphocytic perivascular cuffing was present in the mutant animals, but no accumulation of epithelioid macrophages was seen. In addition, these animals exhibited only scattered areas of interstitial pneumonia by day 45 of the infection.
TABLE 1.
Lung histology in wild-type and ICAM-1 gene-disrupted mice infected aerogenically with M. tuberculosisa
| Time (days) | Result of analysis
|
|||
|---|---|---|---|---|
| Expt 1
|
Expt 2
|
|||
| Wild type | ICAM KO | Wild type | ICAM KO | |
| 15 | + | + | + | + |
| + | + | + | − | |
| + | + | + | + | |
| + | + | + | + | |
| 30 | +++ | + | +++ | ++ |
| +++ | − | + | + | |
| + | + | + | + | |
| ++ | ++ | +++ | + | |
| 45 | +++ | − | +++ | + |
| +++ | + | +++ | ++ | |
| − | + | + | ++ | |
| ++ | − | + | + | |
| 90 | ND | ND | + | + |
| ND | ND | ++ | + | |
| ND | ND | + | ++ | |
| ND | ND | ++ | ++ | |
Four mice per group were infected, and the lower left lobe was taken for histological analysis. Each line represents the characteristics of one mouse, wild type or knockout (KO). +, interstitial pneumonia; ++, mild granulomatous disease; +++, severe granulomatous disease; −, normal tissue; ND, not done.
FIG. 4.
Changes in cell accumulation patterns on days 15, 30, and 45 in the lungs of control mice (A, C, and E) and ICAM-1 mutant mice (B, D, and F) following exposure to a low-dose aerosol infection with M. tuberculosis. In control mice, a diffuse interstitial pneumonia (A; day 15) gradually evolved into a granulomatous response characterized by fields of epithelioid macrophages interspersed with organized rafts of lymphocytes (C and E). This pattern was similar in the mutant mice on day 15 (B), but other than some prominent lymphocyte perivascular cuffing, no further cellular accumulation by day 30 or 45 was observed (D and F). In particular, no epithelioid macrophage accumulation was seen at any time. These observations were made in two separate experiments. Hematoxylin-eosin staining; all fields, magnification of ×500.
DISCUSSION
The results of this study support the hypothesis that sensitized T cells migrate from the bloodstream into the lung tissues of the ICAM-truncated mice, where they secrete IFN-γ, leading to the activation of locally infected macrophages and thus control and restraint of the progressively growing pulmonary infection. On the other hand, the histologic data clearly show that macrophages were not accumulating in the lungs of these mice, as evidenced by a complete lack of the epithelioid macrophage masses characteristic of the developing granuloma. Infected macrophages, which we speculate are local tissue cells that move into the interstitium as it swells due to local inflammatory signals (histamine, prostaglandins, etc.), were therefore presumably the source of the IL-12 detected in the lung tissues, which was clearly present in sufficient quantity to drive adequate IFN-γ secretion by incoming protective T cells (3, 5, 6, 13). Taken in concert with the observation that the ICAM-truncated mice did not mount a DTH reaction to tuberculin, whereas control mice gave small but positive reactions, these data support the hypothesis that the activation of infected macrophages by protective T cells and the influx of epithelioid macrophages that slowly accumulate and surround the site of infection (DTH) are separate events within the course of acquired immunity to tuberculosis infection and that the former event is not dependent on the latter.
The difference in abilities of the lymphocytes and macrophages to migrate into the lung tissues probably reflects the fact that the mutant mice do not express the full form of ICAM-1 but instead express three mutant isoforms of ICAM-1 through alternative RNA splicing (9). Each of the three isoforms is missing one or more of the five extracellular immunoglobulin-like domains, resulting in a shorter ICAM-1 molecule expressed on the cell surface. However, in all three isoforms, the LFA-1 binding site is still preserved, which probably explains the observed perivascular cuffing by lymphocytes around local pulmonary blood vessels (9, 19). On the other hand, the binding site for macrophage Mac-1 molecules has a conformation different from that on the intact ICAM-1 molecule (18, 19), probably explaining the inability of macrophages to leave the blood circulation. This is consistent with the importance of the Mac-1 molecule in macrophage recruitment and granuloma formation, as shown by inhibition of this activity by monoclonal antibodies specific for this molecule (14–16).
It has been long known that in virulent mycobacterial infections, protective immunity and DTH are expressed concurrently, leading some workers to speculate that these two functions are mediated by the same cellular mechanism, whereas others have suggested that the two events are dissociable (1, 2, 8, 12). In this study, we have demonstrated that ICAM-1 gene mutant mice were capable of expressing protective immunity in the lungs, as evidenced by expression of IL-12 and IFN-γ and by the control of bacterial growth, but were unable to mount a DTH response to specific antigens. Similarly, accumulation of blood-borne macrophages into infected sites leading to granulomatous structures consisting predominantly of epithelioid macrophages was completely absent yet did not influence the ability of the animal to initially control the infection. These results, therefore, indicate that control of the infection can occur in the absence of macrophage accumulation from the bloodstream and hence demonstrate that expression of cell-mediated immunity in the lungs to M. tuberculosis infection represents two overlapping mechanisms. In the first, incoming protective T cells secrete IFN-γ to activate locally infected macrophages, restricting bacterial growth, while in the second, DTH-like reaction, noninfected macrophages accumulate and differentiate into a field of epithelioid cells, resulting in the containment of the infection within the granulomatous lesion and reduced dissemination. This in turn implies that DTH is not a protective device but is more important in the latter stages of the disease process in preventing reactivation of the infection or quickly containing it at sites of secondary infection. This suggests that the ICAM-1 exon 5 gene-disrupted mouse would be much more prone to reactivation tuberculosis at a later date due to the lack of this containment mechanism, and this possibility is currently being addressed in our laboratory.
ACKNOWLEDGMENTS
This work was supported by grants AI 40488 and HL-55967 from the National Institutes of Health.
REFERENCES
- 1.Chen-Woan M, Sajewski D H, McGregor D D. T cell cooperation in the mediation of acquired resistance to Listeria monocytogenes. Immunology. 1985;56:33–42. [PMC free article] [PubMed] [Google Scholar]
- 2.Cher D J, Mosmann T R. Two types of murine helper T cell clones. J Immunol. 1987;138:3688–3694. [PubMed] [Google Scholar]
- 3.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper A M, Flynn J L. The protective immune response to Mycobacterium tuberculosis. Curr Opin Immunol. 1995;7:512–516. doi: 10.1016/0952-7915(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 5.Cooper A M, Roberts A D, Rhoades E R, Callahan J E, Getzy D M, Orme I M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazzanelli R T, Eltoum I, Wynn T A, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralisation of TNF-α and correlates with the down-regulated expression of inducible nitric oxide synthesis and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 8.Hussein S, Curtis J, Akuffo H, Turk J L. Dissociation between delayed-type hypersensitivity and resistance to pathogenic mycobacteria demonstrated by T-cell clones. Infect Immun. 1987;55:564–568. doi: 10.1128/iai.55.3.564-567.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King P D, Sandberg E T, Selvakumar A, Fang P, Beaudet A L, Dupont B. Novel isoforms of murine intercellular adhesion molecule-1 generated by alternative RNA splicing. J Immunol. 1995;154:6080–6093. [PubMed] [Google Scholar]
- 10.Mackaness G B. The relationship of delayed type hypersensitivity to acquired cellular resistance. Br Med Bull. 1967;23:52–54. doi: 10.1093/oxfordjournals.bmb.a070516. [DOI] [PubMed] [Google Scholar]
- 11.Ordway D J, Sonnenberg M G, Donahue S A, Belisle J T, Orme I M. Drug-resistant strains of Mycobacterium tuberculosis exhibit a range of virulence for mice. Infect Immun. 1995;63:741–743. doi: 10.1128/iai.63.2.741-743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orme I M. The immune response to the cell wall of Mycobacterium bovis BCG. Clin Exp Immunol. 1988;71:388–393. [PMC free article] [PubMed] [Google Scholar]
- 13.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. . (Review.) [DOI] [PubMed] [Google Scholar]
- 14.Patarroyo M. Adhesion molecules mediating recruitment of monocytes to inflamed tissue. Immunobiology. 1994;191:474–477. doi: 10.1016/S0171-2985(11)80453-5. [DOI] [PubMed] [Google Scholar]
- 15.Rosen H, Gordon S. The role of the type 3 complement receptor in the induced recruitment of myelomonocytic cells in vivo. Cell Mol Biol. 1990;3:3–10. doi: 10.1165/ajrcmb/3.1.3. [DOI] [PubMed] [Google Scholar]
- 16.Rosen H, Milon G, Gordon S. Antibody to the murine type 3 complement receptor inhibits T lymphocyte dependent recruitment of myelomonocytic cells in vivo. J Exp Med. 1989;169:535–548. doi: 10.1084/jem.169.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 18.Sligh J E, Ballantyne C M, Rich S S, Hawkins H K, Smith C W, Bradley A, Beaudet A L. Inflammatory and immune responses are impaired in mice deficient in intracellular adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 20.Svetic A, Finkelman F, Jian Y, Dieffenbach C, Scott D, McCarthy K, Steinberg A, Gause W. Cytokine gene expression after in vivo primary immunisation with goat antibody to mouse IgD antibody. J Immunol. 1991;147:2391–2397. [PubMed] [Google Scholar]
- 21.Wynn T A, Eltoum I, Cheever A W, Lewis F A, Gause W C, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]