Abstract
Purpose of review
This review summarizes the recent developments about anti-MDA5 antibody positive dermatomyositis with a focus on its pathogenesis, clinical features and treatment options of rapidly progressive interstitial lung disease, its most ominous complication.
Recent findings
Anti-MDA5+ dermatomyositis has a heterogeneous clinical spectrum with different patient subsets exhibiting widely different outcomes; severe acute interstitial lung disease is the main factor impacting prognosis. The pathogenetic role of anti-MDA5 antibodies is an active area of investigation.
Summary
Anti-MDA5+ dermatomyositis has a wider spectrum of manifestations than previously thought. A high index of suspicion is needed not to miss atypical presentations. In the setting of acute interstitial lung involvement, once a confident diagnosis is made, an aggressive approach with early combined immunosuppression affords the best chances of survival.
Keywords: anti-MDA5 antibodies, dermatomyositis, immunosuppressants, interstitial lung disease, rapidly progressive interstitial lung disease
INTRODUCTION
Immune-mediated inflammatory myopathies (IIM) are increasingly recognized as complex multisystem diseases with a wide spectrum of organ manifestations engendered in different proportions by inflammation, autoimmunity and vasculopathy [1,2,3]. The description and characterization of several myositis-specific and associated antibodies (MSAs and MAAs) has been a key contribution to defining different myositis clinical and pathophysiological subsets [4,5]. Among these, anti-melanoma differentiation antigen 5 (MDA5) antibodies have been associated with a definite subset of dermatomyositis patients showing prominent cutaneous and lung involvements with rapidly progressive interstitial lung disease (RP-ILD). The spectrum of anti-MDA5+DM is being explored further and subdivided into different clinical and prognostic subsets. Anti-MDA5 antibodies may also be found in the context of isolated lung involvement [6]; thus, the term ‘anti-MDA5 syndrome’ has been recently proposed [7▪▪].
Furthermore, a hyperinflammatory and hyperferritinemic state can be documented at the time of clinical worsening in some of these patients, bearing resemblance to severe cases of human SARS-CoV2 infection [8–11].
In contrast with classical forms of dermatomyositis, no strong association is consistently reported between MDA5+DM and malignancy. Recent research acquisitions have focused on describing the clinical spectrum associated with anti-MDA5 antibodies in Asian and non-Asian settings, in identifying predictors of RP-ILD and death, and on a deeper understanding of anti-MDA5 antibodies, whether as a directly pathogenic entity or as a marker of an underlying pathological process.
Box 1.
no caption available
THE BENCH: MDA5 AND ANTI-MDA5 ANTIBODIES
Originally described in melanoma cells and thence deriving its namesake, MDA5 is an antiviral pattern recognition receptor in humans. MDA5 is a cytosolic receptor that recognizes long strands of double-stranded RNA, a foreign molecular structure in eukaryotic cells. The origin of such molecules stems mainly from RNA viruses and DNA viruses, but dsRNA can also have an endogenous mitochondrial origin. Upon binding to dsRNA, through interaction with mitochondrial antiviral signalling protein (MAVS), MDA5 enhances the transcription of interferon-dependent (IFN) genes. In turn, MDA5 itself is encoded by a IFN-inducible gene (IFIH-1). Therefore, MDA5 sits at the origin of a positive proinflammatory and interferogenic feedback loop, occupying a critical regulatory position.
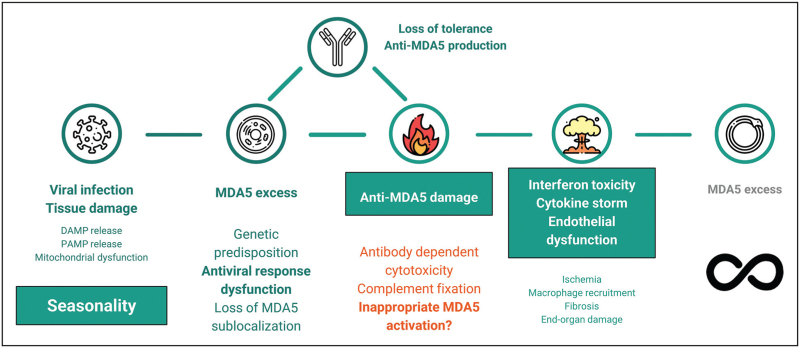
Hyperfunction of MDA5 due to gain-of-function mutations results in a spectrum of diseases sharing malformations, chronic inflammation and features of an interferonopathy with several rheumatological manifestations [12,13]. Furthermore, hyperstimulation of MDA5 by defective clearance mechanisms for mitochondrial dsRNA – for example in hypomorphic polynucleotide phosphorylase mutations – also results in an interferonopathy [14]. Importantly, the range of MDA5 subcellular localizations is not yet entirely clear: indeed, although MDA5 is classically described as a cytosolic receptor, it may relocate when abundant [15]. An overexpression of MDA5 in response to an index event may promote a shift in its subcellular localization, and it may encourage loss of tolerance to MDA5 and production of anti-MDA5 antibodies.
Anti-MDA5 could exert pathological effects on both ends of their functional spectrum: Anti-MDA5 antibodies that inactivate MDA5 may compromise antiviral responses, altering them to the point of indirectly producing an excessive, inefficient and damaging multisystemic inflammation to sustain viral clearance. On the opposite end, anti-MDA5 antibodies may stabilize MDA5 in an ‘active’ configuration, thus creating a constant danger signal at the origin of a pernicious positive feedback, producing the same hyperinflammatory state [16]. Several other mechanisms may be implicated in a direct anti-MDA5-mediated damage, such as formation of immune complexes together with MDA5, cell penetration with downstream pathway disruptions and antibody-dependent cytotoxicity. Anti-MDA5 could also simply be a marker of a dysfunctional antiviral response, with overexpression of MDA5 and loss of tolerance towards it as an epiphenomenon. However, it is increasingly clear that not all anti-MDA5 antibodies are made equal: in a recent study, Anti-MDA5 IgG-1 were found to be associated with RP-ILD and Anti-MDA5 IgA were found to be common, while the IgM isotype was more unusual [17]. In a different study, IgG1 and IgG3 anti-MDA5 antibodies were found to be independently associated with death and with RP-ILD, in contrast with IgG2 and IgG4 [18]. Titres of anti-MDA5 antibodies also seem to be higher in nonsurvivors and in RP-ILD patients, although this is not a universal finding [19,20]. Therefore, anti-MDA5 antibodies have potential roles both as markers and makers of a potentially devastating disease. In a general pathogenetic model (Fig. 1): an index event – presumably a viral infection – is met by a genetically susceptible host with an exuberant production of MDA5, loss of its subcellular localization, tissue damage and break of tolerance. A late immune response with delayed IFN production may promote this maladaptive process, whereas a rapid and orderly virus clearance through a timely initial burst of IFN production may avert further complications, in a similar manner to that described in COVID-19 [21,22]. Anti-MDA5 antibodies, once produced, may further exacerbate the process, leading to more inflammation and tissue damage, and engendering a cytokine storm in which high levels of IFN may mediate a vasculopathy through endothelial toxicity [23,24]. The healing response to the ongoing damage and ischemia would promote macrophage recruitment [25], fibrosis [26] and irreversible organ damage, especially in the lungs.
FIGURE 1.
Proposed general pathogenetic model of the anti-MDA5 syndrome. DAMP, damage-associated molecular pattern; MDA5, melanoma differentiation antigen 5; PAMP, pathogen-associated molecular pattern. Icons made by Freepik from Flaticon.com.
This conceptual framework bears several similarities with the human infection by SARS-CoV-2. Of note, anti-MDA5 antibodies have been found in COVID-19 patients, and their presence and titre showed an association to mortality [27]. On the contrary, nonspecific positive antibody tests are commonplace during viral infections, and anti-MDA5 titres were rather low compared with true anti-MDA5+DM patients.
THE BEDSIDE: CLINICAL CLUSTERS
The first descriptions of anti-MDA5+ dermatomyositis entailed a combination of clinically amyopathic dermatomyositis (CADM) with RP-ILD [28,29]. The cutaneous manifestations included hallmarks of dermatomyositis such as heliotrope rash, Gottron's papules and sign, and other typical dermatomyositis rashes such as V-neck and shawl signs. The presence of prominent cutaneous vasculopathy with skin ulcers was also an outstanding clinical feature.
Since then, the picture has evolved with the availability of retrospective data from both Asian and non-Asian cohorts [30–33]. In a recent unsupervised analysis on a French nationwide multicentre retrospective cohort [34], three clinical phenotypes were proposed: a ‘rheumatoid cluster’ exhibiting mostly arthritis and dermatologic involvement, with infrequent RP-ILD, a female predominance and a good overall prognosis; a male-predominant ‘vasculopathic DM cluster’ displaying severe vasculopathy in the form of Raynaud's phenomenon, skin ulcers and necrosis in addition to typical dermatomyositis rashes; in this group, rates of RP-ILD were intermediate (22.7%), as was the overall prognosis. Clinically relevant myositis (proximal weakness and high creatine kinase) was more prevalent in this subgroup. A ‘RP-ILD cluster’ with a grievous prognosis, high prevalence of ICU admission and very high rates of RP-ILD and death.
Some of these clusters are similar to other reports. In a recent single-centre retrospective Chinese cohort [35], three clusters emerged of which two were comparable to the French study: one mainly showing arthritis and mechanic's hands with low rates of RP-ILD and a good prognosis; one enriched in RP-ILD which was also exhibiting fever, hyperferritinemia and a far worse prognosis. In contrast, a different third cluster identified patients with high rates of typical cutaneous signs and enriched in clinically relevant myositis, with very low rates of RP-ILD (Table 1) [65].
Table 1.
Focus on recent descriptive cohorts and salient clinical characteristics of anti-MDA5+DM and non-DM patients
| Reference | Salient clinical involvement | RP-ILD rate | Prognosis | Comment |
| Allenbach et al.n = 121[39] | Cluster 1ILD 100%Skin 100%• mechanic's hands 73.3% | RP-ILD 93.3% | 3-month mortality 80% | |
| Cluster 2Skin 82.6%• Skin ulcers 37%ILD 82.6%Arthritis/arthralgia 82.6% | RP-ILD 17.4% | 3-month mortality 0% | ||
| Cluster 3Skin 95.4%• Skin ulcers 77.3%• Digital necrosis 31.8%Raynaud phenomenon 81.8%Proximal weakness 68.2%ILD 50% | RP-ILD 22.7% | 3-month mortality 4.5% | ||
| Yang et al.n = 96[35] | Cluster 1Arthritis 84.6%Mechanic's hands 51.3% | RP-ILD 7.7% | 24-week mortality 2.6% | |
| Cluster 2V-neck sign 69.2%Muscle weakness 92.3% | RP-ILD 7.7% | 24-week mortality = 0 | ||
| Cluster 3Fever 77.3%Elevated CRP 100%Hyperferritinemia > 1000 ug/L 75% | RP-ILD 77.3% | 24-week mortality 54% | ||
| Cavagna et al.n = 149[7▪▪] | OverallSkin involvement 74%Symptomatic muscle involvement 49%Joint involvement 51%• symmetric polyarticular in 70%Skin ulcers 15%Raynaud phenomenon 30%Fever 29% | RP-ILD 21.5% | 17% mortality at 36 months• 42% directly due to RP-ILD• 19% due to infection superimposed on RP-ILD | Focused on Anti-MDA5+ overall (10% diagnosed with IPAF) |
| At presentationSkin alone 14%Skin + ILD 13% | ||||
| Hensgens et al.n = 20[65] | OverallILD 95%Skin findings 87%Arthritis/arthralgia 60% | RP-ILD 45% | 1-year mortality 45% | Higher Anti-MDA5 titres in RP-ILD although with shorter disease duration |
The rates of RP-ILD, overall or in different clusters depending on the study, are reported. CRP, C-reactive protein; FU, follow-up; ILD, interstitial lung disease; IPAF, interstitial pneumonia with autoimmune features; RP-ILD, rapidly progressive interstitial lung disease.
In a retrospective analysis of the AENEAS group focusing on anti-MDA5+ patients as a whole [7▪▪], 89% of patients were diagnosed with myositis (dermatomyositis 43%, CADM 31%, polymiositis 5%, overlap myositis 11%); interestingly, the remainder 10% was diagnosed with interstitial pneumonia with autoimmune features (IPAF), not satisfying any other classification criterion. ILD was the main manifestation (72%); skin, joint and muscle involvement also showed a significant prevalence (74, 51 and 56%, respectively). Notably, rates of RP-ILD (21.5%) were lower than in Japanese reports, but in line with other European reports [32]. Onset of ILD was not confined to the first stages of the disease, but it could be diagnosed after a long course and, importantly, after prior treatment with potent immunosuppressants. Although the methodology differs, clinical clusters were not as clear-cut in this study, and arthralgia/arthritis and Raynaud phenomenon did not show a clear segregation in particular subgroups. Importantly, more than half of the patients did not show a positive antinuclear titre, stressing the need to actively look for Anti-MDA5 antibodies whenever clinical suspicion arises.
In severe cases, the disease may be complicated by signs of an hyperinflammatory, hyperferritinemic syndrome similar to severe COVID-19 [8]; this subset is often represented by acutely ill patients with RP-ILD, peripheral cytopaenias, high ferritin, elevated liver enzymes and haemostatic imbalances with both bleeding events and a proclivity towards disseminated intravascular coagulation. For example, spontaneous intramuscular haemorrhages have been described in acutely ill anti-MDA5+ patients, often carrying a grave prognosis [36]. Some of these severe cases may satisfy criteria for macrophage activation syndrome [37], including the presence of haemophagocytosis at bone marrow examination [38]. Awareness of such haematologic manifestations as part of the clinical picture is of critical importance, because these may otherwise lead clinicians astray in what appears to be a time-sensitive and difficult-to-treat disease.
Taken together, recent evidence suggests that any patient presenting with a suspicion or a known positivity for anti-MDA5 antibodies should prompt the treating physician to perform an assessment of a full patient history and a thorough examination of skin, muscle, joints and lungs; chest imaging with high-resolution computed tomography (HRCT) should be obtained expeditiously if any clinical signs of lung involvement are present; if not, at least pulmonary function tests (PFTs) and first-line chest imaging are advisable. Once any level of lung involvement is diagnosed, appropriate therapy and a tight multidisciplinary follow-up by Rheumatology, Pneumology and, if possible, Radiology should be arranged.
INTERSTITIAL LUNG DISEASE, RAPIDLY PROGRESSIVE- INTERSTITIAL LUNG DISEASE AND PREDICTORS OF POOR OUTCOME
RP-ILD is the main factor impacting prognosis in anti-MDA5+DM. Although ILD and RP-ILD can ensue at any point in the disease course, RP-ILD peaks in the first 6–12 months from diagnosis, and it drives mortality in this early period [39,40]. Predictors of both RP-ILD and mortality are therefore of great clinical interest.
The available data, derived from multivariate analyses of retrospective cohorts, point to the following factors as independently associated with ILD in the setting of anti-MDA5+DM: older age, a high neutrophil-to-lymphocyte ratio and/or lymphopenia, elevated LDH, elevated ferritin. The exact ferritin cut-off is variable among studies, with the majority reporting levels in excess of 1000 μg/l. Fever and elevated CRP have also been implicated in portending a worse prognosis (Table 2) [66–68]. These thought-provoking findings reinforce the notion of a dysfunctional antiviral response or a cytokine storm as the underlying substrate of the disease, at least in severe cases.
Table 2.
Focus on recent studies reporting on associated factors to rapidly progressive interstitial lung disease and mortality in Anti-MDA5+DM
| References | Outcome | Risk factors (except RP-ILD) |
| Zuo et al.[43] | RP-ILD | Fever OR 3.672 (1.794–7.516)Elevated ALT OR 2.355 (1.153–4.813)Elevated LDH OR 3.083 (1.517–6.266)Lymphopenia OR 2.141 (1.013–4.528)Elevated Ferritin OR 4.965 (1.973–12.498)Elevated CEA OR 2.276 (1.128–4.591)Elevated CA 15.3 OR 3.305 (1.502–7.272)Protective:Arthralgia OR 0.281 (0.138–0.570) |
| Mortality | Ferritin > 2200 ng/ml AUC 0.66 (0.51–0.80) | |
| So et al.[66] | RP-ILD | Age > 50 years HR 2.640 (1.277–5.455)LDH > 300 U/L HR 3.189 (1.469–6.918)Fever HR 1.903 (0.956–3.790)NLR > 7 HR 1.967 (0.942–4.107) |
| Mortality | Age > 52 years HR 4.750 (1.692–13.333)LDH > 400 U/L HR 2.290 (1.009–5.198)Ferritin > 2800 pmol/l HR 3.042 (1.323–6.997) | |
| Ouyang et al.[44▪] | Mortality | Fever HR 24.6 (2.3–260.7)Ferritin > 1250 μg/l HR 51.1 (3.5–747.5)Elevated CEA HR 85 (1.1–6516.2) |
| Zhou et al.[67] | Mortality | Advanced ageLymphopeniaLow serum albuminHigh LDHHigh ferritin |
| Lian et al.[68] | Mortalitya | Ferritin > 636 ng/ml HR 2.62 (1.18–5.83)LDH > 355 U/l HR 3.59 (1.83–7.01)HRCT score HR 6.24 (1.47–12.56) |
Where available, adjusted ORs, hazard ratios, AUCs and 95% confidence intervals are reported.
AUC, area under the curve; CA15.3, Cancer-Antigen 15.3; CEA, carcinoembryonic antigen; HR, hazard ratio; HRCT, high-resolution CT; LDH, lactic dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio.
Analysis on a cohort of CADM-ILD patients, with Anti-MDA5+ as a subset.
The co-presence of anti-Ro52 (SSA) antibodies has repeatedly been reported to be enriched in ILD and RP-ILD patients [41,42], confirming the not-so-benign profile of this antibody in the setting of autoimmune lung involvement. In recent reports, higher peripheral CD5-CD19+ B-cell counts and elevated carcinoembryonic antigen (CEA) and CA 15.3. were remarked on as independently associated with RP-ILD [43], in addition to the previously mentioned factors. Moreover, in a recent matrix prediction analysis [44▪], three factors (ferritin, CEA, fever) successfully predicted mortality at 6 months. The elevation of oncomarkers may raise suspicion of malignancy being implicated: conversely, CEA levels are heightened in many forms of lung injury such as in idiopathic pulmonary fibrosis and in active smokers [45]; moreover, no cases of adenocarcinoma were reported by the authors at extended follow-up in patients with elevated CEA who survived. Radiological patterns vary between reports but frequently show a combination of nonspecific interstitial pneumonia (NSIP) and organizing pneumonia findings with basal involvement and a rapidly progressive consolidative pattern [46,47]; a UIP-like pattern has also been reported [7▪▪]. Quantification of lung involvement at HRCT contributes to inform prognosis [48–50].
Importantly, although radiology may offer some crucial clues during the diagnostic stage, it remains challenging for any single radiological pattern to uniformly clinch the diagnosis of anti-MDA5 lung involvement a priori without supporting clinical and serological evidence; this reinforces the importance of actively looking for anti-MDA5 antibodies whenever clinically indicated.
THERAPEUTIC DEVELOPMENTS in RAPIDLY PROGRESSIVE-INTERSTITIAL LUNG DISEASE
No universal recommendations exist for treatment of anti-MDA5+DM. Outside of RP-ILD, current therapies are targeted towards the prevailing clinical manifestations whether it be arthritis, myositis, cutaneous rashes and vascular/vasomotor manifestations. In observational studies, employed drugs include glucocorticoids, antimalarials, methotrexate, mycophenolate mofetil, calcineurin inhibitors and azathioprine [7▪▪]. Intravenous immunoglobulins (IvIGs) and rituximab also have a role, especially as second-line interventions.
In the setting of RP-ILD, glucocorticoids in isolation do not seem to offer benefit and recent evidence supports early combined immunosuppression, with a low threshold for therapy escalation, and consideration to therapeutic plasma exchange (PEx) as salvage therapy in unresponsive cases (Table 3) [51,52▪]. The main strategy, supported by retrospective and prospective data, entails the combined use of high-dose glucocorticoids, for example intravenous methylprednisolone pulses 500 mg to 1 g/day for at least three consecutive days followed by 1 mg/kg/day, a calcineurin-inhibitor (CNI) and intravenous cyclophosphamide (CYC) 0.5–1.0 g/m2. In Japanese studies, early combination therapy yielded a better survival rate when compared with step-up therapy [53,54▪]. PEx could afford some incremental survival in cases not responding to combination therapy [55▪,56]. Of note, PEx outside of a combined immunosuppressive regimen appears to be of little value [54▪]. Combination therapy with glucocorticoids and a CNI, especially Tacrolimus, without CYC may yield similar results to triple therapy [57]. Among CNIs, Tacrolimus may perform better than Cyclosporin A [58]. Retrospective evidence suggests that the use of Rituximab as an add-on therapy to background immunosuppression could be a valid option [59]; an ultra-low dose regimen (100 mg single dose) also showed a nonstatistically significant trend towards response [60].
Table 3.
Focus on selected key recent evidence on treatments of Anti-MDA5+-ILD. Studies employing control groups are reported
| References | Design and intervention | Study population | Result |
| Shirakashi et al.[55▪] | Retrospective case-controladd-on PEx vs. no PEx | Anti-MDA5+ RP-ILDn = 38of which progressing under combined immunosuppressionn = 13 | 3-year survival of 62.5% in PEx group vs. 0% in no PEx group (P = 0.04, significant) |
| Abe et al.[56] | Retrospective case-controladd-on PEx vs. no PEx | Anti-MDA5+ RP-ILD under combined immunosuppressionn = 10 | 1-year survival 100% in PEx group vs. 25% in no PEx group (P = 0.033, significant) |
| Mao et al.[60] | Retrospective case-controlsingle 100 mg RTX infusion with or without CYC vs. CYC | Anti-MDA5+ ILD, RP-ILD in 92.5%n = 40 | 180-day mortality 36.4% in RTX group vs. 63.2% in CYC alone group (P = 0.26, nonsignificant) |
| Tsuji et al.[54▪] | Prospective single-arm with historical control groupCombined immunosuppression vs. traditional high-dose GCs with or without add-on PEx | Anti-MDA5+ ILDn = 44 | 12-month survival 85% in combined immunosuppression group vs. 33% in traditional immunosuppression(P < 0.001, significant)12-month survival 85% in add-on PEx vs. 71% in no add-on PEx (P = 0.17, nonsignificant) |
| Fujisawa et al.[58] | Prospective, randomized open-label 52 weeks trialTacrolimus vs. Cyclosporine | Myositis-associated ILD, subgroup for Anti-MDA5+ patientsn = 58 | Survival 88% in TAC group vs. 80% in CsA group (P = 0.63, nonsignificant)Progression-free survival 63% in TAC group vs. 40% in CsA group (P = 0.32, nonsignificant) |
| Chen et al.[63] | Prospective open-label with historical control groupTofacitinib vs. no Tofactinib | Anti-MDA5+ ILD, early (< 3 months)n = 50 | 6-month survival of 100% in Tofacitinib group vs. 78% in control group (significant at P = 0.04) |
CYC, cyclophosphamide; PEx, plasma exchange; RTX, rituximab.
Apart from PEx, other salvage therapies include Polymyxin B Hemoperfusion, which unfortunately has not shown encouraging results [61]. Extracorporeal membrane oxygenation (ECMO), while not a disease-modifying therapy per se, can act as a bridge to recovery or bridge to transplantation through the most critical stages of lung dysfunction [62].
Obviously, an aggressive combined immunosuppression has the drawback of being at odds with the main other confounding factor at the diagnostic and follow-up stages: infection. In fact, infections remain an important cause of death in anti-MDA5+DM patients [7▪▪]. A swift microbiologic workup and close collaboration and shared decision-making between different specialist figures are therefore key to avert unfavourable outcomes in this difficult disease.
Lastly, JAK inhibitors have been reported to be effective, especially in early cases [63]. Isolated reports of a combined use of JAKis with RTX with good effect are also available [64]. Further controlled studies are needed to properly assess the treatment hierarchy.
CONCLUSION
The spectrum of disease manifestations associated with anti-MDA5 antibodies is complex and expanding. Anti-MDA5+DM encompasses different patient groups with different prognoses, with RP-ILD being the main prognostic watershed. Several challenges lie ahead, including obtaining a better understanding of the role of anti-MDA5 antibodies, and achieving clarity on which treatment is the most indicated within and outside the setting of RP-ILD. Collaboration between the different medical specialties of Rheumatology, Pulmonology, Intensive Care and Radiology is paramount to achieve better outcomes.
Acknowledgements
The authors would like to thank Francesca Faustini, Antonella Notarnicola and Lara Dani (Rheumatology Unit, Karolinska Institutet, Stockholm, Sweden) for useful discussions and comments.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Franco C, Gatto M, Iaccarino L, et al. Lymphocyte immunophenotyping in inflammatory myositis: a review. Curr Opin Rheumatol 2021; 33:522–528. [DOI] [PubMed] [Google Scholar]
- 2.Musset L, Allenbach Y, Benveniste O, et al. Anti-HMGCR antibodies as a biomarker for immune-mediated necrotizing myopathies: a history of statins and experience from a large international multicenter study. Autoimmun Rev 2016; 15:983–993. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell S, Cooper RG, Lundberg IE, et al. Myositis Genetics Consortium. Dense genotyping of immune-related loci in idiopathic inflammatory myopathies confirms HLA alleles as the strongest genetic risk factor and suggests different genetic background for major clinical subgroups. Ann Rheum Dis 2016; 75:1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghirardello A, Borella E, Beggio M, et al. Myositis autoantibodies and clinical phenotypes. Auto Immun Highlights 2014; 5:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghirardello A, Rampudda M, Ekholm L, et al. Diagnostic performance and validation of autoantibody testing in myositis by a commercial line blot assay. Rheumatology 2010; 49:2370–2374. [DOI] [PubMed] [Google Scholar]
- 6.González-Moreno J, Raya-Cruz M, Losada-Lopez I, et al. Rapidly progressive interstitial lung disease due to anti-MDA5 antibodies without skin involvement: a case report and literature review. Rheumatol Int 2018; 38:1293–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪▪.Cavagna L, Meloni F, Meyer A, et al. Clinical spectrum time course in non-Asian patients positive for anti-MDA5 antibodies. Clin Exp Rheumatol 2022; 40:274–283. [DOI] [PubMed] [Google Scholar]; This observational retrospective cohort is the largest, multicentre, non-Asian cohort to date.
- 8.Mehta P, Machado PM, Gupta L. Understanding and managing anti-MDA 5 dermatomyositis, including potential COVID-19 mimicry. Rheumatol Int 2021; 41:1021–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian J, Xu H. COVID-19 disease and dermatomyositis: a mini-review. Front Immunol 2022; 12:747116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Du G, Zhang G, et al. Similarities and differences between severe COVID-19 pneumonia and anti-MDA-5-positive dermatomyositis-associated rapidly progressive interstitial lung diseases: a challenge for the future. Ann Rheum Dis 2020; doi: 10.1136/annrheumdis-2020-218594. [DOI] [PubMed] [Google Scholar]
- 11.Giannini M, Ohana M, Nespola B, et al. Similarities between COVID-19 and anti-MDA5 syndrome: what can we learn for better care? Eur Respir J 2020; 56:2001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crow YJ, Stetson DB. The type I interferonopathies: 10 years on. Nat Rev Immunol 2021; 20:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Carvalho LM, Ngoumou G, Park JW, et al. Musculoskeletal disease in MDA5-related type I interferonopathy: a Mendelian mimic of Jaccoud's arthropathy. Arthritis Rheumatol 2017; 69:2081–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhir A, Dhir S, Borowski LS, et al. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature 2018; 560:238-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger M, Hsieh CY, Bakele M, et al. Neutrophils express distinct RNA receptors in a noncanonical way. J Biol Chem 2012; 287:19409–19417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nombel A, Fabien N, Coutant F. Dermatomyositis with anti-MDA5 antibodies: bioclinical features, pathogenesis and emerging therapies. Front Immunol 2021; 12:773352.Published 2021 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M, Zhao Q, Diao L, et al. Distribution of antimelanoma differentiation associated gene 5 (MDA5) IgG subclasses in MDA5+ dermatomyositis. Rheumatology 2021; 61:430–439. [DOI] [PubMed] [Google Scholar]
- 18.Xu YT, Zhang YM, Yang HX, et al. Evaluation and validation of the prognostic value of anti-MDA5 IgG subclasses in dermatomyositis-associated interstitial lung disease. Rheumatology (Oxford) 2022; keac229.[Online ahead of print]. doi: 10.1093/rheumatology/keac229. [DOI] [PubMed] [Google Scholar]
- 19.Moghadam-Kia S, Oddis CV, Sato S, et al. Antimelanoma differentiation-associated Gene 5 antibody: expanding the clinical spectrum in North American patients with dermatomyositis. J Rheumatol 2017; 44:319–325. [DOI] [PubMed] [Google Scholar]
- 20.Matsushita T, Mizumaki K, Kano M, et al. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br J Dermatol 2017; 176:395–402. [DOI] [PubMed] [Google Scholar]
- 21.Sposito B, Broggi A, Pandolfi L, et al. The interferon landscape along the respiratory tract impacts the severity of COVID-19. Cell 2021; 184:4953–4968. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JS, Shin EC. The type I interferon response in COVID-19: implications for treatment. Nat Rev Immunol 2020; 20:585–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funauchi M, Shimadsu H, Tamaki C, et al. Role of endothelial damage in the pathogenesis of interstitial pneumonitis in patients with polymyositis and dermatomyositis. J Rheumatol 2006; 33:903–906. [PubMed] [Google Scholar]
- 24.He C, Chen J, Luo X, Yan B. Evaluation of biomarkers related to endothelial dysfunction: proof of vasculopathy in antimelanoma differentiation-associated gene 5 dermatomyositis. Clin Exp Rheumatol 2021; 39:151–157. [DOI] [PubMed] [Google Scholar]
- 25.Horiike Y, Suzuki Y, Fujisawa T, et al. Successful classification of macrophage-mannose receptor CD206 in severity of anti-MDA5 antibody positive dermatomyositis associated ILD. Rheumatology 2019; 58:2143–2152. [DOI] [PubMed] [Google Scholar]
- 26.Wang K, Zhao J, Chen Z, et al. CD4+CXCR4+ T cells as a novel prognostic biomarker in patients with idiopathic inflammatory myopathy-associated interstitial lung disease. Rheumatology 2019; 58:557. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Wang Q, Wang Y, et al. Presence of anti-MDA5 antibody and its value for the clinical assessment in patients with COVID-19: a retrospective cohort study. Front Immunol 2021; 12:791348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005; 52:1571–1576. [DOI] [PubMed] [Google Scholar]
- 29.Fujikawa K, Kawakami A, Kaji K, et al. Association of distinct clinical subsets with myositis-specific autoantibodies towards anti155/140-kDa polypeptides, anti140-kDa polypeptides, and antiaminoacyl tRNA synthetases in Japanese patients with dermatomyositis: a single-centre, cross-sectional study. Scand J Rheumatol 2009; 38:263–267. [DOI] [PubMed] [Google Scholar]
- 30.Hall JC, Casciola-Rosen L, Samedy LA, et al. Antimelanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res 2013; 65:1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labrador-Horrillo M, Martinez MA, Selva-O’Callaghan A, et al. Anti-MDA5 antibodies in a large Mediterranean population of adults with dermatomyositis. J Immunol Res 2014; 2014:290797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ceribelli A, Fredi M, Taraborelli M, et al. Prevalence and clinical significance of anti-MDA5 antibodies in European patients with polymyositis/dermatomyositis. Clin Exp Rheumatol 2014; 32:891–897. [PubMed] [Google Scholar]
- 33.Chen Z, Cao M, Plana MN, et al. Utility of antimelanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res (Hoboken) 2013; 65:1316–1324. [DOI] [PubMed] [Google Scholar]
- 34.Toquet S, Granger B, Uzunhan Y, et al. The seasonality of dermatomyositis associated with anti-MDA5 antibody: an argument for a respiratory viral trigger. Autoimmun Rev 2021; 20:102788. [DOI] [PubMed] [Google Scholar]
- 35.Yang Q, Lyu K, Li J, et al. Antimelanoma differentiation-associated 5 gene antibody-positive dermatomyositis exhibit three clinical phenotypes with different prognoses. Clin Exp Rheumatol 2022; 40:304–308. [DOI] [PubMed] [Google Scholar]
- 36.Xu Z, Lv X, Xu W, et al. Spontaneous intramuscular hemorrhage in anti-MDA5 positive dermatomyositis: a case series and literature review. Front Med 2021; 8:802753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding Y, Ge Y. Antimelanoma differentiation-associated gene 5 antibody-positive dermatomyositis complicated with macrophage activation syndrome. Ther Adv Chronic Dis 2022; 13:20406223221098128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune-associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol 2020; 49:244–246. [DOI] [PubMed] [Google Scholar]
- 39.Allenbach Y, Uzunhan Y, Toquet S, et al. Different phenotypes in dermatomyositis associated with anti-MDA5 antibody: study of 121 cases. Neurology 2020; 95:e70–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu L, Wang L, Lv C, Tan W. POS0879 clinical features, prognostic factors, and outcome of anti-MDA5 positive dermatomyositis with rapidly progressive interstitial lung disease: a multicenter study of 238 patients. Ann Rheum Dis 2021; 80:696.1–696. [Google Scholar]
- 41.Sabbagh S, Pinal-Fernandez I, Kishi T, et al. Anti-Ro52 autoantibodies are associated with interstitial lung disease and more severe disease in patients with juvenile myositis. Ann Rheum Dis 2019; 78:988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi K, Yamaguchi A, Onuki Y, et al. Clinical features of dermatomyositis associated with anti-MDA5 antibodies by age. Mod Rheumatol 2021; 31:177–185. [DOI] [PubMed] [Google Scholar]
- 43.Zuo Y, Zuo Y, Ye L, et al. Different multivariable risk factors for rapid progressive interstitial lung disease in anti-MDA5 positive dermatomyositis and anti-synthetase syndrome. Front Immunol 2022; 13:845988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44▪.Ouyang ZM, Lin JZ, Tang AJ, et al. A matrix prediction model for the 6-month mortality risk in patients with anti-melanoma differentiation-associated protein-5-positive dermatomyositis. Front Med 2022; 9:860798. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, a prediction of mortality is proposed on the basis of simple clinical and laboratory values in anti-MDA5+DM.
- 45.d’Alessandro M, Bergantini L, Torricelli E, et al. Systematic review and metanalysis of oncomarkers in IPF patients and serial changes of oncomarkers in a prospective Italian real-life case series. Cancers (Basel) 2021; 13:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mira-Avendano I, Abril A, Burger CD, et al. Interstitial lung disease and other pulmonary manifestations in connective tissue diseases. Mayo Clin Proc 2019; 94:309–325. [DOI] [PubMed] [Google Scholar]
- 47.Chino H, Sekine A, Baba T, et al. Radiological and pathological correlation in anti-MDA5 antibody-positive interstitial lung disease: rapidly progressive perilobular opacities and diffuse alveolar damage. Intern Med 2016; 55:2241–2246. [DOI] [PubMed] [Google Scholar]
- 48.Wang M, Fan C. Mortality risk prediction in amyopathic dermatomyositis associated with interstitial lung disease: perhaps some potential details to consider. Chest 2021; 159:1686–1687. [DOI] [PubMed] [Google Scholar]
- 49.Xu W, Wu W, Zhang D, et al. A novel CT scoring method predicts the prognosis of interstitial lung disease associated with anti-MDA5 positive dermatomyositis. Sci Rep 2021; 11:17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi K, Nakajima T, Yamaguchi A, et al. Quantitative CT analysis of interstitial pneumonia in antimelanoma differentiation-associated gene 5 antibody-positive dermatomyositis: a single center, retrospective study. Clin Rheumatol 2022; 41:1473–1481. [DOI] [PubMed] [Google Scholar]
- 51.Romero-Bueno F, Diaz Del Campo P, Trallero-Araguás E, et al. Recommendations for the treatment of antimelanoma differentiation-associated gene 5-positive dermatomyositis-associated rapidly progressive interstitial lung disease. Semin Arthritis Rheum 2020; 50:776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52▪.McPherson M, Economidou S, Liampas A, et al. Management of MDA-5 antibody positive clinically amyopathic dermatomyositis associated interstitial lung disease: a systematic review. Semin Arthritis Rheum 2022; 53:151959. [DOI] [PubMed] [Google Scholar]; This systematic review focuses on treatment options for ILD in anti-MDA5+DM and compiles a list of retrospective and clinical trial evidence to date.
- 53.Motegi SI, Sekiguchi A, Toki S, et al. Clinical features and poor prognostic factors of antimelanoma differentiation-associated gene 5 antibody-positive dermatomyositis with rapid progressive interstitial lung disease. Eur J Dermatol 2019; 29:511–517. [DOI] [PubMed] [Google Scholar]
- 54▪.Tsuji H, Nakashima R, Hosono Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol 2020; 72:488–498. [DOI] [PubMed] [Google Scholar]; In this prospective study with historical controls, combined immunosuppression showed superiority to conventional therapy; PEx also showed a trend towards benefit in the combined immunosuppression group. Importantly, PEx was allowed in the conventional therapy group, but was rarely performed.
- 55▪.Shirakashi M, Nakashima R, Tsuji H, et al. Efficacy of plasma exchange in anti-MDA5-positive dermatomyositis with interstitial lung disease under combined immunosuppressive treatment. Rheumatology (Oxford) 2020; 59:3284–3292. [DOI] [PubMed] [Google Scholar]; This retrospective study reinforces the notion of PEx as an adjunct rescue therapy in MDA5+ RP-ILD patients already treated with combined immunosuppression.
- 56.Abe Y, Kusaoi M, Tada K, et al. Successful treatment of anti-MDA5 antibody-positive refractory interstitial lung disease with plasma exchange therapy. Rheumatology 2020; 59:767–771. [DOI] [PubMed] [Google Scholar]
- 57.Fujisawa T. Management of myositis-associated interstitial lung disease. Medicina (Kaunas) 2021; 57:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujisawa T, Hozumi H, Kamiya Y, et al. Prednisolone and tacrolimus versus prednisolone and cyclosporin A to treat polymyositis/dermatomyositis-associated ILD: a randomized, open-label trial. Respirology 2021; 26:370–377. [DOI] [PubMed] [Google Scholar]
- 59.Ge Y, Li S, Tian X, et al. Antimelanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis responds to rituximab therapy. Clin Rheumatol 2021; 40:2311–2317. [DOI] [PubMed] [Google Scholar]
- 60.Mao MM, Xia S, Guo BP, et al. Ultra-low dose rituximab as add-on therapy in anti-MDA5-positive patients with polymyositis /dermatomyositis associated ILD. Respir Med 2020; 172:105983. [DOI] [PubMed] [Google Scholar]
- 61.Okabayashi H, Ichiyasu H, Hirooka S, et al. Clinical effects of direct hemoperfusion using a polymyxin B-immobilized fiber column in clinically amyopathic dermatomyositis-associated rapidly progressive interstitial pneumonias. BMC Pulm Med 2017; 17:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bay P, Pineton de Chambrun M, Roux A, et al. Extracorporeal life support allows lung transplant in anti-MDA5+ rapidly progressive interstitial lung disease. Eur Respir J 2022; 59:2102968. [DOI] [PubMed] [Google Scholar]
- 63.Chen Z, Wang X, Ye S. Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease. N Engl J Med 2019; 381:291–293. [DOI] [PubMed] [Google Scholar]
- 64.Yen TH, Tseng CW, Wang KL, Fu PK. Combination therapy with rituximab, tofacitinib and pirfenidone in a patient with rapid progressive interstitial lung disease (RP-ILD) due to MDA5 antibody-associated dermatomyositis: a case report. Medicina (Kaunas) 2021; 57:1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hensgens MPM, Delemarre EM, Drylewicz J, et al. Clinical features and immune-related protein patterns of anti-MDA5 positive clinically amyopathic dermatomyositis Dutch patients. Rheumatology (Oxford) 2022; 20:keac030. [DOI] [PubMed] [Google Scholar]
- 66.So J, So H, Wong VTL, et al. Predictors of rapidly progressive- interstitial lung disease and mortality in patients with autoantibodies against melanoma differentiation-associated protein 5 dermatomyositis. Rheumatology (Oxford) 2022; 14:keac094. [DOI] [PubMed] [Google Scholar]
- 67.Zhou J, Huang W, Ren F, et al. Evaluation of prognostic factors in anti-MDA5 antibody-positive patients in Chongqing, China: a retrospective study. Int J Gen Med 2021; 14:4775–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lian X, Zou J, Guo Q, et al. Mortality risk prediction in amyopathic dermatomyositis associated with interstitial lung disease: the FLAIR Model. Chest 2020; 158:1535–1545. [DOI] [PubMed] [Google Scholar]