Introduction
Steatocystoma multiplex (SM, also known as steatocystomatosis, sebocystomatosis, or epidermal polycystic disease) is a rare benign intradermal true sebaceous cyst of various sizes.[1,2] It is considered a nevoid or hamartomatous malformation of the pilosebaceous junction.[3] The classification of SM includes localized, generalized, facial, flexural, acral, syndromal, and suppurative types.[4] This review intends to draw attention to this unique dermatological condition.
History
The term SM translates to “a bag of fat.” In 1873, SM was first described by Jamieson in a case with numerous cysts distributed throughout the body. The term ‘steatocystoma multiplex’ was coined in 1899 by Pringle.[4] In 1982, Brownstein described steatocystoma simplex as a distinct entity.[5]
Epidemiology
The exact prevalence of SM is not known in the general population, with no gender or ethnic predilection.[6] In acral subcutaneous SM, a female preponderance is reported.[7] The occurrence of SM is common in the second and third decades of life. However, the skin lesions can occur at any age, as evidenced by their occurrence at birth or as old as 78 years of age.[8] SM in four successive generations in the same family have been reported.[9]
Aetiopathogenesis
The exact pathogenesis of SM is not known. According to the retention cyst theory, an initial sebaceous duct blockage with a keratinous plug results in cyst formation. However, it fell out of favor, as on numerous occasions, researchers were not able to show the blockade histopathologically.[10] Kligman and Kirchbaum hypothesized that pluripotent ectodermal cells retain the embryonic capacity to form appendages or naevi rather than inclusion or retention cysts.[1]
The keratin 17 gene (KRT17) encodes for a type 1 intermediate filament (K17) that is predominantly expressed in the hair follicles and sebaceous glands. The missense mutation of the K17 gene is associated with nevoid cyst formation in SM.[10,11] Most of the cases of SM are sporadic and are rarely inherited in an autosomal dominant trait that is linked to a mutation in exon 1 of keratin 17.[12] Other mutations rarely associated with SM include N92S, R94C, and R94H.[6] The different mutations can result in the same clinical phenotypes, whereas the same mutations can cause different clinical phenotypes.[13] Factors such as infections, trauma, or immunological episodes might be responsible as a trigger factor in SM.[3]
Clinical features
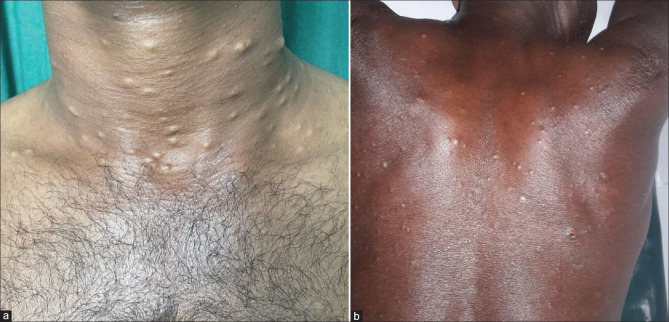
Clinically, SM presents as asymptomatic, numerous, round, smooth, firm, mobile, cystic papules, and nodules.[1,10] The lesions are uniform, with a size of a few millimeters to centimeters along the long axis.[14] The superficial lesions are yellowish, and deeper lesions tend to be skin-colored.[1] The fluid in SM is odorless, oily, clear or opaque, milky or yellow.[15] The overlying epidermal skin is often normal, with no central punctum.[1] SM can occur anywhere in the body but is more frequently seen in areas rich in pilosebaceous units such as the trunk (especially the presternal region), neck, scalp, axilla, proximal extremities, and inguinal region [Figures 1 and 2].[10]
Figure 1.
(a) Steatocystoma multiplex over the neck. Note the yellowish nature of the lesions (b) Steatocystoma multiplex over the posterior trunk
Figure 2.
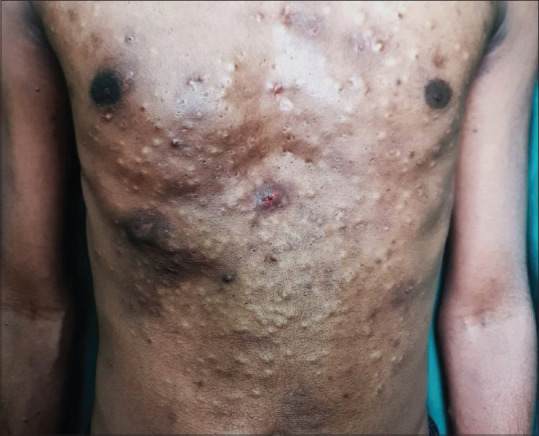
Extensive steatocystoma multiplex over the anterior trunk
SM can transform into steatocystoma multiplex suppurativum (SMS, also known as steatocystoma multiplex conglobatum) at any point in time during its natural course [Figure 3a].[16] It can have associated pain, pruritus, and pyrexia.[17] Cysts often become inflamed, rupture, and drain, resulting in scarring, thus sharing overlapping features with hidradenitis suppurativa. The secondary bacterial infection leads to malodourous discharge and abscess.[2,16]
Figure 3.
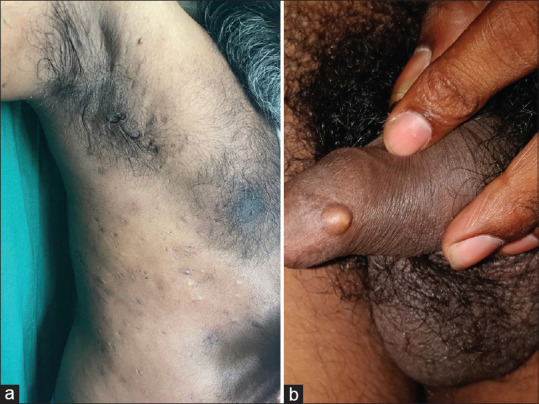
(a) A case of steatocystoma multiplex suppurativum. Note the simultaneous presence of active steatocystoma multiplex lesions along with scars clinically mimicking acne conglobata (b) Steatocystoma simplex in the shaft of the penis
The sporadic solitary tumor counterpart to SM is steatocystoma simplex [Figure 3b]. The atypical clinical presentations include giant SM, linear SM, palatal SS, and extensive calcification of SM.[1,12,18,19] Vulvar SM affecting the sexual activity of women is known.[20] SM of the scalp with concurrently acquired alopecia secondary to trichotillomania has been reported.[21] The occurrence of multiple SM is reported in a psoriasis patient on ustekinumab treatment.[11]
Associations
SM has been associated with hidradenitis suppurativa (HS), pachyonychia congenita, ichthyosis, koilonychia, acrokeratosis verruciformis of Hopf, hypotrichosis, hypohidrosis, hypothyroidism, hypertrophic lichen planus, multiple keratoacanthomas, rheumatoid arthritis, preauricular sinus, pili torti, pili canaliculi, neurofibromatosis 1, and polycystic kidney disease. Patients should be screened for any other ectodermal abnormalities.[14,15,22,23,24]
Co-occurrence of SM with hidradenitis suppurativa, eruptive vellus hair cysts, trichofolliculomas, trichoepitheliomas, and trichoblastomas have been reported.[15] Syndromal associations include X-linked recessive Lowe syndrome, Gardner syndrome, Noonan syndrome, Alagille-Watson syndrome, and Favre-Racouchot syndrome.[4]
Diagnosis
Differential diagnosis
SM is often confused with eruptive vellus hair cyst (EVHC), whose differences are mentioned in Table 1.[25,26] The other differential diagnosis of SM includes multiple epidermoid cysts, neurofibromatosis, xanthomatosis, lipomatosis, milia, follicular infundibular tumors, severe nodulocystic acne, pseudofolliculitis. The salient clinical and histopathological features of the diseases that can mimic SM and its variants are shown in Table 2. When the lesions are inflamed, folliculitis, acne conglobata, and hidradenitis suppurativa should be considered.[3,4,17,25,27]
Table 1.
Differences between steatocystoma multiplex and eruptive vellus hair cyst
| Features | Steatocystoma multiplex | Eruptive vellus hair cyst |
|---|---|---|
| Synonym | Steatocystomatosis, sebocystomatosis, epidermal polycystic disease | Vellus hair cyst |
| Age | Second and third decade | Common in the second decade |
| Gender | No gender predilection | No gender predilection |
| Pathogenesis | Initial sebaceous duct blockage with a keratinous plus result in cyst formation Pluripotent ectodermal cells retain the embryonic capacity to form appendages | Developmental abnormality of vellus hair follicles that predisposes them to occlusion at the infundibulum level Retention of hairs, cystic dilation of the proximal part of the follicle, and secondary atrophy of the hair bulbs |
| Cytokeratin expression | Express both cytokeratin 10 and 17 | Express cytokeratin 10 |
| Clinical features | Asymptomatic, multiple, round, firm, skin-colored to yellowish, mobile cystic papules and nodules of size 2 mm to 20 mm | Asymptomatic, small follicular red or brown papules of 1 to 2 mm diameter |
| Surface changes | Often normal with no surface changes Can suppurate | Some can have central puncta, umbilicated, or a hyperkeratotic crust |
| Site | Common in the trunk, neck, scalp, axilla, proximal extremities, inguinal region | Chest and axilla |
| Course | No spontaneous resolution | Spontaneous resolution in 25% of lesions |
| Dermoscopy | Yellowish structureless areas with diffuse margins | Erythematous maroon halo with occasional irregular radiating capillaries at the periphery Vellus hairs open into the dermis |
| Histopathology | Well-encapsulated cysts whose walls contain several layers of intricately folded epithelial cells and flattened sebaceous gland globules within or in close proximity to cyst wall A thick eosinophilic cuticle protrudes into the lumen | Squamous epithelium-lined cysts in the mid-dermis containing vellus hair and keratin debris |
Table 2.
Characteristics of the differential diagnosis of variants of steatocystoma
| Condition | Age and gender | Clinical features | Site | Investigations |
|---|---|---|---|---|
|
| ||||
| Steatocystoma simplex | ||||
| Solitary apocrine hidrocystoma | Common in adults aged 30–70 years. No gender predilection | Solitary, intradermal, firm, dome-shaped, translucent, bluish, cystic nodule of size ranges from 3 to 15 mm. | Head, face, and neck | Histopathological examination (HPE): Inner cyst wall lined by columnar, eosinophilic cells that show prominent luminal blebbing |
| Solitary eccrine hidrocystoma (Smith type) | Common in adults without any gender predilection | Clinically, they are solitary, dome-shaped, have an amber, brown, or bluish tint, and range from 1 to 6 mm in diameter. Cysts grow in size in summer | Around eyelid skin | HPE: Unilocular cyst in the dermis lined by a thin epithelial layer consisting of 1–2 layers. The cells are bilaminar and have slightly eosinophilic cytoplasm |
| Dermoid cyst | Common in children with no gender predilection | It presents as an asymptomatic, pale, flesh-colored, pearly, dome-shaped, firm, deep-seated, subcutaneous nodule | Head and neck region | HPE: Well-defined wall lined by stratified squamous epithelium and a lumen that may be filled with hair follicles and shafts, sebaceous and eccrine glands. |
|
| ||||
| Steatocystoma multiplex | ||||
|
| ||||
| Epidermoid or infundibular cyst | Young and middle-aged adults with no sex predilection | Slowly-growing, yellowish, white, or skin-colored, firm, smooth, dome-shaped cysts with a central keratin-filled punctum. | Scalp, face, neck, upper trunk | HPE: Early cysts lined by stratified squamous epithelium with a granular layer Cyst is filled with horny material arranged in laminated layers |
| Milia | Common in all age groups | Multiple, superficially located, firm, white, globoid lesions of 1–2 mm in diameter | Face, cheeks, and eyelids | HPE: Cyst with a stratified squamous epithelial lining with a granular layer and contains lamellated keratin located in the superficial dermis |
| Trichilemmal or pilar cyst | More common in middle-aged women | Smooth, mobile, firm, and rounded nodules without punctum Inflamed cysts become tender and rupture following an infection. | Scalp | HPE: Cysts lined by stratified squamous epithelium that show a distinct peripheral palisading. No granular layer Cyst contents are homogenous and eosinophilic and contain cholesterol clefts |
| Neurofibromatosis | Onset in childhood | Soft, lilac-pink tumors, sessile and dome-shaped ranging from a few millimeters to several centimeters | Common in trunk and limbs | HPE: Circumscribed tumors of reticular dermis composed of thin spindle cells with elongated nuclei spaced regularly among thin, wavy, collagenous strands. Hypercellular and enlarged small nerves |
| Familial multiple lipomatosis | Common in the third decade without any gender predilection | Multiple, discrete, round-to-oval, rubbery, encapsulated lipomas with variable tenderness on the trunk and extremities | Trunk and extremities | HPE: Proliferation of mature adipocytes with paucicellular fibrous septa |
| Pseudofolliculitis | After puberty in males | Multiple, small papules and pustules with a chronic relapsing and remitting course Papules may scar, form keloid and leaves hyperpigmentation | Beard area, particularly over the jaw and neck | HPE: Acute inflammation, microabscesses, and foreign body giant cell granuloma formation in the follicle and perifollicular areas |
|
| ||||
| Steatocystoma multiplex suppurativum | ||||
|
| ||||
| Acne conglobata | Common in second and third decade | Multiple and extensive inflammatory papules, tender nodules, and abscesses coalesce to form malodorous draining sinus tracts that heal with hypertrophic and atrophic scars | Trunk and upper limbs extending to the buttocks | No consistent laboratorial abnormalities Bacterial culture from the discharge fluid |
| Severe nodulocystic acne | Seen in second decade Common in males | Severe form of acne characterized by multiple nodules, sinuses, and scarring | Face, chest, and back | Clinical diagnosis Hormonal evaluation |
|
| ||||
| Steatocystoma multiplex suppurativum | ||||
|
| ||||
| Hidradenitis suppurativa | Onset in the second and third decades Common in females | Recurrent and chronic lesions characterized by painful subcutaneous nodules or abscesses, fistula, sinus tracts, and scarring | Axilla, inguinal, ano-genital, sub- and intermammary areas | Microbiology (swabs, purulent exudate and tissue) HPE: Follicular hyperkeratosis, follicular epithelial hyperplasia, perifolliculitis, and dense mixed inflammatory infiltrates in early lesions. Neutrophilic abscess that may connect with squamous epithelium-lined cysts and sinus tracts in advanced lesions Imaging (Ultrasound and magnetic resonance imaging) defines extension of the disease |
Diagnostic methods
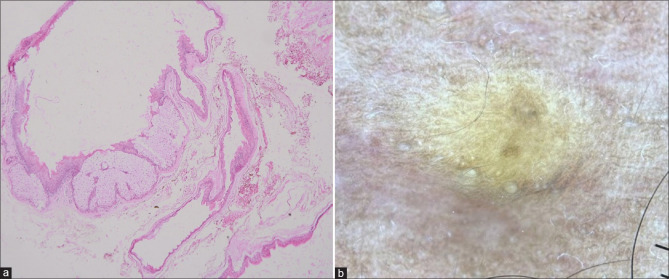
A diagnosis of SM should always be confirmed with histopathology. Histologically, the cysts are well encapsulated, the walls have several layers of epithelial cells that intricately fold and flattened sebaceous gland globules within or close to the cyst wall. A thick, homogenous, eosinophilic cuticle without an intervening granular layer lines the inside wall of the cyst and protrudes irregularly into the lumen [Figure 4a]. The lumen often contains oil, hair, and keratin.[1,10]
Figure 4.
(a) Cyst lining in a wavy, homogenous, eosinophilic horny layer with several layers of epithelial cells collapsed around the cystic space. Note the embedded lobules of sebaceous glands among the epithelial cells (H&E, 20x) (b) Dermoscopy showing the yellowish structureless area (Dermlite D4, polarized, original magnification x10)
In immunohistochemistry, the inner epithelial lining of the cysts shows positive staining with calretinin, the upper layer cells in the upper cyst wall express keratin 17, and basal and suprabasal layers express keratin 14.[20,28] The fine needle aspiration cytology of the cyst fluid shows predominantly acellular, granular debris, crystalline structures, rare anucleated squamous cells, and cholesterol crystals.[29]
Dermoscopy of SM shows yellowish structureless areas (corresponds to sebum contained in the cyst cavity) with diffuse margins [Figure 4b].[4] Mammographs typically reveal multiple oil cysts that have a central fat density. Ultrasound imaging shows multiple anechoic cysts with posterior acoustic enhancement.[6] Ultrasonography with color Doppler is a non-invasive tool that can differentiate SM and HS.[16] Magnetic resonance imaging shows hyperintense lesions.[30]
Treatment
The goals of SM management include a substantial reduction of cyst size, good cosmesis, and good patient satisfaction. Most of the time, patients seek medical advice for cosmetic reasons.[8] There is no specific gold standard treatment option, and it should be geared toward the patient’s clinical presentation.[6] A wide variety of treatment modalities have been tried for SM.[8] The treatment response for this condition is generally not satisfactory.[17] The eventual recurrence of the lesions after treatment is the rule.[1]
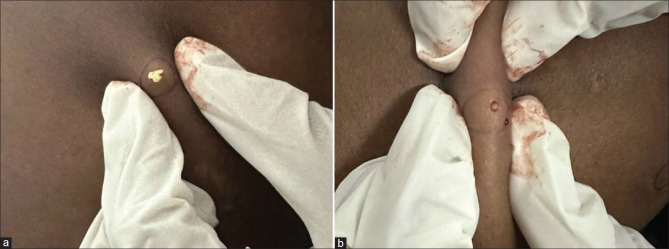
Surgical management includes simple aspiration with an 18-gauge needle, incision and expression of the cyst contents, and radiofrequency probe-mediated extrusion of the contents [Figure 5].[1,8] The use of tissue adhesives for skin closure following the surgical removal of the cyst has good efficacy.[31] For localized variants of SM, local destructive methods such as cryotherapy have been reported to be efficacious.[32] The complete surgical excision of the cysts followed by skin grafting has been tried in the past.[33] Tricarboxylic acid treatment of the cyst walls can be administered after the aspiration of contents.[34]
Figure 5.
(a) Extrusion of cheesy, oily material after a small nick in a lesion (b) Sac protruding out of the steatocystoma multiplex
For non-infectious inflammatory lesions, a short course of oral tetracyclines, topical benzoyl peroxide wash, or clindamycin might be considered. Systemic isotretinoin, due to its anti-inflammatory effect, decreases the size of pre-existing cysts and prevents the formation of new lesions.[35] However, some authors have reported worsening or exacerbating the condition in patients with SM and severe inflammation. Few have observed that systemic isotretinoin had no effect on non-inflamed lesions of SM. A combination of oral rifampicin and clindamycin has shown efficacy in the case of SMS.[36] The treatment with adalimumab in patients with coexistent SMS and HS has shown good efficacy.[2]
Lasers are advantageous because they are minimally invasive. Ablative laser therapies, such as erbium: yttrium-garnet laser, fractionated and non-fractionated Co2 laser, have been tried.[37] A combination of two non-ablative lasers with complementary mechanisms, such as the 1550-nm fractionated Erbium-doped fiber laser that targets dermal cysts and the 1450-nm diode laser that targets abnormal sebaceous glands have shown dramatic improvement in a patient with recalcitrant SM.[38] The 1927-nm fiber-optic diode laser has demonstrated excellent clinical outcomes with minimal adverse effects in a patient with facial SM.[39] A summary of the treatment options for SM is mentioned in Table 3.[31,35,37,38,39,40,41,42]
Table 3.
Summary of various treatment modalities in steatocystoma multiplex
| Treatment modality | Advantages | Disadvantages |
|---|---|---|
|
| ||
| Medical management | ||
| Isotretinoin – administered orally at 1 mg/kg/daily for six months | Decreases the size of pre-existing cysts Prevents the formation of new lesions | Recurrence Results are limited only to inflamed lesions Can produce exacerbation of flare of disease initially |
| Tetracyclines – administered orally | Benefit for suppurative variant of SM | Poor efficacy |
|
| ||
| Surgical management | ||
|
| ||
| Cryotherapy | Treatment of multiple lesions in a single setting Suitable for larger lesions, even calcified ones | Extremely low efficacy cosmetic disfigurement Blister formation Scarring Hypopigmentation |
| Radiofrequency - Expression of the contents with mini-incisions (1–2 mm) made on the cyst | Produces bloodless field No sutures and scar formation No recurrence | Time consuming |
| Needle aspiration with extirpation of the contents | Inexpensive Well-tolerated | Requires a skilled operator. Does not work well on very large (>15 mm) and small (3 mm) cysts High recurrence rate |
| Surgical excision - surgery with elliptical excisions, flaps, or grafts | Followed in older days | Time consuming Scarring Not practical for widespread lesions |
| Surgical techniques – Fine incision followed by cyst wall extraction with forceps, curette, or vein hooks | Shows excellent cosmetic results Cost effective | Time consuming and invasive Transient post-inflammatory hyperpigmentation |
|
| ||
| Lasers | ||
|
| ||
| Fractional Co2 laser - For cyst opening, drainage, and vaporization of the cyst wall and remnants | Can treat multiple lesions in a single session with good cosmetic outcomes Minimal scarring Low recurrence | Not suitable for larger cysts |
| Erbium-YAG (Yttrium Aluminium Garnet) laser | Good toleration No scarring Improved quality of life | Not cost effective Not easily accessible |
Conclusion
SM should be considered as one of the differential diagnoses in patients with multiple asymptomatic intradermal cysts. Early disease recognition and appropriate counseling might help alleviate the psychological implications associated with the disease. It is a rare clinical entity with poor treatment outcomes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to thank Dr. Sai Kiran Attili, Consultant dermatopathologist at Vishaka Institute of Skin and Allergy, Vishakhapatnam, India, for providing the histopathology image.
References
- 1.Rahman MH, Islam MS, Ansari NP. Atypical steatocystoma multiplex with calcification. ISRN Dermatol 2011. 2011 doi: 10.5402/2011/381901. 381901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atzori L, Zanniello R, Pilloni L, Rongioletti F. Steatocystoma multiplex suppurativa associated with hidradenitis suppurativa successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2019;33:42–4. doi: 10.1111/jdv.15848. [DOI] [PubMed] [Google Scholar]
- 3.Jain M, Puri V, Katiyar Y, Sehgal S. Acral steatocystoma multiplex. Indian Dermatol Online J. 2013;4:156–7. doi: 10.4103/2229-5178.110644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma A, Agrawal S, Dhurat R, Shukla D, Vishwanath T. An unusual case of facial steatocystoma multiplex: A clinicopathologic and dermoscopic report. Dermatopathology (Basel) 2018;5:58–63. doi: 10.1159/000488584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SJ, Park HJ, Oh ST, Lee JY, Cho BK. A case of steatocystoma multiplex limited to the scalp. Ann Dermatol. 2009;21:106–9. doi: 10.5021/ad.2009.21.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reick-Mitrisin V, Reddy A, Shah BA. A breast imaging case of steatocystoma multiplex: A rare condition involving multiple anatomic regions. Cureus. 2022;14:e27756. doi: 10.7759/cureus.27756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marzano AV, Tavecchio S, Balice Y, Polloni I, Veraldi S. Acral subcutaneous steatocystoma multiplex: A distinct subtype of the disease? Australas J Dermatol. 2012;53:198–201. doi: 10.1111/j.1440-0960.2011.00851.x. [DOI] [PubMed] [Google Scholar]
- 8.Varshney M, Aziz M, Maheshwari V, Alam K, Jain A, Arif SH, et al. Steatocystoma multiplex. BMJ Case Rep 2011. 2011 doi: 10.1136/bcr.04.2011.4165. bcr0420114165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur T, Kanwar AJ. Steatocystoma multiplex in four successive generations. J Dermatol. 2003;30:559–61. doi: 10.1111/j.1346-8138.2003.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 10.Rollins T, Levin RM, Heymann WR. Acral steatocystoma multiplex. J Am Acad Dermatol. 2000;43:396–9. doi: 10.1067/mjd.2000.100048. [DOI] [PubMed] [Google Scholar]
- 11.Marasca C, Megna M, Donnarumma M, Fontanella G, Cinelli E, Fabbrocini G. A case of steatocystoma multiplex in a psoriatic patient during treatment with anti-IL-12/23. Skin Appendage Disord. 2020;6:309–11. doi: 10.1159/000507657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alotaibi L, Alsaif M, Alhumidi A, Turkmani M, Alsaif F. Steatocystoma multiplex suppurativa: A case with unusual giant cysts over the scalp and neck. Case Rep Dermatol. 2019;11:71–6. doi: 10.1159/000498882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JY, Park JH, Sohng C, Jang YH, Lee SJ, Lee WJ. Huge steatocystoma multiplex with new point mutation in the Exon 1 of KRT 17 Gene. Ann Dermatol. 2018;30:633–5. doi: 10.5021/ad.2018.30.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin NY, Kang JH, Kim JE, Symkhampa K, Huh KH, Yi WJ, et al. Steatocystoma multiplex: A case report of a rare entity. Imaging Sci Dent. 2019;49:317–21. doi: 10.5624/isd.2019.49.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bridges AG, Erickson LA. Co-occurrence of steatocystoma multiplex, eruptive vellus hair cysts, and trichofolliculomas. Cutis. 2017;100:E23–6. [PubMed] [Google Scholar]
- 16.Fletcher J, Posso-De Los Rios C, Jambrosic J, Alavi A. Coexistence of hidradenitis suppurativa and steatocystoma multiplex: Is it a new variant of hidradenitis suppurativa? J Cutan Med Surg. 2021;25:586–90. doi: 10.1177/12034754211010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santana CN, Pereira DD, Lisboa AP, Leal JM, Obadia DL, Silva RS. Steatocystoma multiplex suppurativa: Case report of a rare condition. An Bras Dermatol. 2016;91:51–3. doi: 10.1590/abd1806-4841.20164539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Almeida HL, Jr, Basso P. Linear unilateral steatocystoma multiplex. J Eur Acad Dermatol Venereol. 2009;23:213–4. doi: 10.1111/j.1468-3083.2008.02796.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaya S, Zimmer S, Kämmerer PW. Palatal steatocystoma simplex-a rare oral finding at an even rarer location. J Surg Case Rep 2020. 2020 doi: 10.1093/jscr/rjaa347. rjaa347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batycka-Baran A, Baran W, Maj J, Szepietowski JC. Cystic nodules affecting sexual activity: A quiz. Steatocystoma multiplex. Acta Derm Venereol. 2010;90:445–7. doi: 10.2340/00015555-0825. [DOI] [PubMed] [Google Scholar]
- 21.Lee D, Chun JS, Hong SK, Seo JK, Choi JH, Koh JK, et al. Steatocystoma multiplex confined to the scalp with concurrent alopecia. Ann Dermatol. 2011;23:S258–60. doi: 10.5021/ad.2011.23.S2.S258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietrzak A, Bartosinska J, Filip AA, Rakowska A, Adamczyk M, Szumilo J, et al. Steatocystoma multiplex with hair shaft abnormalities. J Dermatol. 2015;42:521–3. doi: 10.1111/1346-8138.12837. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Fang M, Ding X, Tang L, Zhang X. Familial neurofibromatosis type 1 has diverse manifestations in skin and is associated with steatocystoma multiplex. Clin Exp Dermatol. 2021;46:1166–9. doi: 10.1111/ced.14618. [DOI] [PubMed] [Google Scholar]
- 24.Yoneda K, Nakai K, Demitsu T, Kubota Y. Polycystic kidney disease with steatocystoma multiplex: Evidences for a disruptive effect of mutated polycystin-1 on keratin 17 polymerisation. Acta Derm Venereol. 2015;95:353–4. doi: 10.2340/00015555-1934. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths C, Barker J, Bleiker T, Chalmer R, Creamer D, editors. 9th ed. United Kingdom: Wiley Blackwell; 2016. Rook's Textbook of Dermatology. [Google Scholar]
- 26.Alfaro-Castellón P, Mejía-Rodríguez SA, Valencia-Herrera A, Ramírez S, Mena-Cedillos C. Dermoscopy distinction of eruptive vellus hair cysts with molluscum contagiosum and acne lesions. Pediatr Dermatol. 2012;29:772–3. doi: 10.1111/j.1525-1470.2012.01771.x. [DOI] [PubMed] [Google Scholar]
- 27.Jiang M, Zhang M, Gu H, Chen X. A case of late onset steatocystoma multiplex. Postepy Dermatol Alergol. 2020;37:117–8. doi: 10.5114/ada.2018.78999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riedel C, Brinkmeier T, Kutzne H, Plewig G, Frosch PJ. Late onset of a facial variant of steatocystoma multiplex-calretinin as a specific marker of the follicular companion cell layer. J Dtsch Dermatol Ges. 2008;6:480–2. doi: 10.1111/j.1610-0387.2007.06603.x. [DOI] [PubMed] [Google Scholar]
- 29.Elhence P, Bansal R, Sharma S, Jain V. Steatocystoma multiplex--an uncommon lesion with special emphasis on cytological features and cyto-histological correlation. J Postgrad Med. 2012;58:210–1. doi: 10.4103/0022-3859.101405. [DOI] [PubMed] [Google Scholar]
- 30.Chotai N, Lim SK. Imaging features of steatocystoma multiplex-back to basics. Breast J. 2021;27:389–90. doi: 10.1111/tbj.14179. [DOI] [PubMed] [Google Scholar]
- 31.Jiang L, Yan J, Chen X, Chen Y, Tang Y. A simple modified surgical technique combined with tissue adhesive for steatocystoma multiplex. J Cosmet Dermatol. 2021;20:218–21. doi: 10.1111/jocd.13438. [DOI] [PubMed] [Google Scholar]
- 32.Adams B, Shwayder T. Steatocystoma multiplex suppurativum. Int J Dermatol. 2008;47:1155–6. doi: 10.1111/j.1365-4632.2008.03698.x. [DOI] [PubMed] [Google Scholar]
- 33.Feinstein A, Trau H, Movshovitz M, Schewach-Millet M. Steatocystoma multiplex. Cutis. 1983;31:425–7. [PubMed] [Google Scholar]
- 34.Sato K, Shibuya K, Taguchi H, Kitano Y, Yoshikawa K. Aspiration therapy in steatocystoma multiplex. Arch Dermatol. 1993;129:35–7. [PubMed] [Google Scholar]
- 35.Georgakopoulos JR, Ighani A, Yeung J. Numerous asymptomatic dermal cysts: Diagnosis and treatment of steatocystoma multiplex. Can Fam Physician. 2018;64:892–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed G, Prabha N, Ganguly S. Familial steatocystoma multiplex generalisita suppuritiva: Oral rifampicin and clindamycin combination worth a trial. Indian J Dermatol. 2021;66:553–5. doi: 10.4103/ijd.IJD_117_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassira S, Korta DZ, de Feraudy S, Zachary CB. Fractionated ablative carbon dioxide laser treatment of steatocystoma multiplex. J Cosmet Laser Ther. 2016;18:364–6. doi: 10.1080/14764172.2016.1188212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moody MN, Landau JM, Goldberg LH, Friedman PM. 1,450-nm diode laser in combination with the 1550-nm fractionated erbium-doped fiber laser for the treatment of steatocystoma multiplex: A case report. Dermatol Surg. 2012;38:1104–6. doi: 10.1111/j.1524-4725.2012.02391.x. [DOI] [PubMed] [Google Scholar]
- 39.Cheon DU, Ko JY. 1927-nm fiber-optic diode laser: A novel therapeutic option for facial steatocystoma multiplex. J Cosmet Dermatol. 2019;18:1326–9. doi: 10.1111/jocd.12849. [DOI] [PubMed] [Google Scholar]
- 40.AlSabbagh MM. Steatocystoma multiplex: A review. J Dermatol Surg. 2016;20:91–9. [Google Scholar]
- 41.Senel E. Answer: Can you identify this condition? Can Fam Physician. 2010;56:672. [PMC free article] [PubMed] [Google Scholar]
- 42.Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25–8. doi: 10.4103/0974-2077.63284. [DOI] [PMC free article] [PubMed] [Google Scholar]