Abstract
Background and Objective:
Tranexamic acid (TXA) has recently shown promising results in the treatment of melasma. The objective of this study was to generate statistical evidence on the efficacy of TXA with different routes.
Materials and Methods:
We searched studies in PubMed, Cochrane, ClinicalTrials.gov, and Scopus using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines. A change in melasma area and severity index (MASI)/modified MASI score from the baseline at the end of 8 and 12 weeks was seen. Inverse variance method was used for continuous data to measure standard mean difference (SMD) at a 95% confidence interval (CI). RevMan version 5.4 was used for analysis, and statistical heterogeneity across studies was reported using I2 statistics. P < 0.05 was considered significant.
Results:
Totally, 28 randomized control trials were included. At 8 weeks, oral TXA showed a significant change in SMD of 1.61, 95% CI 0.44–2.79, P = 0.007; at 12 weeks, oral TXA showed SMD of 2.39, 95% CI 1.42–3.35, P < 0.00001 compared to adjuvant treatment. At 8 weeks, topical TXA did not show a significant change with SMD of -0.05, 95% CI -1.08–0.97, P = 0.92; at 12 weeks, topical TXA did not show a significant change with SMD of 0.66, 95% CI -0.10–1.42, P = 0.09 compared to adjuvant treatment. Similarly, for intradermal TXA at 8 weeks, results were not significant with SMD of 1.21, 95% CI -0.41–2.83, P = 0.14, and at 12 weeks, SMD was -0.55, 95% CI -2.27–1.18, P = 0.54 compared to adjuvant treatment.
Conclusion:
Tranexamic acid in an oral formulation can be used along with adjuvant treatment for the management of melasma. Data are still required for topical and intradermal routes. Owing to the fact that our included studies had a lot of heterogeneity, more research is needed along with addressing the adverse effects of tranexamic acid as well as its variation in different skin colors.
Keywords: Intradermal, melasma, oral, topical, tranexamic acid
Introduction
Melasma is a common skin condition that causes blue-gray to brown patches and spots, usually on the sun-exposed areas of the face and neck, predominantly affecting women with darker skin types such as Fitzpatrick skin types IV, V, and VI.[1,2] The exact pathogenesis is unknown; however, it has been hypothesized that melasma is induced by biologically active melanocytes, and increased vascularity and elevated expression of angiogenic factors in the affected epidermis have also been found.[3,4,5] These factors may play an important role in the development of melasma. Melasma is associated with some factors like genetic susceptibility, ultraviolet (UV) light exposure, pregnancy, sex hormones, contraceptive pills, cosmetics, phototoxic drugs, and inflammatory processes.[6,7,8,9,10] Melasma significantly affects a patient’s appearance and quality of life.[11,12,13] For many years, the treatment of melasma has been centered around topical creams, avoiding sunlight, use of vitamins, or even use of lasers to help decrease the pigmentation. Melasma area and severity index (MASI) is the most commonly used outcome measure for melasma. While the MASI score is a subjective assessment, it is calculated as a combination of three separate factors (total area of involvement, darkness, and homogeneity) and is considered a reliable, semi-objective tool in the analysis of melasma. Lately, Pandya and colleagues have proposed the modified MASI score (mMASI), which removes the homogeneity in its calculation because it had the lowest inter-rater agreement. Hence, MASI or mMASI, either of the scores, can be used for melasma.[14]
Recently, the US Food and Drug Administration has approved a modified combination of Kligman’s formulation containing 4% hydroquinone, 0.05% tretinoin, and 0.01% fluocinolone acetonide.[15] Broad-spectrum sunscreens’ protection from UV radiation is useful in the management of melasma, but they usually fail to prevent relapse.[16] The use of tranexamic acid (TXA) has been a recent therapy for treating it. Tranexamic acid (trans-4-aminomethyl cyclohexane carboxylic acid) is a synthetic lysine analog that is mainly used for its antihemorrhagic and antifibrinolytic properties. Tranexamic acid can inhibit UV-induced pigmentation by reducing plasmin via reversible blockade of the lysine-binding site on plasminogen to these cells, decreasing free arachidonic acid, diminishing the production of prostaglandins, and eventually reducing melanogenesis in melanocytes; it also has anti-inflammatory and whitening effects.[17,18,19,20] According to recent studies, different routes of administration, such as oral, topical, intradermal injections, micro-needling, and iontophoresis, as well as different formulations of tranexamic acid, have been used for treating melasma with promising results.[21] Through a meta-analysis, we aimed to assess the therapeutic efficacy of different formulations of tranexamic acid in treating patients with melasma.
Materials and Methods
A meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA) guidelines.[22]
Search strategy and selection criteria
We systematically searched PubMed, Cochrane Library, ClinicalTrials.gov, Google Scholar database, and ctri.nic.in for including relevant clinical trials published till 2021. Search terms included the following: ““Tranexamic acid,” “TXA,” “Melasma,” “Melasma Area and Severity Index (MASI),” “Modified Melasma Area and Severity Index (mMASI),” “Hyperpigmentation,” “Chloasma,” “Oral tranexamic acid,” “Topical tranexamic acid,” “Intradermal tranexamic acid,” “Hydroquinone,” “Vitamin C,” “Triple regimen/Modified Kligman’s regimen,” “Cysteamine,” “Mesotherapy,” and “randomized clinical studies.”
In the primary screening, all the studies that were relevant and were using tranexamic acid in any one of the routes (oral, topical, or intradermal injection) with ongoing medical treatment in a study group of 18 years or older with clinically diagnosed melasma, which were double-blind, single-blind, or unblinded, were included.
Studies with secondary screening with those who included comparison of tranexamic acid in doses of “oral 250 mg twice or thrice daily with or without other treatment in the study group,” “topical tranexamic acid in 2%, 3%, 4%, 5%, or 10% cream twice or thrice daily with or without other treatment in the study group,” “intradermal injection of tranexamic acid at every 4-week interval with medical treatments which included any one of the following: hydroquinone cream, vitamin C, 5% cysteamine, triple regimen/modified Kligman’s regimen which includes giving hydroquinone cream 2% w/w + tretinoin 0.05% w/w + fluocinolone 0.01% w/w, normal saline or distilled water, sunscreen, 1064-nm Q-switched neodymium-doped yttrium aluminum garnet laser (QSNY laser) in the study group” and those studies which provided any available information on MASI or mMASI in quantitative terms like frequency, numbers or percentages were included in the analysis.[14] Those studies in which tranexamic acid was the only treatment, studies without any control group, any group including pregnant patients, lactating females, patients with a history of thrombosis or an abnormal bleeding profile in the study group, any unpublished research work, preclinical studies, or observational studies were excluded from the analysis.
Study selection
After the removal of duplicate and irrelevant studies, each potential article was examined by two investigators independently to see if the study fit the predetermined inclusion criteria. Any disagreement was settled through discussion.
Data collection
From the included studies, data regarding patient characteristics and outcome of interest were collected. The study characteristics, which were publication year, country of origin, sample size, age, and outcome data, were extracted.
Endpoint
The primary efficacy endpoint was assessed by a change in the mean value of MASI or mMASI scores. MASI is a clinical score used for grading pigmentation in melasma ranging from 0 to 48, taking into consideration “area of involvement,” “degree of pigmentation,” and “homogeneity,” while modified MASI (mMASI) eliminates the homogeneity of MASI, at the same time not affecting the reliability or validity of the measures.[14] Change in MASI/mMASI was obtained between pre- and post-treatment with oral, topical, and intradermal tranexamic acid compared to the change in the MASI/mMASI scores with adjuvant treatment at the end of 8 weeks and 12 weeks from the baseline.
Quality assessment of included studies
The quality of the included studies was reviewed independently by two reviewers following the guidelines mentioned in the Cochrane Handbook for Systematic Reviews of Interventions, and the risk of bias was assessed which included different parameters such as selection bias, allocation concealment, blinding of participants and the personnel, attrition bias as incomplete outcome data and selection reporting bias, and other biases were graded as low, unclear, or high risk.
Statistical analysis
Inverse variance method was used for continuous variables to obtain standard mean difference (SMD) along with a 95% confidence interval (95% CI) to describe the change in MASI/mMASI score from the baseline at the end of 8 and 12 weeks as our primary endpoint.[14] Review Manager version 5.4 was used for analysis, and statistical heterogeneity across studies was reported using I2 statistics. I2 statistics of >75% were considered significant. Random effect models were used to estimate the overall effect and to give an accurate estimation of SMDs and 95% CI irrespective of the heterogeneity. P < 0.05 was considered significant.
Results
Baseline characteristics
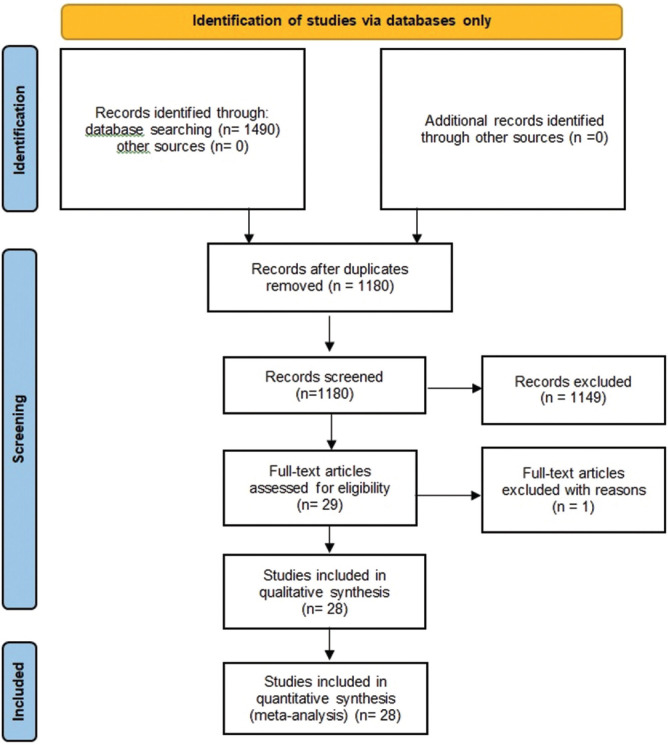
Initially, a total of 29 studies were included through an electronic or manual search and were selected for a full-text review based on the title and abstract details. However, a study done by Wanitphakdeedecha et al. (2020) was excluded as they only had a graphical representation of MASI/mMASI score.[23] Ultimately, 28 studies met the inclusion criteria.[24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] Among these, a study by Sahu et al. used both the oral and topical routes of tranexamic acid.[34] All the studies provided data relevant to the primary outcome. We chose three routes of administration of tranexamic acid: oral, topical, and intradermal injection. The relevant features of the studies included in the meta-analysis are provided in Figure 1 and Supplementary Table. In the end, thirteen studies evaluated the efficacy of oral TXA, nine studies evaluated the efficacy of topical TXA, and seven studies evaluated the efficacy of intradermal injections of TXA.[24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]
Figure 1.
PRISMA flow diagram of included studies
Supplementary Table.
Baseline characteristics of the included studies for meta-analysis
| Study | Country | Sample size (n) | Study design | Intervention and other treatment | Mean or Median age (in years) |
|---|---|---|---|---|---|
| Karn et al. 2012[24] | Nepal | 260 | Prospective, interventional, randomized controlled trial | Oral TXA 250 mg bid and topical HQ and sunscreen versus HQ+sunscreen | 17-55 |
| Shin et al. 2013[25] | Korea | 44 | Randomized prospective trial | Oral TXA 750 mg per day plus QSNY laser versus QSNY laser | 18-55 |
| Padhi et al. 2015[26] | India | 40 | Prospective, parallel, randomized open-label comparative clinical study | Oral TXA 250 mg bid and FbTC cream versus FbTC cream | 24-55 |
| Lajervadi et al. 2017[27] | Iran | 88 | Single center, parallel group, assessor and analyst blinded randomized control trial | Oral TXA 250 mg bid and 4% HQ versus 4% HQ | 18-65 |
| Rafi et al. 2017[28] | Pakistan | 140 | Randomized controlled trial | Topical 2% HQ and oral TXA 500 mg daily versus topical 2% HQ | 15-45 |
| Colferai et al. 2018[29] | Brazil | 37 | Mono centric, randomized, double-blind, controlled trial | Oral TXA 250 mg bid and sunscreen versus sunscreen | 43.97 |
| Del Rosario et al. 2018[30] | USA | 44 | Single center, randomized study | Oral TXA 250 mg bid and sunscreen versus sunscreen | >18 |
| Malik et al. 2019[31] | Pakistan | 100 | Interventional comparative study | Oral TXA 250 mg bid and 3% topical TXA bid versus oral TXA 250 mg bid and azelaic acid | 12-50 |
| Yaghoobi et al. 2019[32] | Iran | 69 | Prospective randomized clinical trial | Oral TXA 250 mg versus 4% topical HQ cream | 18-60 |
| Minni et al. 2020[33] | India | 130 | Parallel designed, prospective, interventional triple blind randomized controlled study | Oral TXA 250 mg bid and topical FbTC versus topical FbTC | 36 |
| Sahu et al. 2020[34] | India | 60 | Prospective, comparative, interventional study | Oral TXA 250 mg bid versus topical TXA versus modified Kligman’s regimen | 18-50 |
| Shihab et al. 2020[35] | Indonesia | 50 | Randomized trial | Oral TXA 250 mg bid and 4% HQ versus 4% HQ | 21-64 |
| Basit et al. 2021[36] | Pakistan | 60 | Randomized trial | Oral TXA 250 mg bid and topical FbTC versus topical FbTC | 18-60 |
| Banihashemi et al. 2015[37] | Iran | 23 | Double-blind clinical trial | 5% liposomal TXA on one side of the face and 4% topical hydroquinone cream on the opposite side of the face once a night, for 12 weeks. | 25-47 |
| Chung et al. 2016[38] | Korea | 13 | Single center, randomized, split-face (internally controlled) study | Four monthly sessions of IPL to both sides of the face along with 2% TXA to one side of the face (topical TXA side) and vehicle without TXA to the other side | 41.38±4.37 years |
| Atefi et al. 2017[39] | Tehran | 60 | Randomized double-blinded clinical trial | 5% TXA versus 2% topical HQ | 38±6.27 years in TXA group and 39.97±7.86 in HQ |
| Laothaworn et al. 2018[40] | Thailand | 25 | Randomized, prospective, split-face, controlled trial | Topical 3% TXA on one side of the face and the vehicle treatment on the other side of the face for 8 weeks and 1064-nm QSNY laser to the entire face at baseline and after 4 weeks. | 30-63 |
| Khuraiya et al. 2019[41] | India | 23 | Prospective comparative study | Topical TXA 5% cream and triple combination of tretinoin, hydroquinone, fluocinolone on each half of face | 18-50 |
| El-Hussiney et al. 2020[42] | Egypt | 100 | Prospective split-faced comparative study | 5% TXA cream on right side face lesions versus 4% HQ cream on left side lesions | 22-40 |
| Kaur et al. 2020[43] | India | 40 | Split-face, prospective, randomized, open-label study | 10% topical TXA solution application on one side of the face and distilled water on the other side of face. | 26-48 |
| Shamsi et al. 2020[44] | Iran | 60 | Single-blind randomized clinical trial | One group micro-needling plus topical 4% TXA, monthly and another group topical 4% HQ, nightly. | 18-35 |
| Menon et al. 2019[45] | India | 30 | Split-face comparative study | Micro-needling with 1 mL (4 mg/mL) TXA on left side, micro-needling with 20% vit C on right side | 35-39 |
| Kaleem et al. 2020[46] | Pakistan | 60 | Non-randomized clinical trial | Intradermal TXA (4 mg/mL) on one side of face, multiple microinjections of 0.9% NS on opposite side of face | 36±7.9 |
| Karrabi et al. 2020[47] | Iran | 54 | Single-blind, randomized, parallel-group clinical trial study | Intradermal microinjections of TXA | 34.29±7.45 |
| Zhao et al. 2020[48] | China | 17 | Split-face randomized controlled trial | Localized Myjet-assisted injection of TXA (0.5 g: 5 mL) and Vit C (1 g: 2.5 mL) | 39.47±6.05 |
| Tahoun et al. 2021[49] | Egypt | 30 | Prospective split-face comparative study | Micro-needling with 1.5 mL (100 mg/mL) of TXA on right side, micro-needling with 1.5 mL of 20% vit C on left side | 40±6.11 |
| Mumtaz et al. 2021[50] | Pakistan | 64 | Non-randomized controlled trial | Intradermal TXA (4 mg) in group B and 1 mL PRP in group A | Group A : 24.63±9.87 Group B: 23.94±8.93 |
| Zakey et al. 2021[51] | Egypt | 50 (27 completed the study) | Prospective, randomized study | Micro-needling with topical 4% TXA every other week in group B, topical 4% HQ cream at night in group A | 31.16±19.16 |
TXA=tranexamic acid, HQ=hydroquinone, QSNY laser=Q-switched neodymium-doped yttrium aluminum garnet laser , FbTC=fluocinolone-based triple combination cream, IPL=intense pulsed light laser, NS=Normal saline, PRP=platelet-rich plasma
Primary efficacy endpoints in oral TXA
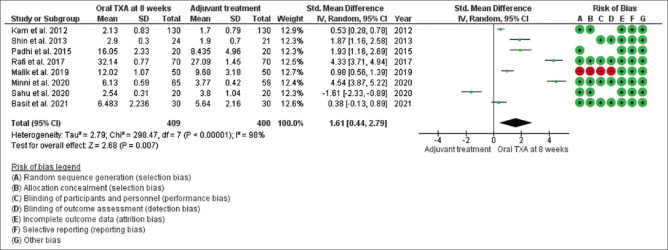
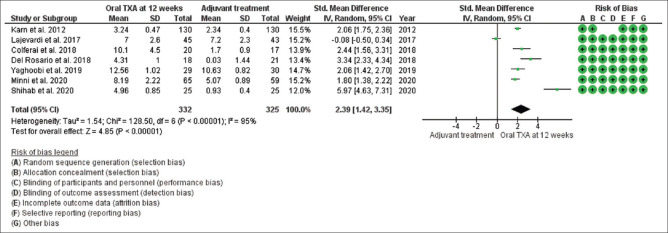
Mean changes in MASI/mMASI score were reported in all 13 studies at 8 weeks and 12 weeks after the beginning of treatment with oral tranexamic acid compared to the other treatment. At 8 weeks, in random effect, SMD was 1.61 and 95% CI was 0.44 to 2.79. P value in random effect at 8 weeks was 0.007, which was significant, as shown in Figure 2. The heterogeneity between studies was 98% (P < 0.00001). At 12 weeks, in random effect, SMD was 2.39 and 95% CI was 1.42 to 3.35. P value in random effect at 12 weeks was < 0.00001, which was again found significant, as shown in Figure 3. The heterogeneity between studies was 95% (P < 0.00001).
Figure 2.
Funnel plot results of the efficacy of oral tranexamic acid in patients with melasma at 8 weeks
Figure 3.
Funnel plot results of the efficacy of oral tranexamic acid in patients with melasma at 12 weeks
Primary efficacy endpoints in topical TXA
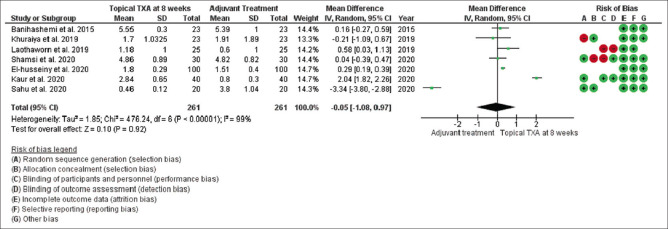
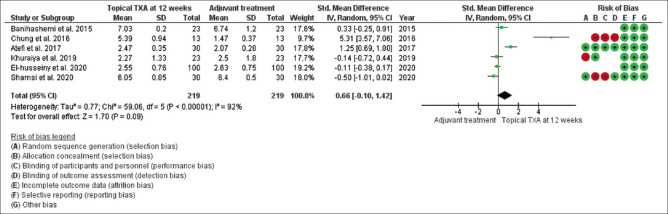
Mean changes in MASI/mMASI score were reported in all nine studies at 8 weeks and 12 weeks after the beginning of treatment with topical tranexamic acid compared to the other treatment. At 8 weeks, in random effect, SMD was -0.05 and 95% CI was -1.08 to 0.97. P value in random effect at 8 weeks was 0.92, which was not significant, as shown in Figure 4. The heterogeneity between studies was 99% (P < 0.00001). At 12 weeks, in random effect, SMD was 0.66 and 95% CI was -0.10 to 1.42. P value in random effect at 12 weeks was 0.09, which was again not significant, as shown in Figure 5. The heterogeneity between studies was 92% (P < 0.00001) as shown in Figure 5.
Figure 4.
Funnel plot results of the efficacy of topical tranexamic acid in patients with melasma at 8 weeks
Figure 5.
Funnel plot results of the efficacy of topical tranexamic acid in patients with melasma at 12 weeks
Primary efficacy endpoints in the intradermal injection of TXA
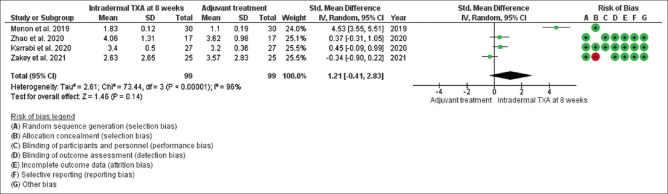
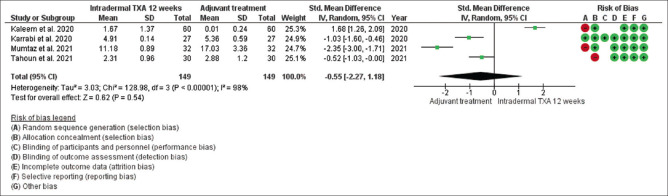
Mean changes in MASI/mMASI score were reported in all seven studies at 8 weeks and 12 weeks after the beginning of treatment with intradermal injection of TXA compared to the other adjuvant treatment. At 8 weeks, in random effect SMD, was 1.21 and 95% CI was -.041 to 2.83. P value in random effect at 8 weeks was 0.14, which was not significant, as shown in Figure 6. The heterogeneity between studies was 96% (P < 0.00001). At 12 weeks, in random effect, SMD was -0.55 and 95% CI was -2.27 to 1.18. P value in random effect at 12 weeks was 0.54, which was also not significant, as shown in Figure 7. The heterogeneity between studies was 98% (P < 0.00001) as shown in Figure 7.
Figure 6.
Funnel plot results of the efficacy of intradermal tranexamic acid in patients with melasma at 8 weeks
Figure 7.
Funnel plot results of the efficacy of intradermal tranexamic acid in patients with melasma at 12 weeks
Discussion
In 1979, Sadako first showed the use of oral tranexamic acid for melasma in Japan.[23] It can also be administered as an intradermal injection or a topical formulation.[38,52,53,54] The role of tranexamic acid in human cell cultures was studied by Maeda et al., in which they revealed that tranexamic acid inhibits melanin synthesis in epidermal melanocytes by blocking the interaction of melanocytes and keratinocytes by inhibition of the plasminogen system.[55] Tranexamic acid has currently become more widely used in the treatment of disorders of pigmentation, especially in Japan.[56] Even in the Chinese population, the use of oral tranexamic acid for melasma has been tried for its effectiveness and safety, as recommended by some authors. However, the dose of oral tranexamic acid used in the treatment of melasma is far less than the dose used for its hemostatic action.[57]
In our meta-analysis, the clinical effectiveness was considered in terms of showing a reduction in MASI/mMASI score at 8th and 12th week from the baseline. We found that oral route of tranexamic acid showed a significant reduction when compared to other adjuvant treatment (8 weeks, SMD was 1.61 and P < 0.007; 12 weeks, SMD was 2.39 and P < 0.00001), while topical route and intradermal route did not show a significant reduction in MASI/mMASI scores when compared to other adjuvant treatment.
Various routes of administration have been described for the use of tranexamic acid in the treatment of hyperpigmentation, including a topical liposomal formulation and intradermal microinjections.[58,59] Hajime et al. used oral tranexamic acid for 10 weeks, during which they showed a reduction in the severity of melasma.[60] Similarly, results were outlined by Higashi et al.[61] In a study by Cho et al. in 2011 which used 500 mg/day of tranexamic acid as an additional therapy to the patients treated with intense pulsed light (IPL) or Nd:YAG laser as compared to patients treated with only IPL or Nd:YAG laser showed that modified MASI score was lower in the tranexamic acid group (P < 0.005).[62] In a triple-blinded randomized control trial by Minni et al., it was shown that oral tranexamic acid + fluocinolone-based triple combination cream (FbTC) results in early response and higher clearance of melasma at 4th week compared with FbTC alone.[33] Recurrence in melasma patients is well-recognized during or after cessation of any treatment (lasers or oral TXA); however, tranexamic acid patients maintained their MASI score from week 12 to 24th week and also showed an improvement despite stopping treatment and the results when compared in both groups were statistically significant in the study by Minni et al. (P < 0.01).[32] Corresponding findings of recurrence in melasma were also reported by Wu et al., Aamir et al., and Lee et al. in their studies.[57,59,63] In a 2013 study by Shin et al., patients received oral tranexamic acid (750 milligrams/day) for 2 months plus two sessions of low-fluency Q-switched Nd: YAG (1064 nm) laser at a 1-month interval or the laser alone as the control. The results were similar to ours in the case of the intervention group, but not significant in the control group.[25] Sharma et al. conducted a clinical trial on patients with melasma, with one group being given oral tranexamic acid (250 mg twice daily) and the other group receiving 4 mg intradermal tranexamic acid every 4 weeks for a period of 12 weeks, which showed a significant reduction in MASI for oral tranexamic acid.[64] Del Rosario et al. also showed similar results with oral tranexamic acid as an effective drug in treating moderate to severe melisma.[30] The systematic review and meta-analysis by Kim et al. on tranexamic acid-only observational studies with pre- and post-treatment MASI also showed a decrease of 1.60 in MASI (95% CI 1.20–2.00; P < 0.001) and established the safety profile.[65]
Many studies revealed the outcomes of topical tranexamic acid in the treatment of melasma, for instance, Banihashemi et al. and Ebrahimi et al.[37,66] Kanechorn Na Ayuthaya et al. compared topical tranexamic acid to a placebo treatment, although no significant difference was seen, the topical medications were able to drastically decrease pigmentation as compared to baseline data.[67] An uncontrolled study conducted in 2007 by Kondou et al. studied the effects of 2% topical emulsion of tranexamic acid in 25 patients for 18 weeks which showed that it was effective in 80% of patients within 8 weeks with no side effects.[68] A recent study by Chung JY et al. in which only IPL was compared with topical tranexamic acid in combination with IPL given randomly to any of the assigned cheeks showed a marked decrease in pigmentation and prevented rebound hyperpigmentation in topical TXA side.[38] Moreover, a pilot study by Laothaworn et al. showed similar results.[40] Ebrahimi et al. also showed significant decrease in MASI score in both groups of topical tranexamic acid and topical HQ plus dexamethasone with no significant difference between the groups.[66]
Lee et al. showed a significant improvement in MASI at the end of treatment following weekly intradermal injection of tranexamic acid.[59] In a study conducted by Budamakuntla et al. in India that evaluated the efficacy of topical TXA with micro-needling in comparison with microinjections of tranexamic acid, there was more reduction in MASI score in micro-needling group than in microinjection group, but the difference was not significant.[69] Studies conducted by Menon et al.[45] and Kaleem et al.[46] showed a significant reduction in the mean MASI score with tranexamic acid. Similar results were also seen with Zaky et al.[51] Another study by Tahoun et al. exhibited significant diminution in dark fine granules, homogeneous pigmentation, and pseudo-reticular brown network with intradermally treated tranexamic acid.[49]
There were a few limitations to the study; we addressed the clinical efficacy of tranexamic acid in melasma and not the adverse events associated with it, and did not address the role of variation in skin color that may have played a role. Furthermore, carrying out a meta-analysis inherently brings bias in terms of heterogeneity of the included studies, which was also seen in our analysis. Further research is needed to compare the clinical efficacy as well as the safety profile of tranexamic acid in melasma through large randomized controlled trials focusing on its clinical implications.
Conclusion
Oral tranexamic acid is superior to the other standard treatments of melasma; it can be used either alone or combined with other standard treatments. Topical and intradermal routes of tranexamic acid were not found superior to the standard treatment. However, a multicentric, larger-scale study is required to support our observation.
Financial support and sponsorship
No financial support in any form was provided.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sarkar R, Arora P, Garg VK, Sonthalia S, Gokhale N. Melasma update. Indian Dermatol Online J. 2014;5:426–35. doi: 10.4103/2229-5178.142484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalaycı MM. Pregnancy Mask: Melasma. JEB Med Sci. 2020;1:65–7. [Google Scholar]
- 3.Lee AY. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015;28:648–60. doi: 10.1111/pcmr.12404. [DOI] [PubMed] [Google Scholar]
- 4.Passeron T. Melasma pathogenesis and influencing factors-An overview of the latest research. J Eur Acad Dermatol Venereol. 2013;27(Suppl 1):5–6. doi: 10.1111/jdv.12049. [DOI] [PubMed] [Google Scholar]
- 5.Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: A review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25 13030/qt47b7r28c. [PubMed] [Google Scholar]
- 6.Patil S, Raj T, Rao R. S, Warnakulasuriya S. Pigmentary Disorders of Oral Mucosa. Pigmentary Disorders, 2, 225 2015 [Google Scholar]
- 7.Lee A-Y. An updated review of melasma pathogenesis. Dermatologica Sinica. 2014;32:233–9. [Google Scholar]
- 8.Filoni A, Mariano M, Cameli N. Melasma: How hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458–63. doi: 10.1111/jocd.12877. [DOI] [PubMed] [Google Scholar]
- 9.Gopichandani K, Arora P, Garga U, Bhardwaj M, Sharma N, Gautam RK. Hormonal profile of melasma in Indian females. Pigment International. 2015;2:85–90. [Google Scholar]
- 10.Tamega Ade A, Miot LD, Bonfietti C, Gige TC, Marques ME, Miot HA. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian Women. J Eur Acad Dermatol Venereol. 2013;27:151–6. doi: 10.1111/j.1468-3083.2011.04430.x. [DOI] [PubMed] [Google Scholar]
- 11.Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in Melasma. Indian J Dermatol. 2015;60:519. doi: 10.4103/0019-5154.164415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Achar A, Rathi SK. Melasma: A clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380–2. doi: 10.4103/0019-5154.84722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freitag FM, Cestari TF, Leopoldo LR, Paludo P, Boza JC. Effect of melasma on quality of life in a sample of women living in southern Brazil. J Eur Acad Dermatol Venereol. 2008;22:655–62. doi: 10.1111/j.1468-3083.2007.02472.x. [DOI] [PubMed] [Google Scholar]
- 14.Pandya AG, Hynan LS, Bhore R, Riley FC, Guevara IL, Grimes P, Nordlund JJ, Rendon M, Taylor S, Gottschalk RW, Agim NG, Ortonne JP. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78–83. doi: 10.1016/j.jaad.2009.10.051. 83.e1-2. [DOI] [PubMed] [Google Scholar]
- 15.Wali V, Parwani H. Comparative study of oral tranexamic acid and triple combination versus tranexamic acid through microneedling in patients of Melisma. Int J Res Dermatol. 2019;5:537–41. [Google Scholar]
- 16.Boukari F, Jourdan E, Fontas E, Montaudié H, Castela E, Lacour JP, et al. Prevention of Melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: A prospective randomized comparative trial. J Am Acad Dermatol. 2015;72:189–90. doi: 10.1016/j.jaad.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Maeda K, Naganuma M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J Photochem Photobiol B. 1998;47:136–41. doi: 10.1016/s1011-1344(98)00212-7. [DOI] [PubMed] [Google Scholar]
- 18.Na JI, Choi SY, Yang SH, Choi HR, Kang HY, Park KC. Effect of tranexamic acid on Melasma: A clinical trial with histological evaluation. J Eur Acad Dermatol Venereol. 2013;27:1035–9. doi: 10.1111/j.1468-3083.2012.04464.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of Melasma: A retrospective analysis. J Am Acad Dermatol. 2016;75:385–92. doi: 10.1016/j.jaad.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Dunn CJ, Goa KL. Tranexamic acid: A review of its use in surgery and other indications. Drugs. 1999;57:1005–32. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 21.Wang JV, Jhawar N, Saedi N. Tranexamic Acid for Melasma: Evaluating the various formulations. J Clin Aesthet Dermatol. 2019;12:E73–4. [PMC free article] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–12. doi: 10.1016/j.jclinepi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Wanitphakdeedecha R, Keoprasom N, Eimpunth S, Manuskiatti W. The efficacy in melasma treatment using a 1410 nm fractional photothermolysis laser. Journal of the European Academy of Dermatology and Venereology. 2014 Mar;28(3):293–7. doi: 10.1111/jdv.12100. [DOI] [PubMed] [Google Scholar]
- 24.Karn D, Kc S, Amatya A, Razouria EA, Timalsina M. Oral tranexamic acid for the treatment of Melasma. Kathmandu Univ Med J (KUMJ) 2012;10:40–3. doi: 10.3126/kumj.v10i4.10993. [DOI] [PubMed] [Google Scholar]
- 25.Shin JU, Park J, Oh SH, Lee JH. Oral tranexamic acid enhances the efficacy of low-fluence 1064-nm quality-switched neodymium-doped yttrium aluminum garnet laser treatment for Melasma in Koreans: A randomized, prospective trial. Dermatol Surg. 2013;39:435–42. doi: 10.1111/dsu.12060. [DOI] [PubMed] [Google Scholar]
- 26.Padhi T, Pradhan S. Oral tranexamic acid with fluocinolone-based triple combination cream versus fluocinolone-based triple combination cream alone in Melasma: An open labeled randomized comparative trial. Indian J Dermatol. 2015;60:520. doi: 10.4103/0019-5154.164416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lajevardi V, Ghayoumi A, Abedini R, Hosseini H, Goodarzi A, Akbari Z, et al. Comparison of the therapeutic efficacy and safety of combined oral tranexamic acid and topical hydroquinone 4% treatment vs. topical hydroquinone 4% alone in Melasma: A parallel-group, assessor- and analyst-blinded, randomized controlled trial with a short-term follow-up. J Cosmet Dermatol. 2017;16:235–42. doi: 10.1111/jocd.12291. [DOI] [PubMed] [Google Scholar]
- 28.Rafi S, Iftikhar U, Rani Z, Hussain I. Comparison of efficacy and safety of topical hydroquinone 2% and oral tranexamic acid 500 mg in patients of melasma. J Pak Assoc Dermatol. 2017;27:204–13. [Google Scholar]
- 29.Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of Melasma. J Cosmet Dermatol. 2019;18:1495–501. doi: 10.1111/jocd.12830. [DOI] [PubMed] [Google Scholar]
- 30.Del Rosario E, Florez-Pollack S, Zapata L, Jr, Hernandez K, Tovar-Garza A, Rodrigues M, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe Melasma. J Am Acad Dermatol. 2018;78:363–9. doi: 10.1016/j.jaad.2017.09.053. [DOI] [PubMed] [Google Scholar]
- 31.Malik F, Hanif MM, Mustafa G. Combination of oral tranexamic acid with topical 3% tranexamic acid versus oral tranexamic acid with topical 20% azelaic acid in the treatment of Melasma. J Coll Physicians Surg Pak. 2019;29:502–4. doi: 10.29271/jcpsp.2019.06.502. [DOI] [PubMed] [Google Scholar]
- 32.Yaghoobi R, Vala S, Pazyar N, Zeinali M, Hesam S. Comparing efficacy and safety of oral tranexamic acid and 4% topical hydroquinone cream in Melasma Treatment: A randomized controlled clinical trial and review of literature. Serbian J Dermatology Venereol. 2019;11:119–28. [Google Scholar]
- 33.Minni K, Poojary S. Efficacy and safety of oral tranexamic acid as an adjuvant in Indian patients with Melasma: A prospective, interventional, single-centre, triple-blind, randomized, placebo-control, parallel group study. J Eur Acad Dermatol Venereol. 2020;34:2636–44. doi: 10.1111/jdv.16598. [DOI] [PubMed] [Google Scholar]
- 34.Sahu PJ, Singh AL, Kulkarni S, Madke B, Saoji V, Jawade S. Study of oral tranexamic acid, topical tranexamic acid, and modified Kligman's regimen in treatment of Melasma. J Cosmet Dermatol. 2020;19:1456–62. doi: 10.1111/jocd.13430. [DOI] [PubMed] [Google Scholar]
- 35.Shihab N, Prihartono J, Tovar-Garza A, Agustin T, Legiawati L, Pandya AG. Randomised, controlled, double-blind study of combination therapy of oral tranexamic acid and topical hydroquinone in the treatment of Melasma. Australas J Dermatol. 2020;61:237–42. doi: 10.1111/ajd.13267. [DOI] [PubMed] [Google Scholar]
- 36.Basit A, Rahman A, Uddin R. Oral Tranexemic Acid with triple combination cream (Flucinolone+Hydroquinone+Tretinoin) versus triple combination cream alone in treatment of Melasma. J Ayub Med Coll Abbottabad. 2021;33:293–8. [PubMed] [Google Scholar]
- 37.Banihashemi M, Zabolinejad N, Jaafari MR, Salehi M, Jabari A. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on Melasma. J Cosmet Dermatol. 2015;14:174–7. doi: 10.1111/jocd.12152. [DOI] [PubMed] [Google Scholar]
- 38.Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in Melasma: Side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373–7. doi: 10.3109/09546634.2015.1115812. [DOI] [PubMed] [Google Scholar]
- 39.Atefi N, Dalvand B, Ghassemi M, Mehran G, Heydarian A. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of Women with Melasma. Dermatol Ther (Heidelb) 2017;7:417–24. doi: 10.1007/s13555-017-0195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laothaworn V, Juntongjin P. Topical 3% tranexamic acid enhances the efficacy of 1064-nm Q-switched neodymium-doped yttrium aluminum garnet laser in the treatment of Melasma. J Cosmet Laser Ther. 2018;20:320–5. doi: 10.1080/14764172.2018.1427869. [DOI] [PubMed] [Google Scholar]
- 41.Khuraiya S, Kachhawa D, Chouhan B, Dua M, Rao P. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of Melasma in Indian population. Pigment International. 2019;6:18. [Google Scholar]
- 42.El-Husseiny R, Rakha N, Sallam M. Efficacy and safety of tranexamic acid 5% cream vs hydroquinone 4% cream in treating Melasma: A split-face comparative clinical, histopathological, and antera 3D camera study. Dermatol Ther. 2020;33:e14240. doi: 10.1111/dth.14240. [DOI] [PubMed] [Google Scholar]
- 43.Kaur A, Bhalla M, Pal Thami G, Sandhu J. Clinical efficacy of topical tranexamic acid with microneedling in Melasma. Dermatol Surg. 2020;46:e96–101. doi: 10.1097/DSS.0000000000002520. [DOI] [PubMed] [Google Scholar]
- 44.Shamsi Meymandi S, Mozayyeni A, Shamsi Meymandi M, Aflatoonian M. Efficacy of microneedling plus topical 4% tranexamic acid solution vs 4% hydroquinone in the treatment of Melasma: A single-blind randomized clinical trial. J Cosmet Dermatol. 2020;19:2906–11. doi: 10.1111/jocd.13392. [DOI] [PubMed] [Google Scholar]
- 45.Menon A, Eram H, Kamath PR, Goel S, Babu AM. A split face comparative study of safety and efficacy of Microneedling with tranexamic acid versus Microneedling with Vitamin C in the Treatment of Melasma. Indian Dermatol Online J. 2020;11:41–5. doi: 10.4103/idoj.IDOJ_22_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaleem S, Ghafoor R, Khan S. Comparison of efficacy of Tranexamic Acid Mesotherapy versus 0.9% normal Saline for Melasma;A split face study in a Tertiary Care Hospital of Karachi. Pak J Med Sci. 2020;36:930–4. doi: 10.12669/pjms.36.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karrabi M, Mansournia MA, Sharestanaki E, Abdollahnejad Y, Sahebkar M. Clinical evaluation of efficacy and tolerability of cysteamine 5% cream in comparison with tranexamic acid mesotherapy in subjects with melasma: A single-blind, randomized clinical trial study. Arch Dermatol Res. 2021;313:539–47. doi: 10.1007/s00403-020-02133-7. [DOI] [PubMed] [Google Scholar]
- 48.Zhao H, Li M, Zhang X, Li L, Yan Y, Wang B. Comparing the efficacy of Myjet-assisted tranexamic acid and vitamin C in treating melasma: A split-face controlled trial. J Cosmet Dermatol. 2020;19:47–54. doi: 10.1111/jocd.13112. [DOI] [PubMed] [Google Scholar]
- 49.Tahoun AI, Mostafa WZ, Amer MA. Dermoscopic evaluation of tranexamic acid versus Vitamin C, with microneedling in the treatment of melasma: A comparative, split-face, single-blinded study. J Dermatolog Treat. 2022;33:1623–9. doi: 10.1080/09546634.2021.1871582. [DOI] [PubMed] [Google Scholar]
- 50.Mumtaz M, Chandio TH, Shahzad MK, Hanif N, Anwar S, Rafique S. Comparing the Efficacy of Patelet-rich Plasma (PRP) versus Tranexamic Acid (4 mg/mL) as Intradermal Treatments of Melasma. J Coll Physicians Surg Pak. 2021;30:502–5. doi: 10.29271/jcpsp.2021.05.502. [DOI] [PubMed] [Google Scholar]
- 51.Zaky MS, Obaid ZM, Khalil EA, Elsaie ML. Microneedling-assisted topical tranexamic acid solution versus 4% hydroquinone for treating melasma: A split-face randomized study. J Cosmet Dermatol. 2021;20:4011–6. doi: 10.1111/jocd.14440. [DOI] [PubMed] [Google Scholar]
- 52.Tse TW, Hui E. Tranexamic acid: An important adjuvant in the treatment of Melasma. J Cosmet Dermatol. 2013;12:57–66. doi: 10.1111/jocd.12026. [DOI] [PubMed] [Google Scholar]
- 53.Rodrigues M, Pandya AG. Melasma: Clinical diagnosis and management options. Australas J Dermatol. 2015;56:151–63. doi: 10.1111/ajd.12290. [DOI] [PubMed] [Google Scholar]
- 54.Kim SJ, Park JY, Shibata T, Fujiwara R, Kang HY. Efficacy and possible mechanisms of topical tranexamic acid in Melasma. Clin Exp Dermatol. 2016;41:480–5. doi: 10.1111/ced.12835. [DOI] [PubMed] [Google Scholar]
- 55.Maeda K, Tomita Y. Mechanism of the inhibitory effect of tranexamic acid on melanogenesis in cultured human melanocytes in the presence of keratinocyte-conditioned medium. J Health Sci. 2007;53:389–96. [Google Scholar]
- 56.Ando H, Matsui MS, Ichihashi M. Quasi-drugs developed in Japan for the prevention or treatment of hyperpigmentary disorders. Int J Mol Sci. 2010;11:2566–75. doi: 10.3390/ijms11062566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu S, Shi H, Wu H, Yan S, Guo J, Sun Y, et al. Treatment of Melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964–70. doi: 10.1007/s00266-012-9899-9. [DOI] [PubMed] [Google Scholar]
- 58.Manosroi A, Podjanasoonthon K, Manosroi J. Development of novel topical tranexamic acid liposome formulations. Int J Pharm. 2002;235:61–70. doi: 10.1016/s0378-5173(01)00980-2. [DOI] [PubMed] [Google Scholar]
- 59.Lee JH, Park JG, Lim SH, Kim JY, Ahn KY, Kim MY, et al. Localized intradermal microinjection of tranexamic acid for treatment of Melasma in Asian patients: A preliminary clinical trial. Dermatol Surg. 2006;32:626–31. doi: 10.1111/j.1524-4725.2006.32133.x. [DOI] [PubMed] [Google Scholar]
- 60.Hajime M, Mineo T, Yoshio T. Oral administration therapy with tranexamic acid for melasma. Nishinihon J Dermatol. 1985;47:1101–4. [Google Scholar]
- 61.Higashi N. Treatment of Melasma with oral tranexamic acid. Skin Res. 1988;30:676–80. [Google Scholar]
- 62.Cho HH, Choi M, Cho S, Lee JH. Role of oral tranexamic acid in Melasma patients treated with IPL and low fluence QS Nd: YAG laser. J Dermatolog Treat. 2013;24:292–6. doi: 10.3109/09546634.2011.643220. [DOI] [PubMed] [Google Scholar]
- 63.Aamir S, Naseem R. Oral tranexamic acid in treatment of Melasma in Pakistani population: A pilot study. J Pak Assoc Dermatol. 2014;24:198–203. [Google Scholar]
- 64.Sharma R, Mahajan VK, Mehta KS, Chauhan PS, Rawat R, Shiny TN. Therapeutic efficacy and safety of oral tranexamic acid and that of tranexamic acid local infiltration with microinjections in patients with Melasma: A comparative study. Clin Exp Dermatol. 2017;42:728–34. doi: 10.1111/ced.13164. [DOI] [PubMed] [Google Scholar]
- 65.Kim HJ, Moon SH, Cho SH, Lee JD, Kim HS. Efficacy and safety of tranexamic acid in Melasma: A meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776–81. doi: 10.2340/00015555-2668. [DOI] [PubMed] [Google Scholar]
- 66.Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for Melasma. J Res Med Sci. 2014;19:753–7. [PMC free article] [PubMed] [Google Scholar]
- 67.Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, Nakakes A. Topical 5% tranexamic acid for the treatment of Melasma in Asians: A double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150–4. doi: 10.3109/14764172.2012.685478. [DOI] [PubMed] [Google Scholar]
- 68.Kondou S. Clinical study of effect of tranexamic acid emulsion on melasma and freckles. Skin Research. 2007;6:309–15. [Google Scholar]
- 69.Budamakuntla L, Loganathan E, Suresh DH, Shanmugam S, Suryanarayan S, Dongare A, et al. A Randomised, Open-label, Comparative Study of Tranexamic Acid Microinjections and Tranexamic Acid with Microneedling in Patients with Melasma. J Cutan Aesthet Surg. 2013;6:139–43. doi: 10.4103/0974-2077.118403. [DOI] [PMC free article] [PubMed] [Google Scholar]